Introduction
In 1996, the East-West Cancer Center developed an
antitumor agent known as HangAmDan (HAD) (1). Originally, this compound contained 9
antitumor oriental medicinal herbs (Table I) (1). However, it was later modified to
HangAmDan-B (HAD-B; Table II),
which contains 8 herbs (2). The
antitumor effects and safety of HAD have been tested in in
vitro and in vivo studies (2,3). The
toxicity of HangAmDan-B (HAD-B) in mice has been investigated over
short (1-week single oral dose) and long (5-week repeated oral
dose) time periods (4). No
mortalities or significant differences in hematological factors
were observed. HAD-B was demonstrated to be safe, nontoxic and
suitable for use in studies with mice. The results of animal
studies have provided preclinical evidence of the safety of HAD-B,
meaning that it may be used in human studies (4). A traditional Korean medical therapy,
termed Wheel Balance Therapy, also produced positive results in
case studies using HAD (1). The
integrative cancer treatment program, termed wheel balance cancer
therapy (WBCT), incorporates traditional oriental treatments with
conventional cancer treatments. The WBCT program includes
anticancer diet, metabolism activation, antiangiogenic and
immunoenhancing herbal therapy and, meditation and exercise
(5). HAD-B inhibited the cell
motility and invasiveness of NCI-H460 cells, in addition to
inhibiting the activity of matrix metalloproteinase (MMP)-2 and
MMP-9, in a non-cytotoxic dose-dependent manner (2). HAD-B has also been associated with
the suppression of mRNA and protein expression, and the
upregulation of tissue inhibitors of metalloproteinase (TIMP)-1 and
TIMP-2. Reduced invasion was associated with increased tightness of
tight junctions (2). In addition,
HAD-B treatment led to reduced levels of claudin family members,
which are involved in the control of tight junctions, and are thus
important for the processes underlying paracellular transport.
Tight junctions and MMPs are important targets associated with
HAD-B-mediated suppression of invasiveness in NCI-H460 cells
(2). Human umbilical vein
endothelial cells exhibit significant suppression of bFGF-induced
proliferation, adhesion, migration and capillary tube formation in
response to treatment with water extract of HangAmDan (WEHAD). This
treatment was associated with the upregulation of RAD51, RAD52 and
p73, and the downregulation of pFAK. Chick chorioallantoic
membranes displayed a reduction in the length of blood vessels that
formed following treatment with WEHAD. A role has been suggested
for WEHAD in the inhibition of angiogenesis, as a mechanism that
may underlie the anticancer effects of HAD (6).
 | Table IIngredients of HangAmDan. |
Table I
Ingredients of HangAmDan.
| Herbs (Latin
Botanical Name) | Relative quantity
(mg) |
|---|
| Coix Lachryma
semen | 259.0 |
| Panax notoginseng
radix | 86.0 |
| Hippocampus
kelloggi | 26.0 |
| Cordyceps
militaris | 26.0 |
| Santsigu
tuber | 26.0 |
| Ginseng
radix | 26.0 |
| Bovis
calculus | 17.0 |
| Ipomoea batatas
Margarita | 17.0 |
| Moschus | 17.0 |
| Total quantity (1
capsule) | 500.0 |
 | Table IIIngredients of HangAmDan-B. |
Table II
Ingredients of HangAmDan-B.
| Herbs (Latin
Botanical Name) | Relative quantity
(mg) |
|---|
| Panax notoginseng
radix | 95.2 |
| Cordyceps
Militaris | 71.4 |
| Cremastrae
appendiculata Tuber | 71.4 |
| Panx ginseng
radix | 71.4 |
| Bovis
calculus | 47.6 |
| Ipomoea batatas
Margarita | 47.6 |
| Boswellia
cateri | 47.6 |
| Commiphora
myrrha | 47.6 |
| Total quantity (1
capsule) | 499.8 |
Ovarian cancer is one of the most lethal types of
gynecological cancer. It was the fifth leading cause of
cancer-related mortality in females in the United States in 2008
(7). Each year ~125,000
mortalities occur due to ovarian cancer and 204,000 new cases are
diagnosed worldwide (8). Advanced
disease at the time of diagnosis is one of the primary reasons for
the high fatality rate; 70% females with ovarian cancer are
diagnosed at an advanced stage (7,8).
When the disease is confined to the ovary, the rate of successful
treatment is high (70–90%). However, when diagnosis occurs at an
advanced stage, the five-year survival rates drop to 20–30%. In
view of these statistics, early detection may be the most effective
method for reducing the mortality rate of ovarian cancer (8). Ovarian cancer is the second most
common type of gynecological cancer in Korea. Mortality rates for
ovarian cancer increased 9.5-fold in Korea between 1983 and 2006
(9).
