Introduction
Heparin is a potent blood anti-coagulant drug in
clinical practice, and the underlying mechanisms of its
anti-coagulant function have been extensively studied (1). Aside from its anti-coagulant
capacity, heparin is known to modulate a wide array of inflammatory
responses, partly through inhibiting the production of inflammatory
factors via blocking the p38/mitogen-activated protein kinase
(MAPK) and nuclear factor κ B (NF-κB) activation (2). Although the anti-inflammatory effect
of heparin is well known (3), it
has not been applied as an anti-inflammatory drug, not only due to
the potential risk of bleeding, but also due to its complex
mechanisms of action which are largely elusive. Therefore,
illustrating the underlying mechanisms of the anti-inflammatory
effect of heparin is required for its potential application in the
clinic.
Caveolae are cholesterol- and glycosphingolipid-rich
ampullate invaginations of the plasma membrane, which participate
in a wide range of processes in cells (4). As the coat proteins of caveolae,
caveolins have specific functions, which vary depending on the cell
type. Caveolin-1 was the first protein identified as a prominent
resident of caveolae (5), and it
has been reported to regulate lipid metabolism, apoptosis, and
endocytosis in macrophages (6–8).
Caveolin-1 expressed in different types of immune cell was proved
to have a critical role in modulating inflammatory responses
induced by infection and disease (9,10).
Interestingly, it was also reported that heparin is able to
regulate caveolin-1 expression and subcellular localization in
human vascular smooth muscle cells (11). However, whether the two have any
interaction in the inflammatory process in peritoneal macrophages
has not been studied in detail. Revealing the role of caveolin-1 in
the inflammatory process regulated by heparin can further elucidate
the functions of caveolin-1 and aid in the discovery of novel
treatment strategies for inflammation.
Therefore, the present study investigated the
effects of heparin on LPS-induced murine peritoneal macrophages as
well as the underlying mechanisms. Since caveolin-1 has been linked
with the modulation of the inflammatory response, the present study
tested whether heparin and caveolin-1 are associated in the
LPS-induced inflammatory process. Herein, to better characterize
the role of caveolin-1 in LPS-induced inflammation treated by
heparin, small interfering (si)RNA was used to silence caveolin-1
in murine peritoneal macrophages. The effects and mechanisms of
heparin in inflammation were studied through assessing the levels
of inflammatory cytokines tumor necrosis factor (TNF)-α,
interleukin (IL)-6, IL-8 and IL-1β, as well as signaling molecules
of the p38, extracellular signal-regulated kinase (ERK), c-Jun
N-terminal kinase (JNK) and MAPK pathways, respectively.
Materials and methods
Animals, cell culture and reagents
C57BL/6 mice were provided by the Experimental
Animal Centre of China Medical University (Shenyang, China). The
animal study protocol was approved by the Animal Experimental
Committee of China Medical University, and the mice received humane
care in accordance with the Principles of Laboratory Animal Care.
Peritoneal macrophages were isolated from the 6–8 week-old mice, as
described previously (12). The
macrophages were maintained in 10% fetal bovine serum (FBS;
Hyclone, Logan, UT, USA) Dulbecco's modified Eagle's medium (DMEM;
Gibco-BRL, Grand Island, NY, USA). siRNA of caveolin-1 and a
scrambled non-specific sequence were stably transfected into the
peritoneal macrophages, respectively, using Lipofectamine 2000
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) as per
the manufacturer's instructions. Cells treated with transfection
reagent only were used as the control. The cells were cultured in
DMEM and maintained in an incubator at 37°C with 5% CO2.
The cells were divided into different groups and stimulated with
LPS (100 ng/ml; Sigma-Aldrich, St. Louis, MO, USA) and/or heparin
(20 µg/ml; Qianhong Bio-Pharma Co., Ltd, Changzhou,
China).
Cytokine analysis
The levels of cytokines TNF-α, IL-6, IL-8 and IL-1β
induced by LPS in heparin-stimulated peritoneal macrophages with or
without knockdown of caveolin-1 were measured. Briefly, the cells
were centrifuged at 1,000 × g for 5 min and the supernatants were
collected. The cytokine analysis was conducted using Mouse TNF-α,
IL-6, IL-8 and IL-1β ELISA kits purchased from Multisciences
(Hangzhou, China) according to the manufacturer's instructions.
Transfection of siRNA
Initially, four siRNA sequences targeting the
caveolin-1 gene were designed and synthesized by GenePharma Co.,
Ltd. (Shanghai, China). The efficiency of the siRNAs was tested by
quantitative polymerase chain reaction (qPCR) and western blotting.
Three sequences with improved effects (CGGUUGUACCGUGCAUCAA,
CAGACGAGGUGACUGAGAA and CCUUCACUGUGACAAAAUA) were used. The
scrambled non-specific sequence ACGUGACACGUUCGGAGAA with no effect
was used as a negative control. Cells treated with transfection
reagent alone were used as the control. For transfection, cells
were seeded into six-well plates and transfected with cave-olin-1
siRNA or control plasmids using Lipofectamine 2000 reagent
(Invitrogen Life Technologies) as per the manufacturer's
instructions. Each well received 75 pM siRNA in a volume of 200
µl, with all conditions assessed in triplicate wells. Cells
were incubated for 24 h prior to subsequent treatments.
