Introduction
Vascular endothelial growth factor (VEGF) has a
fundamental role in the physiological angiogenesis and the
vascularization of the follicular luteinizing granulosa layer
during corpus luteum (CL) formation (1–4).
Inhibition of VEGF in vivo during the luteal phase prevents
luteal angiogenesis and subsequent progesterone secretion (5–8),
while an excess VEGF generation during the vascularization of
multiple follicles is also thought to cause ovarian
hyperstimulation syndrome (OHSS) (9–11).
Therefore, the molecular regulation of luteal VEGF expression
becomes more and more important.
A previous study by our group reported that
hypoxia-inducible factor (HIF)-1α contributes to the
transcriptional regulation of VEGF in luteal cells (LCs) (12). HIF-1, a helix-loop-helix
transcriptional factor, which consists of HIF-1α and HIF-1β, has
been cloned and characterized as a transcriptional activator of
numerous oxygen-sensitive genes, including erythropoietin (13,14),
heme oxygenases (15,16), transferrin (16), and several glycolytic enzymes
(13–17). It has been indicated that HIF-1α is
inducible by a decrease in tissue or cellular O2
(15,16). HIF-1β is not inducible, but it can
bind to HIF-1α to form a dimer to activate the transcription of
numerous genes containing cis hypoxia-response element (HRE) in
their promoter or enhancer regions (15,16).
Previously, a chromatin immunoprecipitation (ChIP) assay indicated
that estrogen can simultaneously induce the recruitment of HIF-1α
as well as -β to the upstream HRE and ERα to the proximal GC-rich
region of the VEGF promoter, which mediates transcriptional
activation of the murine VEGF gene (18–20).
It has been demonstrated that PHDs are the major
enzymes to promote the degradation of HIF-1α (21–23).
PHD catalyzes site-specific proline hydroxylation of HIF-1α, and
PHD is recognized and targeted for degradation by the
ubiquitin-proteasome pathway. Three isoforms of PHD, including
PHD1, -2, and -3, have been identified (21–24).
Previous studies by our group have indicated that PHD2 participates
in functional regulation, including sensing high-salt intake and
maintaining normal blood pressure, through regulating HIF-α levels
(25) and also has a role in
angiogenesis (26).
Given the role of PHD2 in the regulation of HIF-1α
levels, it was hypothesized in the present study that the PHD2
signaling pathway has a role in ovarian angiogenesis and that
overexpression of PHD2 attenuates the expression of VEGF induced by
hypoxia in LCs. The present study examined the effect of hypoxia on
the expression of HIF-1α and determined the role of PHD2, the
primary isoform of PHDs, in hypoxia-induced activation of HIF-1α by
transfection of PHD2-expressing vectors into LCs. The effect on
VEGF mRNA expression was also examined. The results indicated that
PHD2 is a mediator of cellular HIF-1α and its target gene VEGF in
LCs, which may be an important regulatory mechanism of
VEGF-dependent angiogenesis during mammalian CL development.
Materials and methods
Animals
25-day-old Female Sprague-Dawley (SD) rats (Fuzhou
Animal Center, Fuzhou, China) were used in the present study. All
animals were maintained under a 12-h light/dark cycle with food and
water available ad libitum. The study was conducted in
accordance with guidelines of the Institutional Animal Care and Use
Committee and was approved by the Ethics Committee on Animal
Experimentation of Fujian Normal University. All efforts were made
to minimize animal discomfort and to reduce the number of animals
used.
Isolation and culture of rat LCs
Rat LCs were isolated and cultured according to
previously described procedures (27–29).
Briefly, the rats received a subcutaneous injection of equine
chorionic gonadotropin [eCG; Sigma-Aldrich, St. Louis, MO, USA; 50
international units (IU)] and an ovulatory dose (25 IU) of human
chorionic gonadotropin (hCG; Sigma-Aldrich) 64 h later. Ovaries was
obtained at day 5 after hCG injection and minced with a razor
blade. Tissue was digested in medium 199 (Gibco-BRL, Invitrogen
Life Technologies, Carlsbad, CA, USA) containing 1% fetal calf
serum (FCS; Gibco-BRL) and 2,000 IU collagenase (Gibco-BRL) plus
3,000 IU of DNase (Sigma-Aldrich) per gram of tissue for 1 h at
37°C under 95% air with 5% CO2. The contents of the
flask were filtered through nylon mesh (BD Biosciences, Franklin
Lakes, NJ, USA) and centrifuged (100 xg, 5 min); the supernatant
fraction was discarded and the pellet was washed three times with
fresh medium. The final cell concentration was 106 cells
per ml and cells were incubated in six-well culture plates. Cell
numbers were determined with a hemocytometer and cell viability was
>90% as assessed by Trypan blue (Sigma-Aldrich) staining
exclusion.
Transfection of plasmids expressing rat
PHD2 into the cells
Plasmids encoding rat full-length PHD2 cDNA were a
generous gift from Dr Ningjun Li (Department of Pharmacology &
Toxicology, School of Medicine, Virginia Commonwealth University,
Richmond, VA, USA). The expression and function of rat PHD2 protein
by the plasmids has been validated by previous studies (25,30–32).
Plasmid transfections were performed using lipids (DOTAP/DOPE;
Avanti Polar Lipids, Inc., Alabaster, AL, USA) according to the
manufacturer's instructions. In brief, 5 µg DNA was mixed
with a lipid solution at a DNA/lipid ratio of 1:10 (w/w) in
serum-free culture medium (5 ml for a 10-cm dish). Cells were
incubated with this transfection medium for 5 h and switched to
normal medium for another 16 h. The cells were then ready for the
subsequent experiments.