Heat-shock protein 27 (HSP27) has a low molecular
weight and is produced in response to pathophysiological stress in
animal cells (10). Malignant
tumors have been demonstrated to express higher levels of HSP27
than benign tumors (11). HSP27 is
an independent marker for survival in ovarian cancer (10). In a study by Elpek et al
(12), it was demonstrated that
HSP70 and HSP90 expression had no significant prognostic
association with epithelial ovarian carcinoma, while HSP27
expression and The International Federation of Gynecology and
Obstetrics (FIGO) staging were reliable indicators of prognosis.
Increased levels of auto-antibodies against HSP27 in patients with
breast, ovarian or endometrial cancer suggest the presence and
increased levels of HSP27 in the circulation of these individuals
(13). HSP27 has been observed to
be exclusively localized to the cytoplasm, and is associated with
high-grade tumors (14).
Heat-shock proteins are important in a number of mechanisms
underlying carcinogenesis and are associated with resistance to
anticancer drug therapy in ovarian carcinoma (15). A previous study demonstrated that
upregulation of HSP27 in human breast cancer cells reduced
susceptibility to herceptin by increasing the stability of the
human epidermal growth factor receptor 2 (Her2) protein (16).
In the present study, the effects of HAD-B treatment
on ovarian cancer cell proliferation, and the possible mechanisms
underlying the effects of HAD-B in this process were
investigated.
Materials and methods
Preparation of HangAmDan-B
HAD-B (Daejeon Oriental Hospital, Daejeon, Korea), a
herbal formula consisting of Radix P. notoginseng, C. militaris,
C. appendiculata, Radix P. ginseng, calculus bovis, I. batatas
Margarita, B. carteri and C. myrrha in powder form, was
prepared as reported in previous studies (1–4) and
stored at −20°C prior to use. The formula was prepared as a stock
solution with a final concentration of 10 mg/ml, and was diluted in
media during subsequent experiments, as required.
Human ovarian cancer cell lines
The NIH:OVCAR-3 and SKOV-3 human ovarian cancer cell
lines were obtained from the American Type Culture Collection
(www.atcc.org).
3-[4,5-dimethylthiazol-2-yl]-
2,5-diphenyltetrazolium bromide (MTT) assay
A colorimetric MTT assay was used to assess cell
proliferation following HAD-B treatment. MTT assays were performed
as described in a previous study (17). Briefly, equal numbers of cells were
incubated in each well in 0.2 ml serum-free or serum-containing
culture medium. Following 2 or 4 days of culture, 0.1 mg MTT
(Sigma-Aldrich, St. Louis, MO, USA) was added to each well, and
incubated at 37°C for a further 4 h. Plates were centrifuged at 450
× g for 5 min at room temperature and the medium was then removed.
Dimethyl sulfoxide (0.15 ml) was added to each well to solubilize
the crystals, and plates were then read at 540 nm using a PowerWave
HT microplate spectrophotometer (Bio-Tek Instruments Inc.,
Winooski, VT, USA). All experiments were performed in
triplicate.
Fluorescence-activated cell sorting
(FACS) analysis
OVCAR-3 and SKOV-3 cells were treated with HAD-B.
Following incubation for 48-h, apoptosis detection was conducted
using a fluorescein isothiocyanate (FITC) Annexin V Apoptosis
Detection kit (cat no. 556547; BD Biosciences, San Diego, CA, USA).
FACS analysis was performed using a BD FACSCalibur using CellQuest
Pro software (BD Biosciences).
Invasion assay
The tumor cell invasion assay was conducted in a BD
BioCoat Matrigel™ Invasion Chamber, with 24-well plates (BD
Biosciences, Franklin Lakes, NJ, USA). The upper chamber was filled
with 2.5×104 cells in serum-free media, which were then
treated with HAD-B for 72 h in an incubator maintained at 37°C in
5% CO2 in a humidified atmosphere. Following treatment
for 72 h in the upper chamber, cells were fixed and stained using
Diff-Quik (cat no. 38721; Sysmex Asia Pacific Pte. Ltd., Tampines
Grande, Singapore). Images were captured using an Olympus BX51
microscope (Olympus Corporation, Tokyo, Japan) at a magnification
of ×100. The number of stained cells was counted, and cell invasion
was expressed as the absolute cell count from a representative
image taken from the lower surface of the filter.