Reverse transcription-qPCR (RT-qPCR)
Total RNA extracted separately from the control,
scrambled siRNA and caveolin-1 siRNA-transfected cells using the
RNAsimple Total RNA kit (Tiangen Biotech Co., Ltd., Beijing, China)
was treated with DNase (Tiangen Biotech Co., Ltd.). The pure RNAs
were used for the synthesis of first-strand cDNA using Super
Moloney's murine leukemia virus reverse transcriptase (BioTeke,
Beijing, China). Table I lists the
specific primers used for qPCR analysis. The amplified products
were quantitated using SYBR Green (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) fluorescence on an Exicycler™
96 (Bioneer Corporation, Daejeon, Korea) under the following
conditions: 95°C for 10 min, followed by 40 cycles at 95°C for 10
sec, 60°C for 10 sec and 72°C for 30 sec. Data were analyzed using
the 2−ΔΔCt method.
 | Table IOligonucleotide primer sets for
polymerase chain reaction. |
Table I
Oligonucleotide primer sets for
polymerase chain reaction.
| Name | Sequence (5′→3′) | Length (bases) | Tm (°C) | Size (kb) |
|---|
| Caveolin-1 F |
CTTTACCAACCTGCTACCTA | 20 | 50.2 | 169 |
| Caveolin-1 R |
CATTTCCCAAGTGATTCTCC | 20 | 54.3 | |
| β-actin-F |
CTGTGCCCATCTACGAGGGCTAT | 23 | 64.5 | 155 |
| β-actin-R |
TTTGATGTCACGCACGATTTCC | 22 | 63.2 | |
| IL8-F |
GCCCAATTACTAACAGGTTCC | 21 | 56.0 | 223 |
| IL8-R |
ACTTCACTGGAGTCCCGTAG | 20 | 53.9 | |
| IL6-F |
ACTTCCATCCAGTTGCCTTCTT | 22 | 59.7 | 174 |
| IL6-R |
TCATTTCCACGATTTCCCAGA | 21 | 60.3 | |
| IL-1β-F |
TTTGAAGTTGACGGACCCC | 19 | 57.9 | 149 |
| IL-1β-R |
ATCTCCACAGCCACAATGAGTG | 22 | 59.8 | |
| TNF-α-F |
TTCTACTGAACTTCGGGGTGAT | 22 | 58.2 | 158 |
| TNF-α-R |
CACTTGGTGGTTTGCTACGA | 20 | 56.9 | |
Western blot analysis
For western blot analysis, protein was extracted
from the transfected and treated cells. After the protein
concentration was determined, aliquots of 40 µg protein were
boiled in phosphate-buffered saline (PBS) with 5X loading buffer
(Beijing Solarbio Science & Technology Co., Ltd.). Proteins
separated by SDS-PAGE (8%, 10% and 12% polyacrylamide gels; Beijing
Solarbio Science & Technology Co., Ltd.) were transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA) using an electroblot apparatus (DYCZ-40D; Beijing, China).
Membranes were blocked in 5% nonfat milk and incubated separately
with the following primary antibodies: Rabbit polyclonal
anti-caveolin-1 (1:100; cat. no. sc-894), rabbit polyclonal
anti-ERK1/2 (1:100; cat. no. sc-292838), mouse monoclonal anti-JNK
(1:100; cat. no. sc-137018), rabbit polyclonal p38 (1:100; cat. no.
sc-535) and their phosphorylated equivalents rabbit polyclonal
p-ERK1/2 (1:100; cat. no. sc-23759-R), mouse monoclonal p-JNK
(1:100; cat. no. sc-6254) and rabbit polyclonal p-p38 (1:1,00; cat.
no. sc-17852-R), all obtained from Santa Cruz Biotechnology, Inc
(Dallas, TX, USA), and mouse horseradish peroxidase
(HRP)-conjugated anti-β-actin (1:10,000; cat. no. KC-5A08; Kangchen
Bio-Tech, Inc., Shanghai, China), at 4°C overnight with gentle
agitation. After washing with Tris-buffered saline containing Tween
20 (TTBS; Beijing Solarbio Science & Technology Co., Ltd.),
membranes were incubated with the appropriate HRP-conjugated goat
anti-mouse (cat. no. A0216) and anti-rabbit (cat. no. A0208)
secondary antibodies (1:5,000; Beyotime Institute of Biotechnology,
Haimen, China) for 45 min at 37°C. Membranes were then washed with
TTBS and protein bands were visualized using enhanced
chemiluminescence (ECL) reagent (7 Seapharmtech, Shanghai, China)
on X-ray film. The protein expression levels was quantified by
Gel-Pro Analyzer Version 3.0 (Media Cybernetics, Silver Spring, MD,
USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation (SD). All raw data were analyzed by one-way analysis of
variance followed by the Bonferroni's post-hoc test. Statistical
analyses were conducted using SPSS version 17.0 (SPSS Inc.,
Chicago, IL, USA). A P-value less than 0.05 was considered to
indicate a statistically significant difference between values.