Cell hypoxia
Cell culture under hypoxia with 3% O2 was
performed as described previously (33,34).
Briefly, LCs were plated in six-well plates 24 h prior to the
experiments to reach sub-confluence. To decrease O2
pressure in the culture medium, the plates and dishes were
transferred to a sealed, humidified modular chamber, the
atmospheric air was evacuated by a vacuum pump, and the chamber was
re-filled with a non-standard gas mixture containing 3%
O2 and 5% CO2 in an N2 base. After
repeating this evacuation and inflow five times, the cells were
cultured for 6 h. After hypoxia, the culture medium was rapidly
replaced by TRIzol solution (Invitrogen Life Technologies), and
total RNA was extracted for determination of HIF-1α and VEGF mRNA.
For HIF-1α protein determination, the cultured cells were scraped
immediately and placed in ice-cold homog-enization buffer [25 mM
Tris-HCl, 300 mM sucrose, 2 mM EDTA, protease inhibitor cocktail
(Roche Diagnostics, Basel, Switzerland), pH 7.4], frozen in liquid
nitrogen and then stored at −80°C for western blot analysis.
RNA extraction and quantitative reverse
transcription polymerase chain reaction (RT-PCR) analysis of PHD1,
2 and 3, HIF-1α and VEGF
Total RNA was extracted using TRIzol solution and
then reverse-transcribed uding a cDNA Synthesis kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The RT products were
amplified using SYBR green (Bio-Rad Laboratories, Inc.) and the
following primers: PHD1 forward, 5′-GCT GCT GCG TTG GTTAC-3′ and
reverse; 5′-GCC TCC TGG TTC TCTTG-3′ (GenBank accession no.:
NM001004083); PHD2 forward, 5′-CTG GGA CGC CAA GGTGA-3′ and
reverse, 5′-CAA TGT CAG CAA ACTGG-3′ (GenBank accession no.:
NM178334); PHD3 forward, 5′-GTT CAG CCC TCC TATGC-3′ and reverse,
5′-ACC ACC GTC AGT CTTTA-3′ (GenBank accession no.: NM019371);
HIF-1α forward, 5′-CTG GCA CGG GGA TGA TAC AGC-3′ and reverse,
5′-TCT CAT CCA TTG ACT GCC CCAG-3′ (GenBank accession no.:
AF057308) and VEGF forward, 5′-ACG AAG CGC AAG AAA TCCC-3′ and
reverse, 5′-TTA ACT CAA GCT GCC TCGCC-3′ (GenBank accession no.:
M32167) with an iCycler iQ Real Time PCR Detection System (Bio-Rad
Laboratories, Inc.). The levels of 18 rRNA [forward, 5′-CGC CGC TAG
AGG TGA AATTC-3′ and reverse, 5′-TCT TGG CAA ATG CTT TCGC-3′
(GenBank accession no.: M11188)] were used as an endogenous
control. The 25 µl PCR reaction mix included 12.5 µl
SYBR Green PCR Master Mix, 2.5 µl 10X primers, 1 µl
cDNA template and 9 µl RNase-free water. The PCR conditions
were as follows: 50°C for 2 min, 95°C for 15 min, followed by 40
cycles at 94°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec.
Relative gene expression was calculated in accordance with the ΔΔCt
method, with relative mRNA levels calculated as
2−ΔΔCt.
Preparation of nuclear extracts and
cytosolic protein, and western blot analysis of protein levels of
HIF-1α and PHD
Nuclear protein was prepared as previously described
(12,25,32).
Cytosolic protein and nuclear protein were collected separately
(Beyotime Institute of Biotechnology, Haimen, China). The cytosolic
protein was used for western blot analysis of PHD2, while the
nuclear fraction was used for western blot analyses of HIF-1α.
Briefly, protein concentration was determined using a Bio-Rad assay
(Bio-Rad Laboratories, Inc.) with bovine serum albumin standards.
The protein samples (30 µg) were separated by 8% SDS-PAGE,
and were electrophoretically transferred to a polyvinylidene
fluoride membrane (Pall Corporation, Pensacola, FL, USA). The
membrane was washed with phosphate-buffered saline with 0.2%
Tween-20 (PBST; Sigma-Aldrich), blocked with 5% nonfat dried milk
in PBST and probed with the following primary antibodies (Novus
Biologicals, Littleton, CO, USA): Mouse monoclonal anti-HIF-1α
(1:300; cat. no. NB100-105), rabbit polyclonal anti-PHD2 (1:300;
cat. no. NB100-137), and rabbit polyclonal anti-β-actin (cat. no.
NB600-503H) overnight at 4°C. After washing, the membranes were
then incubated with horseradish peroxidase-conjugated goat
anti-mouse (1:5,000; cat. no. NB7570) or goat anti-rabbit (1:5,000,
cat. no. NBP2-30348H) immunoglobulin G secondary antibodies (Novus
Biologicals) for 1 h at room temperature. β-actin was used as a
loading control (1:5,000, cat. no. NB600-501H). To detect the
immunoblotting signal 2 ml enhanced chemiluminescence detection
solution (Thermo Scientific, Rockford, IL, USA) was used, and the
membrane was exposed to Kodak OMAT film (Eastman Kodak, Rochester,
NY, USA). The blots were quantified using ImageJ software (National
Institutes of Health, Bethesda, MD, USA)
Determination of HIF prolyl hydroxylase
activity
HIF-1α peptide-specific conversion of 2-oxoglutarate
into succinate provides a hydroxyl group for HIF-1α to be prolyl
hydroxylated. This reaction has been widely used for the
determination of PHD activity by measuring HIF-1α-dependent
conversion rate of 2-oxoglutarate into succinate (25,35,36).