Western blot analysis
Western blot analysis was performed as described in
a previous study (17). Briefly,
cell homogenates containing equivalent amounts of protein were
centrifuged at 4,000 × g, and the supernatant fractions were
subjected to SDS-PAGE (Invitrogen Life Technologies, Carlsbad, CA,
USA). Following electrophoresis, proteins were transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA) and blocked by incubation for 2 h at 4°C in 1% Tween
20-Tris-buffered saline, containing 1.5% non-fat powdered milk
(Bio-Rad Laboratories, Hercules, CA, USA) and 1 mM MgCl2
(Biochemicals Inc., Gyeonggi, Republic of Korea). Membranes were
incubated for 2 h at room temperature with primary antibodies
against caspase-3 (cat. no. 9664; rabbit monoclonal; 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), PARP (cat. no. 9541;
rabbit polyclonal; 1:1,000; Cell Signaling Technology, Inc.), HSP27
(cat. no. ab1426; rabbit polyclonal; 1:1,000′ Abcam, Cambridge,
UK), p-HSP27 (Ser-15; cat. no. ab5581; rabbit polyclonal; 1:1,000;
Abcam), p-HSP27 (Ser-78; cat. no. ab32501; rabbit monoclonal;
1:1,000; Abcam), p-HSP27 (Ser-82; cat. no. ab90537; rabbit
polyclonal; 1:1,000; Abcam), Her2 (cat. no. ab2428; rabbit
polyclonal; 1:1,000; Abcam), p-AKT (Ser-473; cat. no. 9271; rabbit
polyclonal; 1:1,000; Cell Signaling Technology, Inc.), AKT (cat.
no. 9272; rabbit polyclonal; 1:1,000; Cell Signaling Technology,
Inc.), phosphorylated mechanistic target of rapamycin (p-mTOR;
Ser-2448; cat. no.2971, rabbit polyclonal; 1:1,000; Cell Signaling
Technology, Inc.), mTOR (cat. no. 2972; rabbit polyclonal; 1:1,000;
Cell Signaling Technology, Inc.), phosphorylated extracellular
signal regulated kinases (p-ERK; cat. no. 4370; rabbit monoclonal;
1:1,000; Cell Signaling Technology, Inc.) and actin (cat. no.
sc-69879; mouse monoclonal; 1:1,000; Santa Cruz Biotechnology, Inc.
Dallas, TX, USA). Membranes were then washed 3 times for 15 min
with blocking solution, and incubated with diluted horseradish
peroxidase-conjugated secondary antibodies (SouthernBiotech,
Birmingham, AL, USA) for 1 h at room temperature. This was followed
by 3 washes with blocking solution for 15 min, incubation with
WEST-ZOL® plus chemiluminescence reagent (Intron
Biotechnology, Inc., Gyeonggi-do, Korea) for 1 min, and exposure to
film (Kodak Blue XB-1; Kodak, Rochester, NY, USA).
2-D electrophoresis (E), liquid
chromatography-mass spec- trometry (LC-MS)/MS and database
searching
2-DE analysis was performed as described in a
previous study (17). Proteins
(150 mg) were applied to 13 cm immobilized non-linear gradient
strips (pH 3–10; GE Healthcare, Chalfont, UK), and focused at 8,000
V within 3 h. For 2-D separation, 12% polyacrylamide gels
(chemicals from Serva Electrophoresis GmbH, Heidelberg, Germany and
Bio-Rad Laboratories) were used. 2-DE gels were stained with a
Colloidal Blue Staining kit (Invitrogen Life Technologies) for 24
h, and then destained with deionized water. 2-DE gels containing
proteins of interest were excised, destained with 50% aceto-nitrile
in 0.1 M ammonium bicarbonate, and dried in a Savant SpeedVac
Centrifugal Evaporator (Thermo Fisher Scientific, Waltham, MA,
USA). Dried gel pieces were re-swollen with 30 µl sodium
bicarbonate (25 mM; pH 8.8), containing 50 ng trypsin (Promega
Corporation, Madison, WI, USA) at 37°C overnight. Samples were
desalted using ZipTip C18 pipette tips (EMD Millipore), and
dissolved in 10 µl 2% acetonitrile in 0.1% formic acid.
Analysis was performed using a LTQ XL Linear Ion Trap Mass
Spectrometer system (Thermo Fisher Scientific) in the Proteomics
Core, National Cancer Center (Seoul, Korea). The mass spectrometry
was set for nanospray ionization (NSI) in positive mode. A syringe
pump was used to introduce the calibration solution for automatic
tuning and calibration of the mass spectrometer in NSI positive ion
mode. Infusion of digested samples into the ionization source of
the mass spectrometer was accomplished by liquid chromatographic
separation. The spray voltage was set at +1.8 kV, the temperature
of the capillary was set at 200°C, the capillary voltage was set at
+20 V and the tube lens voltage was set at +100 V. The auxiliary
gas was set to 0 arb. Full scan experiments were performed to
linear trap in the range m/z 150–2000. Systematic MS/MS experiments
were performed by changing the relative collision energy and
monitoring the intensities of the fragment ions. All MS/MS samples
were analyzed using Proteome Discoverer software, version 1.4
(Thermo Fisher Scientific). The Proteome Discoverer was set up to
search the uniprot_sprot database (www.uniprot.org) and IPI human database (www.ebi.ac.uk/IPI). The database searching parameters
included up to two missed cleavages allowing for full trypsin
digestion, fixed modification for carbamidomethyl cysteine and
variable modifications for methionine oxidation.