Results
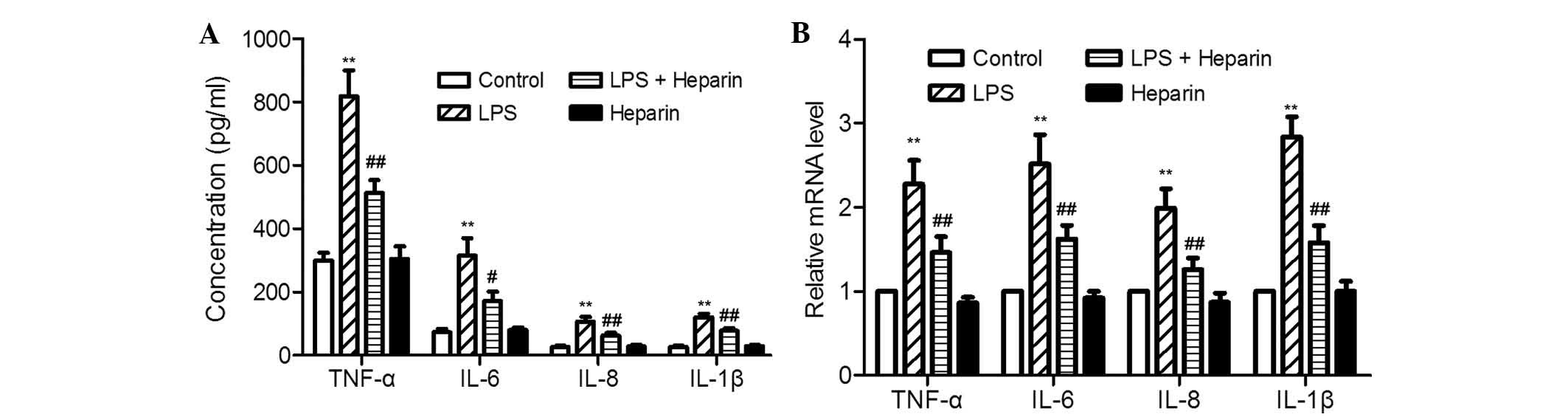
Heparin decreases the transcription and
concentration of LPS-induced inflammatory cytokines in murine
peritoneal macrophages
Heparin is a potential therapeutic inhibitor of
inflammation due to its ability to attenuate LPS-induced production
of inflammatory cytokines in human monocytes (13). The effects of heparin on
LPS-induced transcription and expression of inflammatory cytokines
in murine peritoneal macrophages were assessed at 24 h after LPS
and heparin co-stimulation or separate treatment. As shown in
Fig. 1, TNF-α, IL-6, IL-8 and
IL-1β transcription levels and protein concentrations were found to
be low in untreated and heparin-treated samples, while they were
strongly increased following LPS stimulation, and reduced by
heparin upon LPS and heparin co-stimulation. 100 ng/ml LPS induced
2.74-, 4.30-, 4.04- and 4.54-fold increases in TNF-α, IL-6, IL-8
and IL-1β protein concentration, and 2.28-, 2.52-, 1.99- and
2.84-fold increases in transcription levels, respectively, compared
to those in the control. Co-incubation with heparin and LPS
resulted in a significant reduction of all inflammatory cytokines
compared with those following LPS treatment alone. Protein
concentrations of TNF-α, IL-6, IL-8 and IL-1β were decidedly
decreased by 37.4, 45.5, 42.0 and 35.7%, and the transcription was
correspondingly inhibited by 36, 35.7, 36.7 and 44.4%,
respectively, with the addition of 100 ng/ml LPS and 20