Statistics
Values are expressed as the mean ± standard error.
The significance of differences in mean values within and between
multiple groups was evaluated using analysis of variance followed
by a Duncan's multiple range test. Statistical analysis was
conducted using Sigmastat 3.02 (Sigma-Aldrich). P<0.05 was
considered to indicate a statistically significant difference.
Results
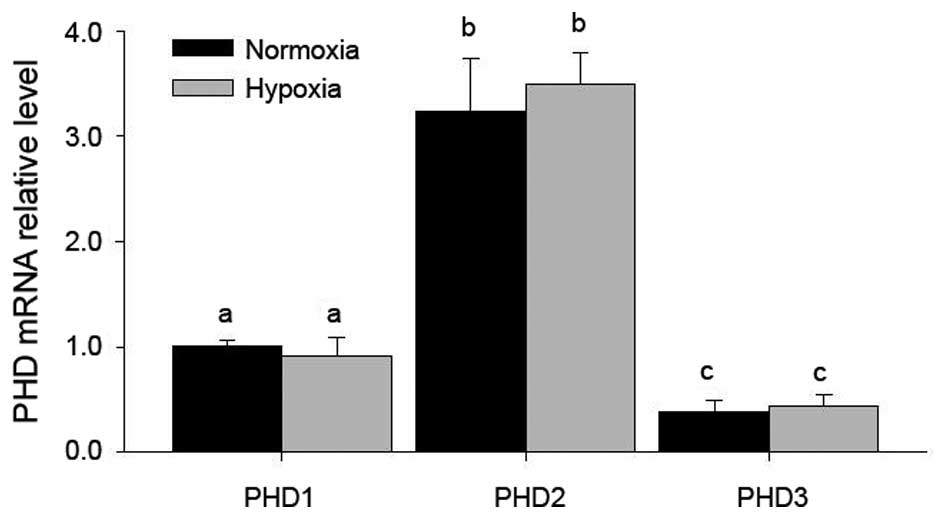
PHD2 is the most abundantly expressed PHD
in LCs, and is not affected by hypoxia
RT-qPCR indicated that all of three PHDs were
present, among which PHD2 was most abundantly expressed in LCs
(Fig. 1), while no significant
changes of these mRNA levels were found after hypoxia treatment
(Fig. 1).
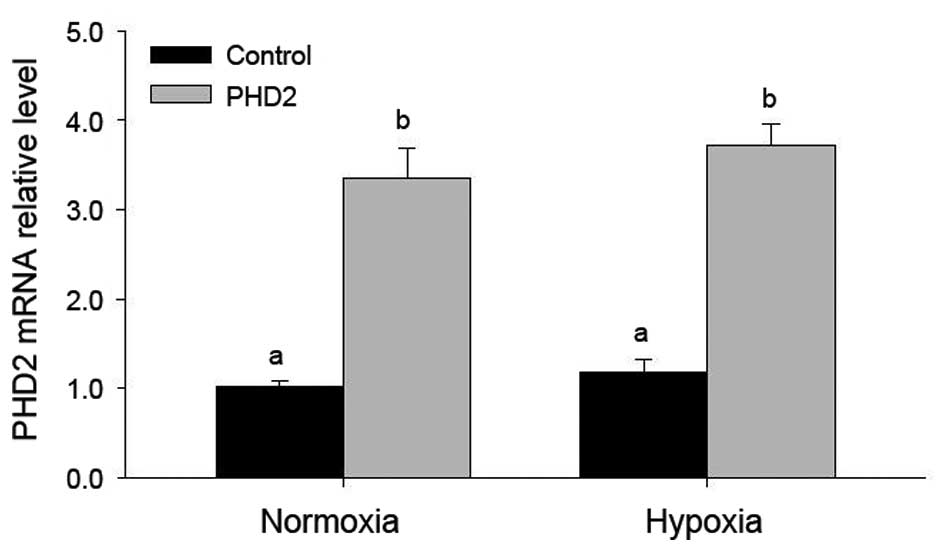
Efficient PHD2 transfection in LCs is not
affected by hypoxia
PHD2 mRNA levels increased significantly after PHD2
transfection, even following hypoxia treatment (Fig. 2). However hypoxia was found to have
no marked effect on PHD2 mRNA expression (Fig. 2), indicating the high transfection
efficiency and the high expression levels of PHD2 plasmid.