Statistical analysis
Differneces between groups were calculated using
Studen's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Suppression of the rate of proliferation
following HAD-B treatment in the absence of serum
In order to study the effect of HAD-B on NIH:OVCAR-3
and SKOV-3 cell lines, an MTT assay was performed (Fig. 1A). In the absence of serum, HAD-B
treatment led to a dose-dependent reduction in the proliferation
rate. With continued treatment, the rate of proliferation decreased
in a time-dependent manner. In the presence of serum, NIH:OVCAR-3
and SKOV-3 cell lines exhibited variable changes in cell
proliferation rates (Fig. 1A)
Shift in the OVCAR-3 cell population from
the viable region to the apoptotic region
FACS analysis (Fig.
1B) indicated that there was a movement of a small population
of OVCAR-3 cells from the viable region towards the apoptotic
region. However, significant changes were not observed in apoptotic
response or in the viable cell population density of OVCAR 3 cells.
SKOV-3 cells did not exhibit a significant change in the proportion
of cells in early or late apoptosis, following treatment with
varying concentrations of HAD-B. The viable cell count was not
altered significantly in the SKOV-3 cell line.
Reduced Matrigel invasion of OVCAR-3
cells following treatment with HAD-B
An in vitro invasion assay (Fig. 1C) using Matrigel indicated that the
invasion rate remained unaltered in the NIH:OVCAR-3 and SKOV-3
cells following treatment with HAD-B.
Reduction in the phosphorylation rate of
signaling molecules affecting proliferation, following HAD-B
treatment
In order to examine the expression patterns of
different signaling molecules following HAD-B treatment, western
blot analysis was conducted in NIH:OVCAR-3 and SKOV-3 cell lines,