µg/ml heparin compared to levels following LPS treatment
alone. Treatment of murine peritoneal macrophages with heparin
alone had little effect on any of the inflammatory cytokines at the
transcription or protein level, with results similar to those of
untreated samples. Overall, heparin was able to decrease the levels
of LPS-induced inflammatory cytokines in murine peritoneal
macrophages.
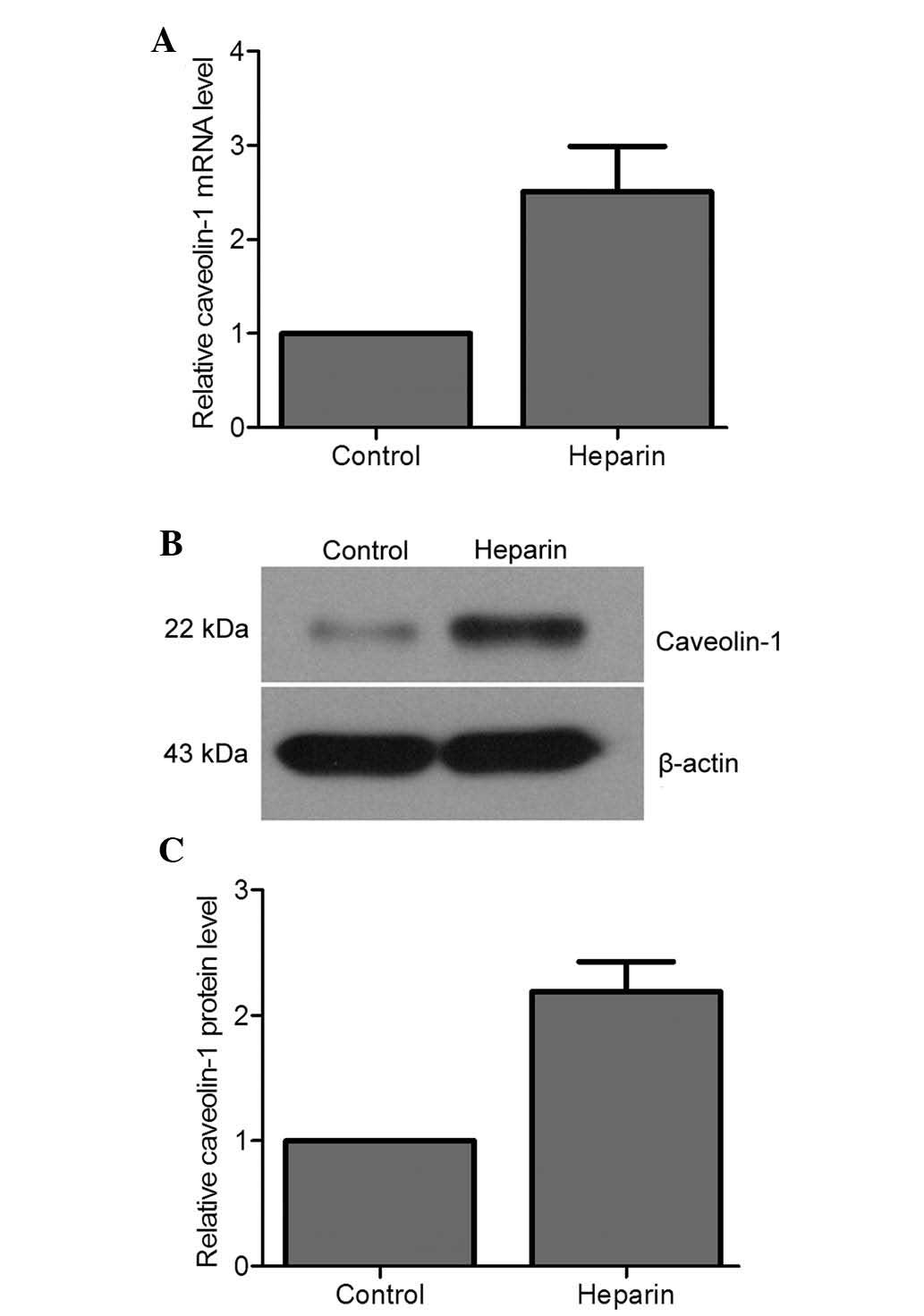
Heparin induces transcription and
expression of caveolin-1 in murine peritoneal macrophages
Heparin has been reported to regulate the expression
and sub-cellular localization of caveolin-1 in human vascular
smooth muscle cells (11). To
determine the association between heparin and caveolin-1 in murine
peritoneal macrophages, the transcription and protein expression
levels of caveolin-1 were quantitated under heparin treatment. It
was shown that heparin markedly enhanced the expression of
caveolin-1 at the mRNA (Fig. 2A)
and protein (Fig. 2B and C)
levels. A 2.51- and a 2.19-fold increase in mRNA and protein
levels, respectively, of caveolin-1 was detected compared with that
in the control. These results suggested that heparin is able to
induce the transcription and expression of caveolin-1 in murine
peritoneal macrophages.
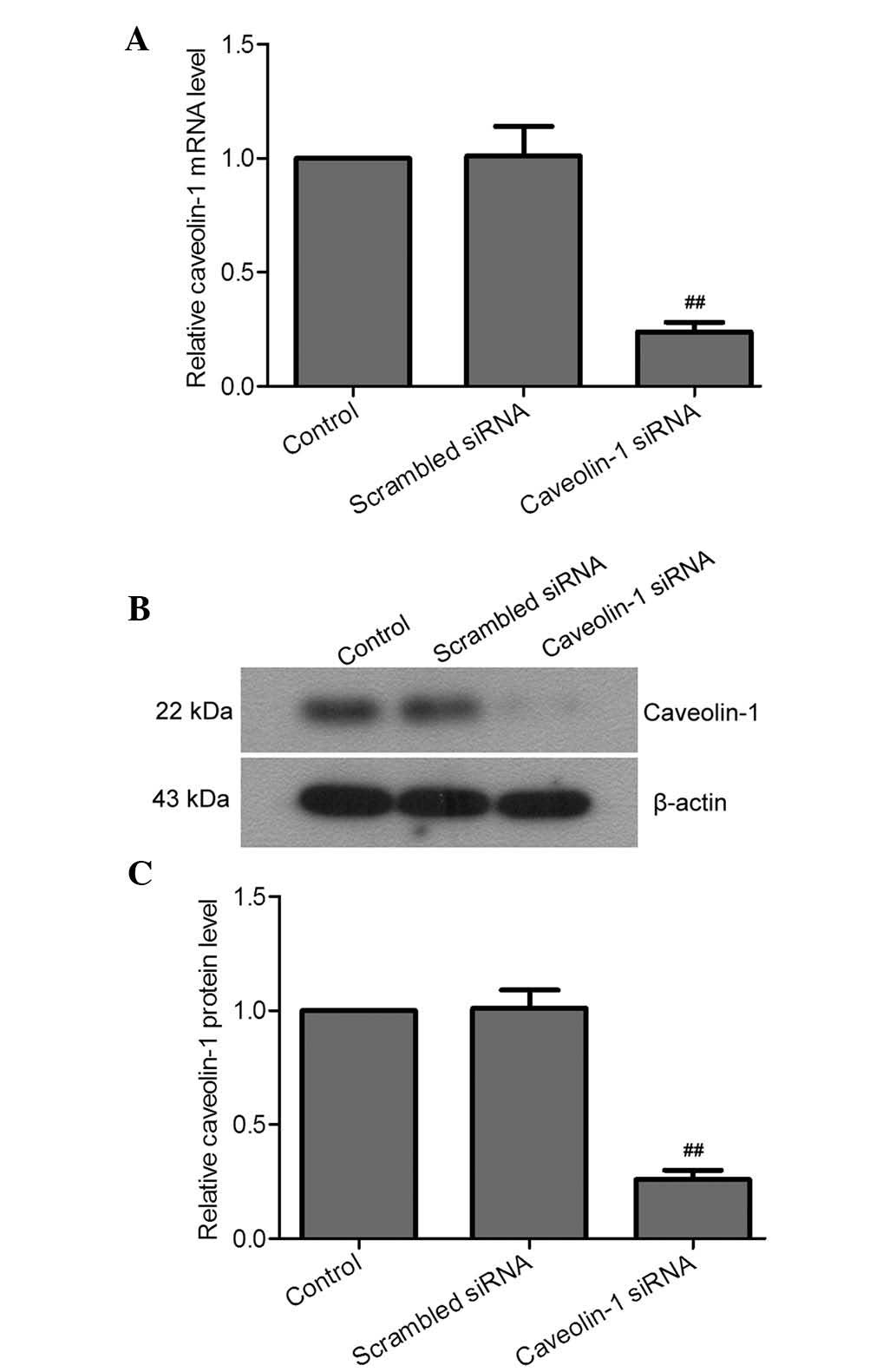
Caveolin-1 is essential for heparin to
decrease LPS-induced inflammatory cytokines in murine peritoneal
macrophages
Caveolin-1 has been reported to confer
anti-inflammatory effects in murine macrophages (12). To assess the function of caveolin-1