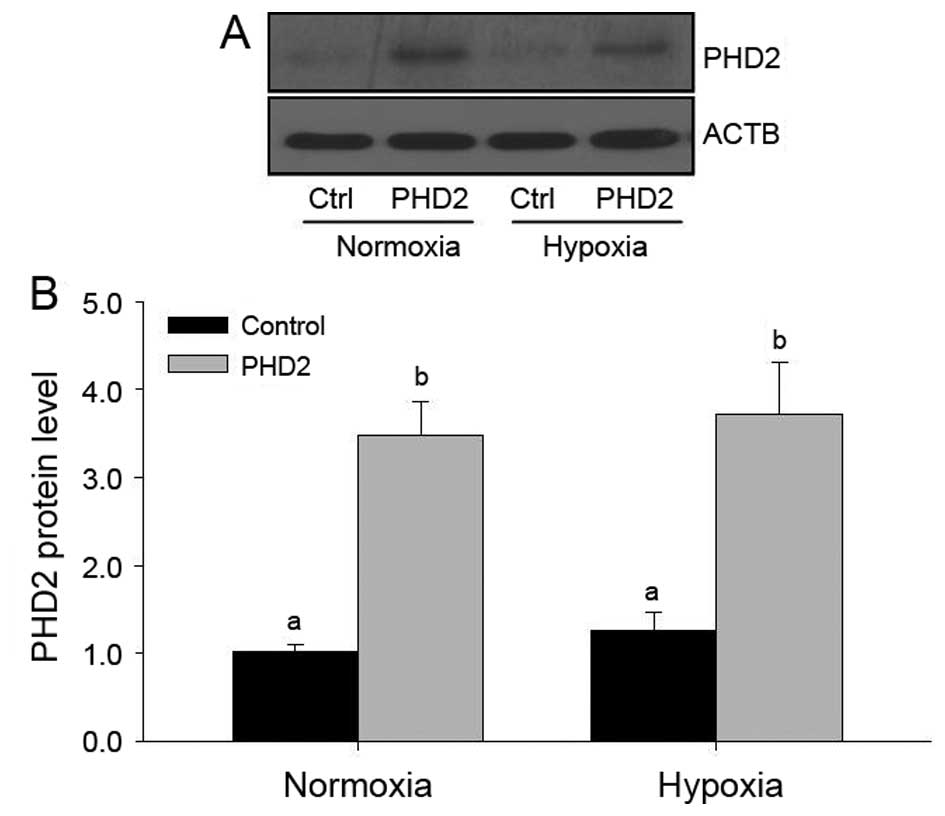
Furthermore, PHD2 protein levels were detected by
western blot analysis (Fig. 3).
The results indicated that, similar to mRNA levels, PHD2 protein
levels were enhanced following transfection, but that hypoxia had
no effects on PHD2 protein levels (Fig. 3).
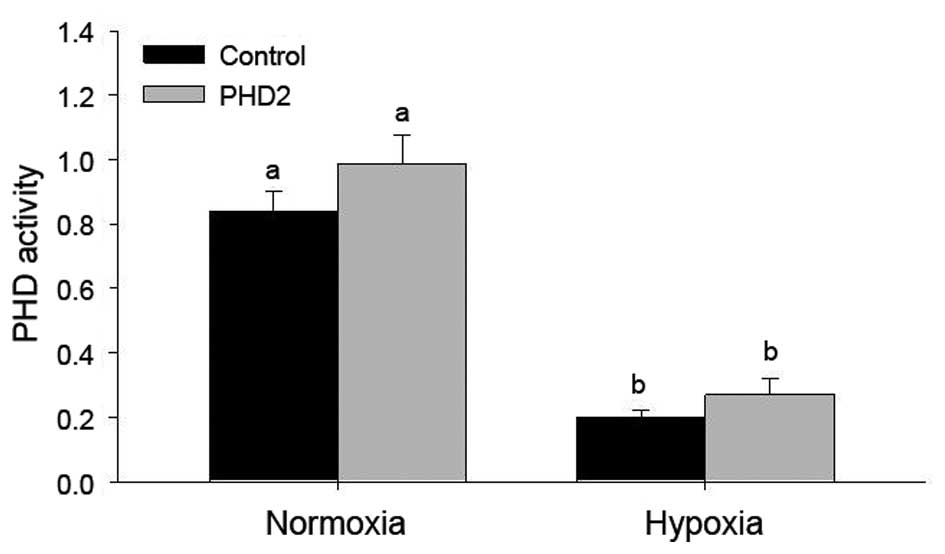
Effects of hypoxia and PHD2 transfection
on PHD2 protein levels and PHD2 activity in LCs
To further confirm stable transfection of PHD2 into
LCs, PHD2 biological activity was tested using the 2-oxoglutarate
conversion assay (Fig. 4). In the
PHD2-transfected groups, 2-oxoglutarate conversion was enhanced,
however, not to a statistically significant extent. Of note, a
significant decrease of PHD activity was found in LCs under hypoxic
conditions, even following transfection with PHD2 plasmids
(Fig. 4).
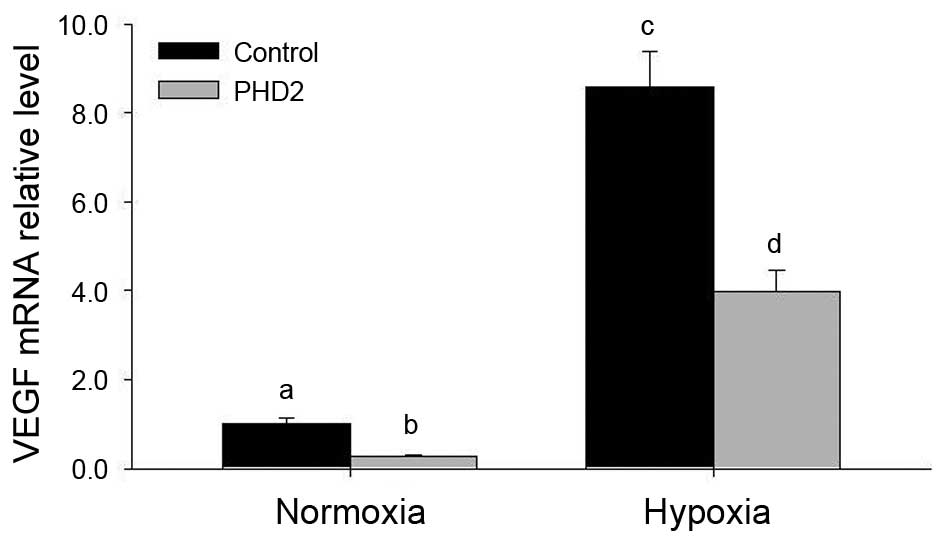
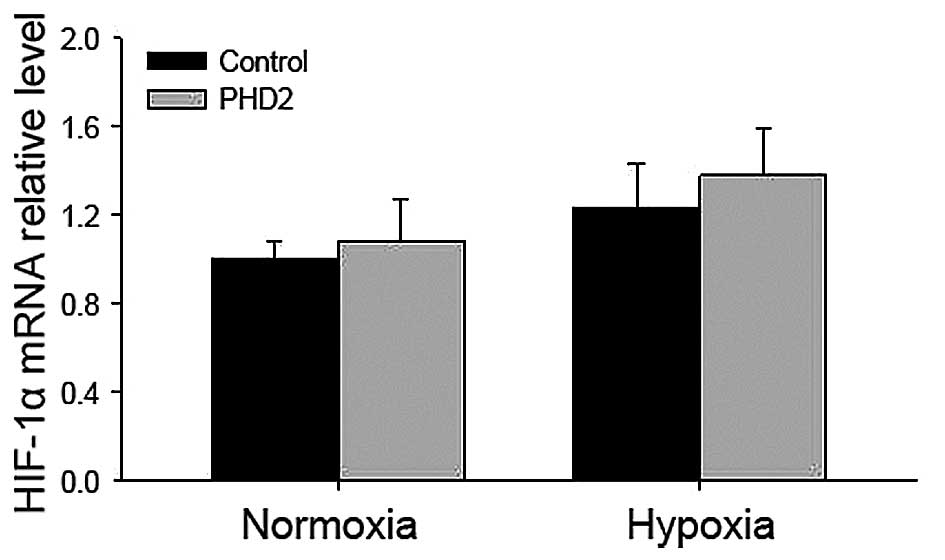
Hypoxia increases and PHD2 transfection
decreases VEGF mRNA levels in LCs, while HIF-1α mRNA is
unaffected
In the present study, hypoxia significantly