in the presence and absence of serum (Fig. 2). In the absence of serum,
NIH:OVCAR-3 and SKOV-3 cell lines exhibited a reduction in the
phosphorylation of mTOR and AKT, in a dose-dependent manner.
However, the phosphorylation of ERK was not suppressed. In the
presence of serum, the NIH:OVCAR-3 cells exhibited no reduction in
the expression of mTOR or AKT. The expression of PARP and caspase-3
was not affected by any of the tested variables, which indicates
that there was no change in the level of apoptosis in NIH:OVCAR-3
and SKOV-3 cell lines following HAD-B treatment.
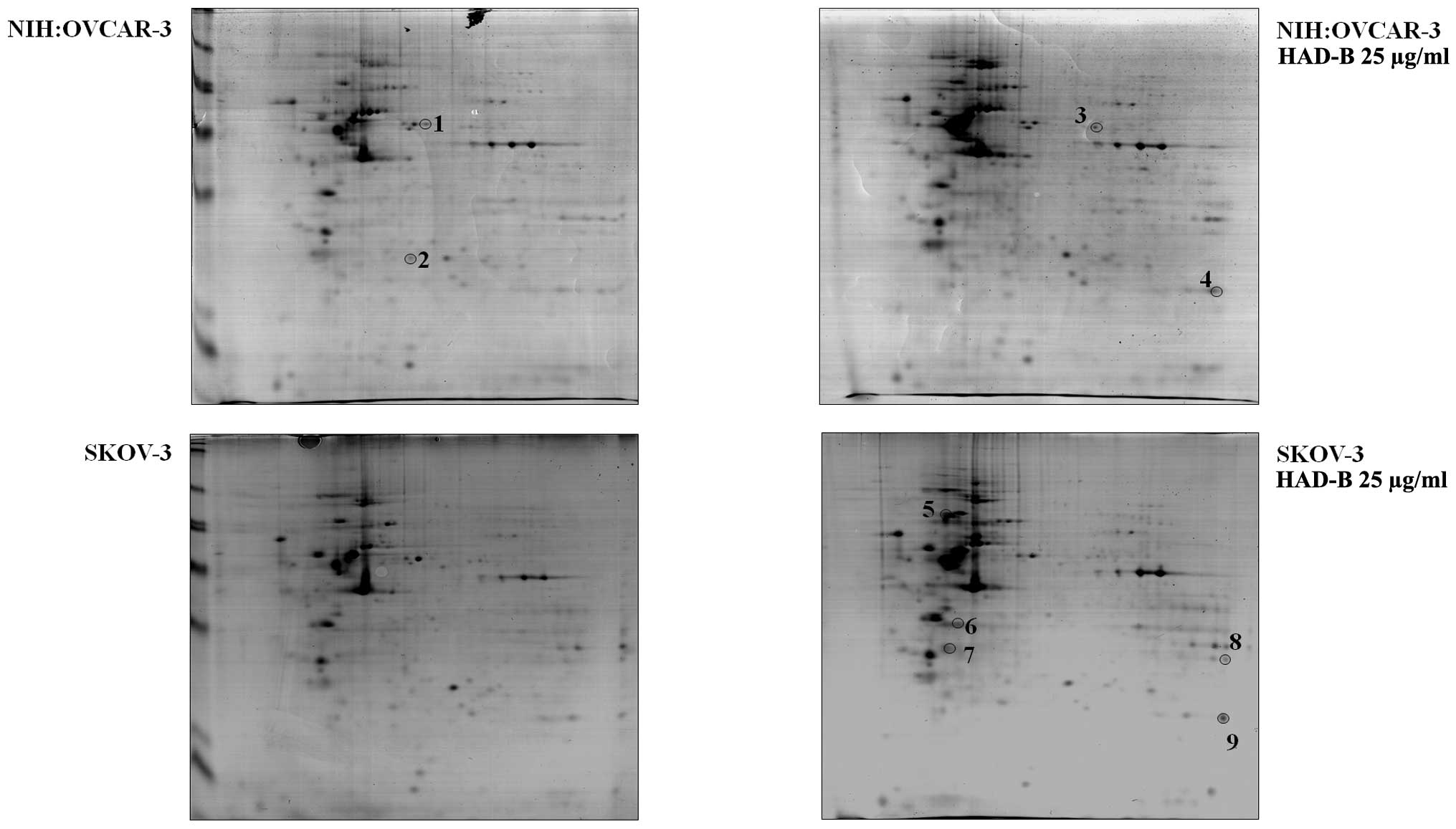
Proteome alteration in ovarian cancer
cells following HAD-B treatment
Proteomic profiling by 2D gel electrophoresis
followed by LC-MS/MS, was conducted in order to investigate
proteins affected by HAD-B treatment in ovarian cancer cells
(Fig. 3). Nine proteins were
altered by HAD-B treatment, as described in Table III. A previous study suggested
that heat-shock protein β-1, also known as HSP27, reduces
susceptibility to herceptin in human breast cancer cells by
increasing the stability of the Her2 protein.
 | Table IIIIdentification of nine proteins by
matrix-assisted laser desorption/ionization-time of flight
analysis. |
Table III
Identification of nine proteins by
matrix-assisted laser desorption/ionization-time of flight
analysis.
| Spot number | Protein name | Accession number | Score | Sequence coverage
(%) | Molecular weight
(kDa) | Calculated pI |
|---|
| 1 | Protein
disulfide-isomerase A3 | P30101 | 30.08 | 13.47 | 56.7 | 6.35 |
| 2 | Heat shock protein
β-1 | P04792 | 23.42 | 30.24 | 22.8 | 6.40 |
| 3 | T-complex protein 1
subunit β | P78371 | 21.56 | 11.03 | 57.5 | 6.46 |
| 4 | Peroxiredoxin-1 | Q06830 | 25.58 | 18.59 | 22.1 | 8.13 |
| 5 | 78 kDa
glucose-regulated protein | P11021 | 110.68 | 32.87 | 72.3 | 5.16 |