in the heparin-mediated reduction of the concentration of the
LPS-induced inflammatory cytokines TNF-α, IL-6, IL-8 and IL-1β by
heparin in murine peritoneal macrophages, caveolin-1 was silenced
through transfection of siRNA specifically targeting mRNA sequences
of caveolin-1 into murine peritoneal macrophages. At 24 h
post-transfection, the mRNA and protein levels of caveolin-1 siRNA
cells were significantly decreased compared to those in the
scrambled siRNA-transfected and control cells (Fig. 3). The transfected cells were
subsequently incubated with LPS and/or heparin, followed by
assessment of mRNA and protein levels of the inflammatory
cytokines. In the caveolin-1 knockdown group, LPS-induced increases
in the inflammatory cytokines TNF-α, IL-6, IL-8 and IL-1β in murine
peritoneal macrophages were greater than those in the scrambled
siRNA group. Of note, co-treatment with heparin LPS-induced
increases of inflammatory cytokines TNF-α, IL-6, IL-8 and IL-1β
were significantly abrogated in the scrambled siRNA group, while
heparin did not significantly decrease the levels of these cytokins
in the caveolin-1 siRNA group (Fig.
4). Treatment of transfected murine peritoneal macrophages with
heparin alone had no effects on the levels of any of the
inflammatory cytokines. Hence, caveolin-1 was essential for heparin
to decrease the concentration of LPS-induced inflammatory cytokines
in murine peritoneal macrophages.