increased VEGF mRNA expression in LCs (Fig. 5). To better understand the role of
PHD2 in LCs, the mRNA levels of VEGF and HIF-1α in LCs transfected
with PHD2 plasmid were also examined (Figs. 5 and 6). Of note, a marked decrease in VEGF
levels was observed in PHD2-transfected LCs (Fig. 5), while HIF-1α mRNA levels were not
significantly altered in LCs following hypoxia treatment (Fig. 6).
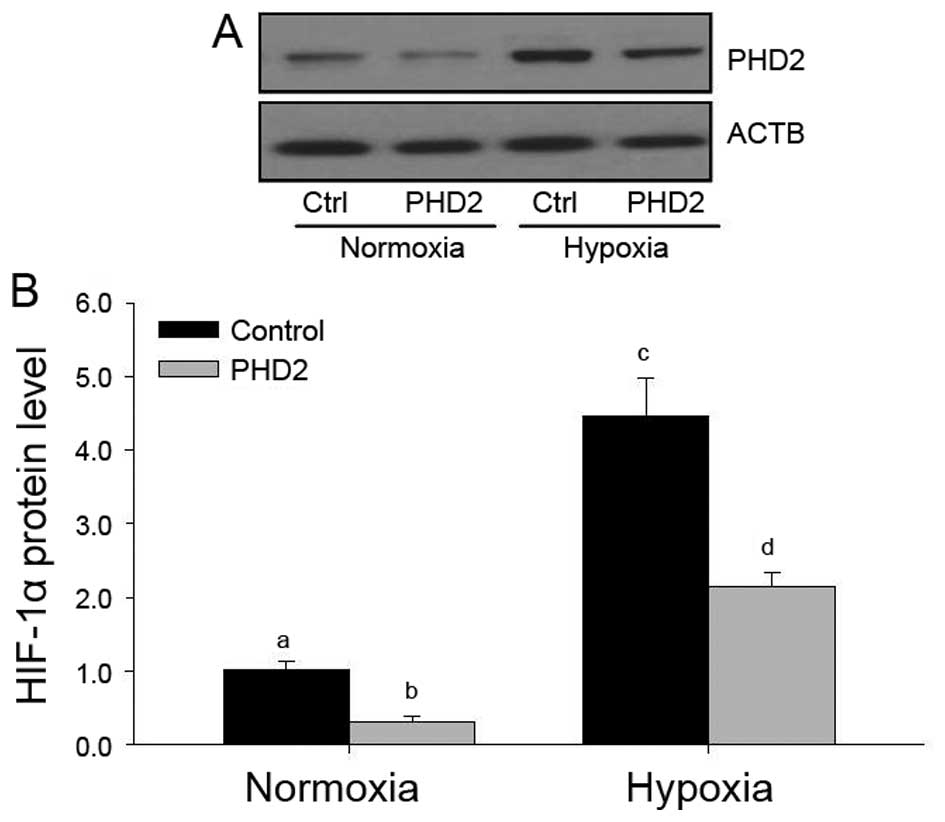
Effects of hypoxia and PHD2 transfection
on HIF-1α protein levels in LCs
It has been demonstrated that PHDs are major enzymes
to promote the degradation of HIF-1α (21–24);
therefore, the present study examined the HIF-1α protein levels in
each group (Fig. 7). Hypoxia
inhibited HIF-1α protein expression under normoxic and hypoxic
conditions (Fig. 7), and a
significant decrease of HIF-1α protein was found in
PHD2-transfected LCs, which was consistent with the results of
previous studies by our group (25,32),
indicating that PHD2 may regulate VEGF expression via the HIF-1α
pathway in LCs during CL development.