| 6 |
Glyceraldehyde-3-phosphate
dehydrogenase | P04406 | 30.54 | 19.40 | 36.0 | 8.46 |
| 7 | Annexin A5 | P08758 | 33.87 | 24.38 | 35.9 | 5.05 |
| 8 | Voltage-dependent
anion-selective channel protein 1 | P21796 | 44.58 | 33.92 | 30.8 | 8.54 |
| 9 |
Peroxiredoxin-1 | Q06830 | 49.37 | 39.70 | 22.1 | 8.13 |
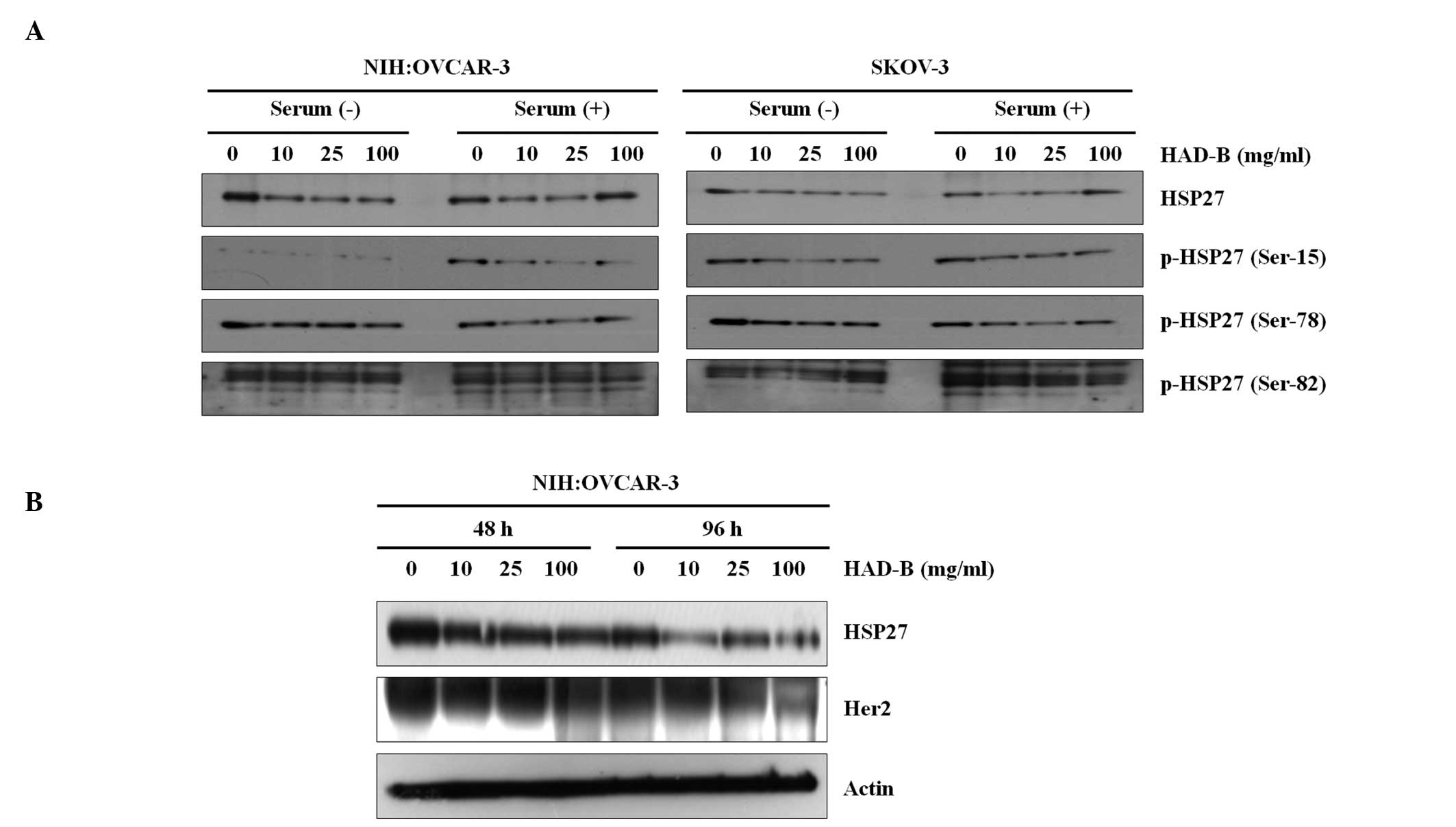
HSP27 and Her2 are suppressed by HAD-B
treatment
In order to investigate the effect of HAD-B
treatment on HSP27 and p-HSP27 expression levels in NIH:OVCAR-3 and
SKOV-3 cell lines, western blotting was performed in the presence
and absence of serum (Fig. 4A).
The results demonstrated that HAD-B reduced HSP27 and p-HSP27
levels in NIH:OVCAR-3 cells in a dose-dependent manner in the
presence of serum. The expression of p-HSP27 (Ser-15) was also
reduced in the absence of serum in these cells. However, no
significant reduction of HSP27 levels was observed in SKOV-3 cells.
In order to examine the association between the expression of HSP27
and Her2, a further western blot assay was performed (Fig. 4B), which indicated that Her2
expression was suppressed in association with reduced HSP27 levels
in a dose-dependent manner, and that this reduction was more marked
following longer duration of treatment.
Discussion
HAD-B has been shown to exhibit anticancer effects,
including the inhibition of cell motility and angiogenesis, with
consequent reduced invasiveness in cancer cell lines (2,6). It
is a potential novel anticancer agent with minimal or no side
effects when administered within the therapeutic dose limits
(4). Previous studies have
demonstrated that a reduction in HSP27 expression is associated
with a more advanced stage of disease, and that HSP27 is an
independent marker of survival in ovarian cancer (10).