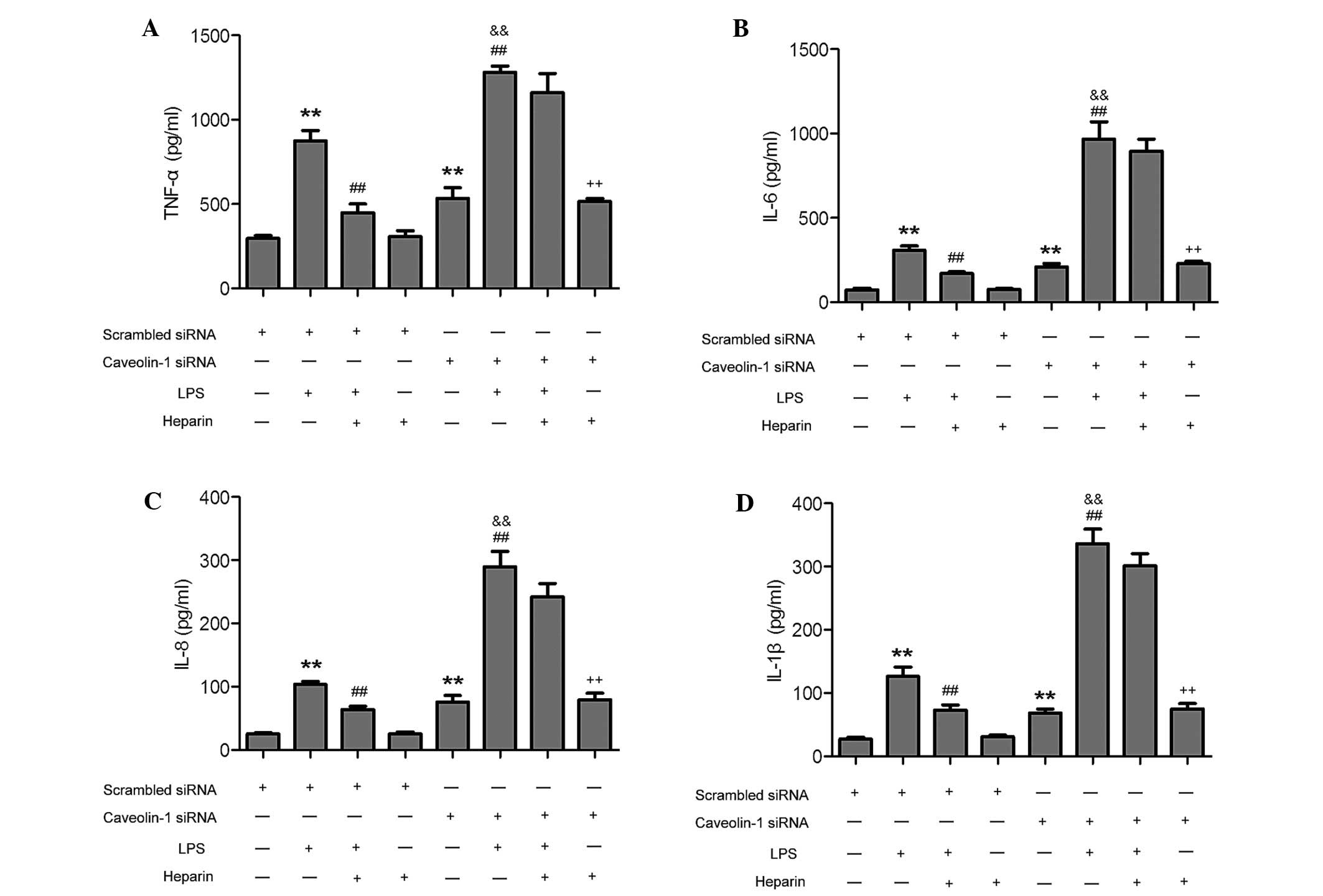 | Figure 4Caveolin-1 knockdown inhibits
heparin-mediated decreases of LPS-induced inflammatory cytokines in
murine peritoneal macrophages. Concentrations of (A) TNF-α, (B)
IL-6, (C) IL-8 and (D) IL-1β in murine peritoneal macrophages
transfected with scrambled and caveolin-1 siRNA were determined by
means of ELISA at 24 h after LPS, heparin and LPS + heparin
treatments. Values are expressed as the mean ± standard deviation
(n=3). **P<0.05, compared with the scrambled siRNA
cells; ##P<0.05, compared with the scrambled siRNA +
LPS-treated cells; &&P<0.05, compared with
the caveolin-1 siRNA cells; ++P<0.05, compared with
the scrambled siRNA + heparin-treated cells. IL, interleukin; TNF,
tumor necrosis factor; LPS, lipopolysaccharide; siRNA, small
interfering RNA. |
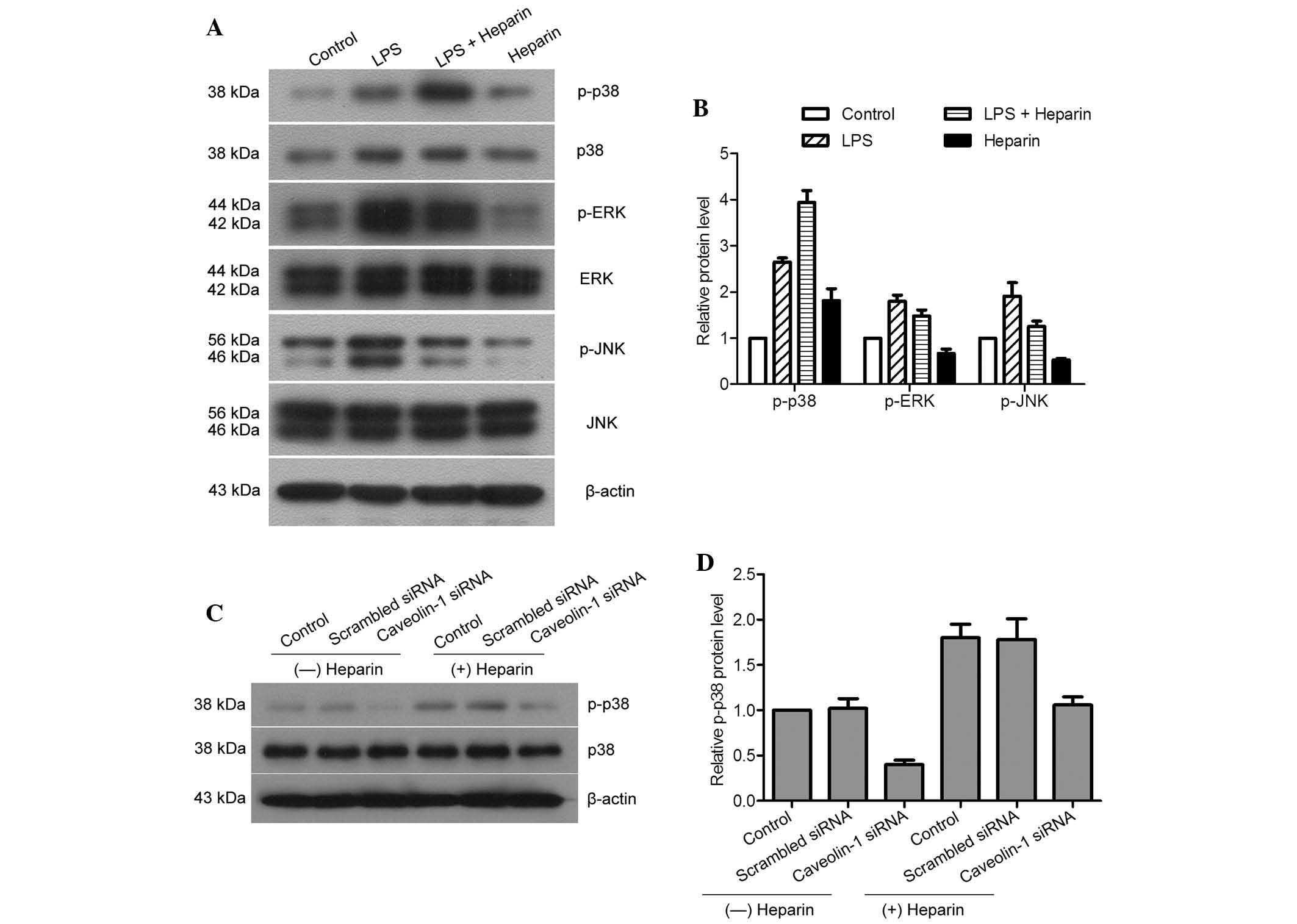
Effect of heparin on the MAPK pathway and
the mechanisms of p38/MAPK activation in murine peritoneal
macrophages
It has been reported that heparin can inhibit the
LPS-induced inflammatory response through blocking the activation
of the p38/MAPK pathway in endothelial cells (2). To clarify the association between
heparin and the MAPK pathway in LPS-induced murine peritoneal
macrophages, the expression levels of p-ERK, ERK, p-JNK, JNK, p-p38
and p38 were assessed in heparin- and/or LPS-treated cells. The
results demonstrated that LPS increased the expression of p-ERK,
p-JNK and p-p38, while heparin could increase the expression of
p-p38 only (Fig. 5A and B).