Discussion
The results of the present study clearly
demonstrated that hypoxia induced VEGF through inhibiting HIF-1α
signaling in LCs, which was attenuated by overexpression of PHD2,
suggesting that PHD2-mediated VEGF expression via the HIF-1α
pathway may be an important mechanism of VEGF-dependent
angiogenesis during mammalian CL development.
The CL is a temporary endocrine structure in
mammals, which has an important role in the female reproductive
cycle and is formed temporarily from a ruptured and ovulated
follicle with rapid angiogenesis (5,6,11,37,38).
VEGF is thought to have a paramount role in the regulation of
normal and abnormal angiogenesis in the ovary (2–4,6,9,11,39,40),
particularly in the newly formed CL. Numerous studies have shown
that reproductive hormones such as hCG also take part in the
primary regulation of VEGF expression in the ovary. For example,
VEGF mRNA expression in human luteinized granulosa cells has been
shown to be dose- and time-dependently enhanced by hCG in
vitro (9,10). Chronic or acute exposure to hCG
directly stimulates VEGF production and secretion by monkey
(1) and human luteinized granulosa
cells (5,9,10,40).
Furthermore, the administration of a gonadotropin-releasing hormone
antagonist decreased VEGF mRNA expression in the CL of monkeys
(41). In addition, luteal
vascularization and the development of ovarian hyperstimulation
syndrome (OHSS) are absolutely dependent on LH/hCG stimulation
(9,10). Furthermore, in a fully formed,
highly vascular CL hCG also up-regulates VEGF expression (5). However, a previous study by our group
has already provided direct evidence that VEGF is transcriptionally
activated by a HIF-1-mediated mechanism in LCs under hypoxia
(12), which is caused by
ovulation of the ruptured follicle with bleeding and an immature
vascula-ture (2,38). Therefore, the present study
examined the induced effect of hypoxia and PHD2 overexpression on
VEGF mRNA expression in LCs. Of note, VEGF expression was induced
by hypoxia in LCs and hypoxia-stimulated HIF-1α protein expression
was highly correlated with VEGF expression, indicating hypoxia
stimulated VEGF expression via the HIF-1α signaling pathway.
Numerous studies have indicated that HIF-1α
regulates the expression of numerous genes whose protein products
have critical roles in developmental and physiological processes,
including angiogenesis, erythropoiesis, glycolysis, iron transport
and cell proliferation/survival (5,38,42–45).
Because HIF-1α activates the transcription of VEGF, which is
required for angiogenesis, it is possible that hypoxia may mediate
angiogenesis via the HIF-1α/VEGF pathway. In addition to a detailed
exploration of the downstream mechanism of HIF-1α (12,46–48),
recent studies have clarified the upstream process modulated by
PHDs (49), which regulates HIF-1α
degradation by the ubiquitin-proteasome pathway. In particular,
PHD2 has been drawing considerable attention because PHD2 is
considered to be the key oxygen sensor of all identified PHD
enzymes (25,26), as knockdown of PHD2 resulted in
elevated HIF protein levels (49),
and several recent studies have also highlighted the importance of
PHD2 in tumourigenesis (26). The
present study reported that hypoxia induced the activation of
HIF-1α and overexpression of PHD2 blocked this activation of HIF-1α
and its target gene VEGF following hypoxia treatment. These results
indicated that PHD2 is involved in HIF-1α-mediated gene activation
in LCs under hypoxia, which may present a novel mechanism of
VEGF-dependent angiogenesis during mammalian CL development.
In conclusion, the present study clearly
demonstrated that hypoxia induces HIF-1α and VEGF expression in
LCs. To the best of our knowledge, the present study was also the
first to provide direct evidence indicating that PHDs are expressed
in rat LCs and that hypoxia-induced VEGF expression can be blocked
by overexpression of PHD2 in LCs. This PHD2-mediated VEGF
expression may be one of the important mechanisms of VEGF-dependent
angiogenesis during CL formation in the mammalian ovary.
Furthermore, this PHD2 antagonism presents an opportunity for the
development of novel treatments for fertility control and for
certain types of ovarian dysfunction (26,44,45),
particularly conditions characterized by pathological angiogenesis
and excessive vascular permeability, including polycystic ovarian
syndrome (PCOS), OHSS and ovarian neoplasia.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (nos. 31101032 and 31271255), the
Program for New Century Excellent Talents in University of the
Ministry of Education of China (no. NCET-120614), the Doctoral
Foundation of the Ministry of Education of China (no.
20113503120002) and Fujian Provincial Science and Technology
Projects of the Department of Education (no. JB14041). The authors
particularly thank Dr Ningjun Li from Department of Pharmacology
& Toxicology, School of Medicine, Virginia Commonwealth
University in the USA for kindly providing the PHD and control
plasmids.
References
|
1
|
Christenson LK and Stouffer RL:
Follicle-stimulating hormone and luteinizing hormone/chorionic
gonadotropin stimulation of vascular endothelial growth factor
production by macaque granulosa cells from pre- and periovulatory
follicles. J Clin Endocrinol Metab. 82:2135–2142. 1997.PubMed/NCBI
|
|
2
|
Kaczmarek MM, Schams D and Ziecik AJ: Role
of vascular endothelial growth factor in ovarian physiology-an
overview. Reprod Biol. 5:111–136. 2005.PubMed/NCBI
|
|
3
|
Shimizu T and Miyamoto A: Progesterone
induces the expression of vascular endothelial growth factor (VEGF)
120 and Flk-1, its receptor, in bovine granulosa cells. Anim Reprod
Sci. 102:228–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimizu T, Jayawardana BC, Tetsuka M and
Miyamoto A: Differential effect of follicle-stimulating hormone and
estradiol on expressions of vascular endothelial growth factor
(VEGF) 120, VEGF164 and their receptors in bovine granulosa cells.