In order to investigate the effect of HAD-B on cell
proliferation in NIH:OVCAR-3 and SKOV-3 cell lines, an MTT assay
was performed. Following treatment with HAD-B, a suppression in the
rate of proliferation was observed in the absence of serum, in a
dose- and time-dependent manner. No significant reduction in the
rate of proliferation was observed in the presence of serum
following treatment with HAD-B. This suggested that a factor that
is present in serum may interact with HAD-B or with a separate
molecule, which inhibits HAD-B. Apoptosis was examined by FACS
analysis, using Annexin V. The OVCAR-3 cell population shifted
slightly from the viable to apoptotic region, as the HAD-B dose was
increased. SKOV-3 cells displayed no alterations in viable or
apoptotic cell density, or a shift in the cell population. Despite
the fact that there was only a small alteration in apoptosis, the
Matrigel in vitro invasion assay indicated a reduction in
the invasive properties of OVCAR-3 cells. By contrast, there was no
change in the invasive capability of SKOV-3 cells.
In the current study the expression profiles of
various signaling molecules in NIH:OVCAR-3 and SKOV-3 cell lines
following treatment with HAD-B, indicated a reduction in the
phosphorylation of mTOR and AKT in the absence of serum, in a
dose-dependent manner, which affected the proliferation rate
following HAD-B treatment. A previous study demonstrated that
inhibition of ovarian cancer cell proliferation is due to
interference with the EGFR-AKT pathway (18). The HIV protease inhibitor,
ritonavir, has been shown to induce cell cycle arrest and apoptosis
in the MDH-2774 and SKOV-3 ovarian cell lines, in a dose-dependent
manner. Ritonavir treatment resulted in G1 cell cycle
arrest in ovarian cancer cells by downregulation of RB
phosphorylation levels and diminution of G1 cyclins and
cyclin-dependent kinases, and augmentation of their inhibitors
(19). Ritonavir has also been
shown to suppress the level of phosphorylated AKT in a
dose-dependent manner. Administration of an AKT inhibitor may
increase the therapeutic efficacy of ritonavir (19). This indicates a role for
phosphorylation in the proliferation of cells.
In the present study, no change in the expression of
PARP or caspase-3 was identified, which indicates that there was no
increase in apoptosis in the NIH:OVCAR-3 and SKOV-3 cell lines
following treatment with HAD-B, and that HAD-B reduced
proliferation levels without inducing apoptosis.
Proteomic profiling demonstrated the suppression of
one protein and the increased expression of eight proteins
following treatment with HAD-B. A further western blot assay
indicated that HAD-B successfully reduced levels of HSP27 in
NIH:OVCAR-3 cells in a dose-dependent manner in the presence of
serum. No significant reduction was observed in the levels of HSP27
in SKOV-3 cells, while in OVCAR-3 cells, Her2 expression was
suppressed, and HSP27 levels were reduced in a dose- and
time-dependent manner. This suggests that NIH:OVCAR-3 cells are
sensitive to HAD-B treatment. It has previously been demonstrated
that the growth arrest and senescence of HER2-expressing cells are
associated with the downregulation of HSP27 (20). In a previous study, upregulation of
HSP27 in human breast cancer cells was demonstrated to reduce
susceptibility to herceptin by increasing Her2 protein stability
(16). These observations suggest
that the reduced cell proliferation of the OVCAR-3 cell population
following HAD-B treatment, was associated with a reduction in
levels of HSP27, which is also associated with Her2 levels, as
demonstrated by the concomitant reduction in HSP27 levels and Her2
expression. By contrast, no significant reduction was detected in
the levels of HSP27 in SKOV-3 cells, following treatment with
HAD-B, which also explains the absence of a change in the
invasiveness of SKOV-3 cells.
In conclusion, HAD-B treatment suppressed the rate
of proliferation of NIH:OVCAR-3 and SKOV-3 cell lines in the
absence of serum, and the mechanism underlying this effect did not
appear to involve the induction of apoptosis. HAD-B treatment was
associated with limited alteration in the proteome. Notably, HSP27
was suppressed by HAD-B treatment in the absence of serum. Reduced
levels of HSP27 were associated with a reduction in Her2
expression, which ultimately resulted in the inhibition of
proliferation in a dose- and time-dependent manner. The reduction
in invasion observed in OVCAR-3 cells was associated with a
reduction in HSP27 levels following HAD-B treatment. HAD-B also
inhibited phosphorylation of the signaling molecules, mTOR and AKT,
which is likely to have contributed to the observed reduced rate of
proliferation.
References
|
1
|
Jeong TY, Park BK, Lee YW, Cho CK and Yoo
HS: Prospective analysis on survival outcomes of nonsmall cell lung
cancer over IIIb treated with HangAm-Dan. Zhongguo Fei Ai Za Zhi.