LPS-induced increases in p-ERK and p-JNK were inhibited by heparin,
while the reduced expression of p-JNK was more severe than that of
p-ERK. Downregulation of caveolin-1 attenuated the expression of
p-p38. However, the expression levels of p-p38 were increased in
the presence of heparin compared with those in the
heparin-untreated groups, even when caveolin-1 was knocked down in
the murine peritoneal macrophages (Fig. 5C and D). Therefore, heparin was
able to activate the p38/MAKP pathway and inhibit ERK and JNK
pathways in LPS-induced inflammation of murine peritoneal
macrophages. However, heparin was also able to activate p38 through
inducing caveolin-1 as well as by itself.
Discussion
Heparin is a soluble glycosaminoglycan, which is
well known for its anti-coagulant properties and anti-inflammatory
effects (14). It has been
reported that heparin can suppress the lethal response to acute
lung injury associated with sepsis through inhibiting inflammatory
responses (15). However, it has
remained elusive whether heparin can regulate inflammatory
responses in murine peritoneal macrophages. In the present study,
the heparin-mediated regulation of the induction of inflammatory
cytokines by LPS was assessed in murine peritoneal macrophages. As
expected, TNF-α, IL-6, IL-8 and IL-1β were upregulated by LPS at
the transcriptional and at the protein level, while all cytokines
were significantly reduced by co-treatment with heparin. This
indicated that heparin induced a strong decrease in inflammation of
murine peritoneal macrophages. The results demonstrated that the
observed reduction of LPS-induced inflammatory cytokines by heparin
treatment in murine peritoneal macrophages is in agreement with
similar studies performed on human cells and mice (16,17).
Therefore, heparin may represent a potential inhibitory agent in
inflammation through reducing the release of inflammatory
cytokines.
Caveolin-1 has numerous functions, including
participation in macromolecular transport and permeability, cardiac
hypertrophy, intercellular calcium, regulation of the vascular
reactivity, blood pressure, redox signaling and function, as well
as mechanotransduction (18,19).
Caveolin-1 was previously shown to be a potent immunomodulatory
molecule in murine macrophages (12), and also to be regulated by heparin
(11). To date, to the best of our
knowledge, the association between caveolin-1 and heparin in
inflammation has not been evidenced. The present study showed that
heparin induced high expression of caveolin-1 in murine peritoneal
macrophages. Furthermore, levels of the inflammatory cytokines
TNF-α, IL-6, IL-8 and IL-1β were higher in caveolin-1-silenced
cells than those in scrambled siRNA-transfected cells, indicating
that caveolin-1 itself had anti-inflammatory effects in murine
macrophages. Hence, induction of caveolin-1 was an important
mechanism of heparin in anti-inflammation. In addition,
downregulation of caveolin-1 inhibited the effects of heparin on
LPS-induced inflammatory cytokines. Upon caveolin-1-silencing,
heparin lost its ability to decrease LPS-induced inflammatory
cytokines, compared with that in scrambled siRNA-transfected cells.
This suggested that caveolin-1 was an essential factor for heparin
during the reduction of the concentration of LPS-induced
inflammatory cytokines in murine peritoneal macrophages. As heparin
can bind to numerous proteins non-specifically, the underlying
mechanisms of its anti-inflammatory action are complex and have
remained to be fully elucidated. Interacting with inflammatory
cytokines and basic fibroblast growth factor (bFGF) as well as
binding to acute phase and complementary proteins may also
contribute to the anti-inflammatory effect of heparin (20). The results of the present study
indicated that caveolin-1 may be a novel and necessary target of
heparin in the efficient anti-inflammatory process.
MAPKs are a family of intracellular kinases, and the
MAPK signal transduction pathway serves as a focal point for
ubiquitous and highly evolutionarily conserved mechanisms of
diverse extracellular stimulating responses and various cellular
process regulations (21). The
ERKs, JNK and p38 are identified as the cores of the MAPK pathway,
and they can be activated by environmental stresses and
inflammatory mediators (22). The
expression levels of caveolin-1 are positively correlated with p38
activation and anti-inflammatory effects, while they are negatively
correlated with ERK or JNK in murine macrophages (12). It was found that heparin had the
same effects in LPS-induced murine peritoneal macrophages and
exerted anti-inflammatory effects through activating p38, while, at
the same time, inhibiting ERK and JNK pathways. The
anti-inflammatory effects of heparin may have been based on
inducing caveolin-1, which actually took charge of activating the
p38/MAPK pathway. However, when caveolin-1 was silenced, heparin
was still able to activate the p38/MAPK pathway as evidenced by
increased expression of p-p38. These results indicated that heparin
had anti-inflammatory effects in LPS-induced murine peritoneal
macrophages via multiple signaling pathways, including the
induction of caveolin-1, activation of p38 and inhibition of ERK
and JNK pathways. By contrast, unfractionated heparin has been
reported to inhibit LPS-induced inflammation through blocking
p38/MAPK and NF-κB activation in endothelial cells (2). This confirmed the anti-inflammatory
effects of heparin, while various mechanisms of action may be
relevant in different cell types. Whenever heparin is used as the
anti-inflammatory agent, however, the effects are mediated via the
p38/MAPK pathway.