J Reprod Dev. 53:105–112. 2007. View Article : Google Scholar
|
|
5
|
Wulff C, Dickson SE, Duncan WC and Fraser
HM: Angiogenesis in the human corpus luteum: simulated early
pregnancy by HCG treatment is associated with both angiogenesis and
vessel stabilization. Hum Reprod. 16:2515–2524. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fraser HM, Bell J, Wilson H, Taylor PD,
Morgan K, et al: Localization and quantification of cyclic changes
in the expression of endocrine gland vascular endothelial growth
factor in the human corpus luteum. J Clin Endocrinol Metab.
90:427–434. 2005. View Article : Google Scholar
|
|
7
|
Fraser HM, Wilson H, Wulff C, Rudge JS and
Wiegand SJ: Administration of vascular endothelial growth factor
Trap during the 'post-angiogenic' period of the luteal phase causes
rapid functional luteolysis and selective endothelial cell death in
the marmoset. Reproduction. 132:589–600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duncan WC, van den Driesche S and Fraser
HM: Inhibition of vascular endothelial growth factor in the primate
ovary up-regulates hypoxia-inducible factor-1alpha in the follicle
and corpus luteum. Endocrinology. 149:3313–3320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neulen J, Yan Z, Raczek S, Weindel K, Keck
C, et al: Human chorionic gonadotropin-dependent expression of
vascular endothelial growth factor/vascular permeability factor in
human granulosa cells: importance in ovarian hyperstimulation
syndrome. J Clin Endocrinol Metab. 80:1967–1971. 1995.PubMed/NCBI
|
|
10
|
Nastri CO, Ferriani RA, Rocha IA and
Martins WP: Ovarian hyperstimulation syndrome: pathophysiology and
prevention. J Assist Reprod Genet. 27:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Z, Neiva KG, Lingen MW, Ellis LM and
Nör JE: VEGF-dependent tumor angiogenesis requires inverse and
reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ.
17:499–512. 2010. View Article : Google Scholar :
|
|
12
|
Zhang Z, Yin D and Wang Z: Contribution of
hypoxia-inducible factor-1α to transcriptional regulation of
vascular endothelial growth factor in bovine developing luteal
cells. Anim Sci J. 82:244–250. 2010. View Article : Google Scholar
|
|
13
|
Wang GL and Semenza GL: Characterization
of hypoxia-inducible factor 1 and regulation of DNA binding
activity by hypoxia. J Biol Chem. 268:21513–21518. 1993.PubMed/NCBI
|
|
14
|
Wang GL and Semenza GL: Desferrioxamine
induces erythropoietin gene expression and hypoxia-inducible factor
1 DNA-binding activity: implications for models of hypoxia signal
transduction. Blood. 82:3610–3615. 1993.PubMed/NCBI
|
|
15
|
Wang GL and Semenza GL: Purification and
characterization of hypoxia-inducible factor 1. J Biol Chem.
270:1230–1237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar
|
|
17
|
Wenger RH, Rolfs A, Marti HH, Guénet JL
and Gassmann M: Nucleotide sequence, chromosomal assignment and
mRNA expression of mouse hypoxia-inducible factor-1 alpha. Biochem
Biophys Res Commun. 223:54–59. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kazi AA, Jones JM and Koos RD: Chromatin
immunoprecipitation analysis of gene expression in the rat uterus
in vivo: estrogen-induced recruitment of both estrogen receptor
alpha and hypoxia-inducible factor 1 to the vascular endothelial
growth factor promoter. Mol Endocrinol. 19:2006–2019. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kazi AA and Koos RD: Estrogen-induced
activation of hypoxia-inducible factor-1alpha, vascular endothelial
growth factor expression and edema in the uterus are mediated by
the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology.
148:2363–2374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Molitoris KH, Kazi AA and Koos RD:
Inhibition of oxygen-induced hypoxia-inducible factor-1alpha
degradation unmasks estradiol induction of vascular endothelial
growth factor expression in ECC-1 cancer cells in vitro.
Endocrinology. 150:5405–5414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bruick RK and McKnight SL: A conserved
family of prolyl-4-hydroxylases that modify HIF. Science.
294:1337–1340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, et al: HIFalpha targeted for VHL-mediated destruction by proline
hydroxylation: implications for O2 sensing. Science.
292:464–468. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jaakkola P, Mole DR, Tian YM, Wilson MI,
Gielbert J, et al: Targeting of HIF-alpha to the von Hippel-Lindau
ubiquitylation complex by O2-regulated prolyl
hydroxylation. Science. 292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Epstein AC, Gleadle JM, McNeill LA,
Hewitson KS, O'Rourke J, et al: C. elegans EGL-9 and mammalian
homologs define a family of dioxygenases that regulate HIF by
prolyl hydroxylation. Cell. 107:43–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Zhu Q, Xia M, Li PL, Hinton SJ, et
al: Hypoxia-inducible factor prolyl-hydroxylase 2 senses high-salt
intake to increase hypoxia inducible factor 1alpha levels in the
renal medulla. Hypertension. 55:1129–1136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chan DA and Giaccia AJ: PHD2 in tumour
angiogenesis. Br J Cancer. 103:1–5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomas JP, Dorflinger LJ and Behrman HR:
Mechanism of the rapid antigonadotropic action of prostaglandins in
cultured luteal cells. Proc Natl Acad Sci USA. 75:1344–1348. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conti M, Harwood JP, Dufau ML and Catt KJ:
Effect of gonadotropin-induced receptor regulation on biological
responses of isolated rat luteal cells. J Biol Chem. 252:8869–8874.