13:1009–1015. 2010.PubMed/NCBI
|
|
2
|
Choi YJ, Shin DY, Lee YW, et al:
Inhibition of cell motility and invasion by HangAmDan-B in NCI-H460
human non-small cell lung cancer cells. Oncol Rep. 26:1601–1608.
2011.PubMed/NCBI
|
|
3
|
Yu T, Moh SH, Kim SB, et al: HangAmDan-B,
an ethnomedicinal herbal mixture, suppresses inflammatory responses
by inhibiting Syk/NF-κB and JNK/ATF-2 pathways. J Med Food.
16:56–65. 2013. View Article : Google Scholar :
|
|
4
|
Yoo HS, Lee HJ, Kim JS, et al: A
toxicological study of HangAmDan-B in mice. J Acupunct Meridian
Stud. 4:54–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park HM, Kim SY, Jung IC, et al:
Integrative tumor board: a case report and discussion from
East-West Cancer Center. Integr Cancer Ther. 9:236–245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bang JY, Kim KS, Kim EY, et al:
Anti-angiogenic effects of the water extract of HangAmDan (WEHAD),
a Korean traditional medicine. Sci China Life Sci. 54:248–254.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zerbini LF, Tamura RE, Correa RG, et al:
Combinatorial effect of non-steroidal anti-inflammatory drugs and
NF-κB inhibitors in ovarian cancer therapy. PLoS One. 6:e242852011.
View Article : Google Scholar
|
|
8
|
Rauh-Hain JA, Krivak TC, Del Carmen MG and
Olawaiye AB: Ovarian cancer screening and early detection in the
general population. Rev Obstet Gynecol. 4:15–21. 2011.PubMed/NCBI
|
|
9
|
Park B, Park S, Kim TJ, et al:
Epidemiological characteristics of ovarian cancer in Korea. J
Gynecol Oncol. 21:241–247. 2010. View Article : Google Scholar
|
|
10
|
Geisler JP, Geisler HE, Tammela J, et al:
Heat shock protein 27: an independent prognostic indicator of
survival in patients with epithelial ovarian carcinoma. Gynecol
Oncol. 69:14–16. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Langdon SP, Rabiasz GJ, Hirst GL, et al:
Expression of the heat shock protein HSP27 in human ovarian cancer.
Clin Cancer Res. 1:1603–1609. 1995.PubMed/NCBI
|
|
12
|
Elpek GO, Karaveli S, Simşek T, Keles N
and Aksoy NH: Expression of heat-shock proteins hsp27, hsp70 and
hsp90 in malignant epithelial tumor of the ovaries. APMIS.
111:523–530. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De AK and Roach SE: Detection of the
soluble heat shock protein 27 (hsp27) in human serum by an ELISA. J
Immunoassay Immunochem. 25:159–170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elstrand MB, Kleinberg L, Kohn EC, Tropé
CG and Davidson B: Expression and clinical role of antiapoptotic
proteins of the bag, heat shock and Bcl-2 families in effusions,
primary tumors, and solid metastases in ovarian carcinoma. Int J
Gynecol Pathol. 28:211–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Langmár Z and Vleskó G: The potential role
of heat shock proteins in the treatment of ovarian cancer. Orv
Hetil. 152:92–95. 2011.In Hungarian. View Article : Google Scholar
|
|
16
|
Kang SH, Kang KW, Kim KH, et al:
Upregulated HSP27 in human breast cancer cells reduces herceptin
susceptibility by increasing Her2 protein stability. BMC Cancer.
8:2862008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoo BC, Hong SH, Ku JL, et al: Galectin-3
stabilizes heterogeneous nuclear ribonucleoprotein Q to maintain
proliferation of human colon cancer cells. Cell Mol Life Sci.
66:350–364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo J, Schally AV, Zarandi M, Varga J and
Leung PC: Antiproliferative effect of gowth hormone-releasing
hormone (GHRH) antagonist on ovarian cancer cells through the
EGFR-Akt pathway. Reprod Biol Endocrinol. 8:542010. View Article : Google Scholar
|
|
19
|
Kumar S, Bryant CS, Chamala S, et al:
Ritonavir blocks AKT signaling, activates apoptosis and inhibits
migration and invasion in ovarian cancer cells. Mol Cancer.
8:262009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng L, Gabai VL and Sherman MY:
Heat-shock transcription factor HSF1 has a critical role in human
epidermal growth factor receptor-2 induced cellular transformation
and tumorigenesis. Oncogene. 29:5204–5213. 2010. View Article : Google Scholar : PubMed/NCBI
|