1977.PubMed/NCBI
|
|
29
|
Pepperell JR, Porterfield DM, Keefe DL,
Behrman HR and Smith PJ: Control of ascorbic acid efflux in rat
luteal cells: role of intracellular calcium and oxygen radicals. Am
J Physiol Cell Physiol. 285:C642–C651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang J, Zhao Q, Mooney SM and Lee FS:
Sequence determinants in hypoxia-inducible factor-1alpha for
hydroxylation by the prolyl hydroxylases PHD1, PHD2 and PHD3. J
Biol Chem. 277:39792–39800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Percy MJ, Zhao Q, Flores A, Harrison C,
Lappin TR, et al: A family with erythrocytosis establishes a role
for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc
Natl Acad Sci USA. 103:654–659. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li N, Yi F, Sundy CM, Chen L, Hilliker ML,
et al: Expression and actions of HIF prolyl-4-hydroxylase in the
rat kidneys. Am J Physiol Renal Physiol. 292:F207–F216. 2007.
View Article : Google Scholar
|
|
33
|
Nishimura R, Sakumoto R, Tatsukawa Y,
Acosta TJ and Okuda K: Oxygen concentration is an important factor
for modulating progesterone synthesis in bovine corpus luteum.
Endocrinology. 147:4273–4280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okuda K, Miyamoto A, Sauerwein H,
Schweigert FJ and Schams D: Evidence for oxytocin receptors in
cultured bovine luteal cells. Biol Reprod. 46:1001–1006. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Richard-Fiardo P, Payen E, Chèvre R, Zuber
J, Letrou-Bonneval E, et al: Therapy of anemia in kidney failure,
using plasmid encoding erythropoietin. Hum Gene Ther. 19:331–342.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yi F, Xia M, Li N, Zhang C, Tang L and Li
PL: Contribution of guanine nucleotide exchange factor Vav2 to
hyperhomocys-teinemic glomerulosclerosis in rats. Hypertension.
53:90–96. 2009. View Article : Google Scholar :
|
|
37
|
Young FM, Rodger FE, Illingworth PJ and
Fraser HM: Cell proliferation and vascular morphology in the
marmoset corpus luteum. Hum Reprod. 15:557–566. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nishimura R and Okuda K: Hypoxia is
important for establishing vascularization during corpus luteum
formation in cattle. J Reprod Dev. 56:110–116. 2010. View Article : Google Scholar
|
|
39
|
van den Driesche S, Myers M, Gay E, Thong
KJ and Duncan WC: HCG up-regulates hypoxia inducible factor-1 alpha
in luteinized granulosa cells: implications for the hormonal
regulation of vascular endothelial growth factor A in the human
corpus luteum. Mol Hum Reprod. 14:455–464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee A, Christenson LK, Patton PE, Burry KA
and Stouffer RL: Vascular endothelial growth factor production by
human luteinized granulosa cells in vitro. Hum Reprod.
12:2756–2761. 1997. View Article : Google Scholar
|
|
41
|
Ravindranath N, Little-Ihrig L, Phillips
HS, Ferrara N and Zeleznik AJ: Vascular endothelial growth factor
messenger ribonucleic acid expression in the primate ovary.
Endocrinology. 131:254–260. 1992.PubMed/NCBI
|
|
42
|
Zhong H, Chiles K, Feldser D, Laughner E,
Hanrahan C, et al: Modulation of hypoxia-inducible factor 1alpha
expression by the epidermal growth factor/phosphatidylinositol
3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells:
implications for tumor angiogenesis and therapeutics. Cancer Res.
60:1541–1545. 2000.PubMed/NCBI
|
|
43
|
Yaba A, Bianchi V, Borini A and Johnson J:
A putative mitotic checkpoint dependent on mTOR function controls
cell proliferation and survival in ovarian granulosa cells. Reprod
Sci. 15:128–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miyazawa M, Yasuda M, Fujita M, Kajiwara
H, Hirabayashi K, et al: Therapeutic strategy targeting the
mTOR-HIF-1alpha-VEGF pathway in ovarian clear cell adenocarcinoma.
Pathol Int. 59:19–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miyazawa M, Yasuda M, Fujita M,
Hirabayashi K, Hirasawa T, et al: Granulosa cell tumor with
activated mTOR-HIF-1alpha-VEGF pathway. J Obstet Gynaecol Res.
36:448–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Z, Yu D, Yin D and Wang Z:
Activation of PI3K/mTOR signaling pathway contrbutes to induction
of vascular endothelial growth factor by hCG in bovine developing
luteal cells. Anim Reprod Sci. 125:42–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang J, Zhang Z, Wu Y, Chen L, Luo Q,
Chen J, Huang X, Cheng Y and Wang Z: Regulatory effect of
hypoxia-inducible factor-1α on hCG-stimulated endothelin-2
expression in granulosa cells from the PMSG-treated rat ovary. J
Reprod Dev. 58:678–684. 2012. View Article : Google Scholar
|
|
48
|
Li N, Chen L, Yi F, Xia M and Li PL:
Salt-sensitive hypertension induced by decoy of transcription
factor hypoxia-inducible factor-1alpha in the renal medulla. Circ
Res. 102:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu Q, Hu J, Han WQ, Zhang F, Li PL, Wang
Z and Li N: Silencing of HIF prolyl-hydroxylase 2 gene in the renal
medulla attenuates salt-sensitive hypertension in Dahl S rats. Am J
Hypertens. 27:107–113. 2014. View Article : Google Scholar
|