In conclusion, the present study demonstrated the
anti-inflammatory effects and mechanisms of heparin in LPS-induced
murine peritoneal macrophages. Heparin has a protective role in
inflammation by suppressing the production of the inflammatory
cytokines TNF-α, IL-6, IL-8 and IL-1β. High expression of
caveolin-1 induced by heparin can enhance the anti-inflammatory
effects, and caveolin-1 is a necessary factor for efficient
anti-inflammatory action by heparin. The present study also
provided evidence that the effects of heparin involve activation of
the p38/MAPK signaling pathway through inducing caveolin-1 as well
as by itself. It was further observed that heparin increased
LPS-induced activation of p38 and decreased activation of ERK and
JNK, which may be further mechanisms underlying its
anti-inflammatory effects. These results indicated that heparin has
the potential to be the drug of first choice in treating
hemorrhagic inflammation.
References
|
1
|
Barrow RT, Parker ET, Krishnaswamy S and
Lollar P: Inhibition by heparin of the human blood coagulation
intrinsic pathway factor X activator. J Biol Chem. 269:26796–26800.
1994.PubMed/NCBI
|
|
2
|
Li X, Zheng Z, Li X and Ma X:
Unfractionated heparin inhibits lipopolysaccharide-induced
inflammatory response through blocking p38 MAPK and NF-κB
activation on endothelial cell. Cytokine. 60:114–121. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lever R and Page C: Non-anticoagulant
Effects of Heparin: An Overview. Heparin-A Century of Progress.
207. Lever R, Mulloy B and Page CP: Springer; Berlin Heidelberg:
pp. 281–305. 2012, View Article : Google Scholar
|
|
4
|
Hansen CG and Nichols BJ: Exploring the
caves: cavins, caveolins and caveolae. Trends Cell Biol.
20:177–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rothberg KG, Heuser JE, Donzell WC, Ying
YS, Glenney JR and Anderson RG: Caveolin, a protein component of
caveolae membrane coats. Cell. 68:673–682. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernández-Rojo MA, Gongora M, Fitzsimmons
RL, et al: Caveolin-1 is necessary for hepatic oxidative lipid
metabolism: Evidence for crosstalk between caveolin-1 and bile acid
signaling. Cell Rep. 4:238–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meyer C, Liu Y, Kaul A, Peipe I and Dooley
S: Caveolin-1 abrogates TGF-[beta] mediated hepatocyte apoptosis.
Cell Death Dis. 4:e4662013. View Article : Google Scholar
|
|
8
|
Li J, Scherl A, Medina F, Frank PG, Kitsis
RN, Tanowitz HB, Sotgia F and Lisanti MP: Impaired phagocytosis in
caveolin-1 deficient macrophages. Cell Cycle. 4:1599–1607. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gadjeva M, Paradis-Bleau C, Priebe GP,
Fichorova R and Pier GB: Caveolin-1 modifies the immunity to
pseudomonas aeruginosa. J Immunol. 184:296–302. 2010. View Article : Google Scholar :
|
|
10
|
Feng H, Guo L, Song Z, et al: Caveolin-1
protects against sepsis by modulating inflammatory response,
alleviating bacterial burden and suppressing thymocyte apoptosis. J
Biol Chem. 285:25154–25160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peterson TE, Kleppe LS, Caplice NM, Pan S,
Mueske CS and Simari RD: The regulation of caveolin expression and
localization by serum and heparin in vascular smooth muscle cells.
Biochem Biophys Res Commun. 265:722–727. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang XM, Kim HP, Song R and Choi AM:
Caveolin-1 confers antiinflammatory effects in murine macrophages
via the MKK3/p38 MAPK pathway. Am J Respir Cell Mol Biol.
34:434–442. 2006. View Article : Google Scholar
|
|
13
|
Hochart H, Vincent Jenkins P, Smith OP and
White B: Low-molecular weight and unfractionated heparins induce a
downregulation of inflammation: decreased levels of proinflammatory
cytokines and nuclear factor-κB in LPS-stimulated human monocytes.
Br J Haematol. 133:62–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin-ping Li IV: Heparin, heparan sulfate
and heparanase in inflammatory reactions. Thrombosis and
haemostasis. 102:799–1006. 2009.
|
|
15
|
Zhao D, Ding R, Mao Y, Wang L, Zhang Z and
Ma X: Heparin rescues sepsis-associated acute lung injury and
lethality through the suppression of inflammatory responses.
Inflammation. 35:1825–1832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spratte J, Meyer zu Schwabedissen H,
Endlich N, Zygmunt M and Fluhr H: Heparin inhibits TNF-α signaling
in human endometrial stromal cells by interaction with NF-κB. Mol
Hum Reprod. 19:227–236. 2012. View Article : Google Scholar
|
|
17
|
Ding R, Zhao D, Guo R, Zhang Z and Ma X:
Treatment with unfractionated heparin attenuates coagulation and
inflammation in endotoxemic mice. Thromb Res. 128:e160–e165. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sowa G: Caveolae, caveolins, cavins and
endothelial cell function: new insights. Front Physiol. 2:1202012.
View Article : Google Scholar
|
|
19
|
Anderson RG: Caveolae: where incoming and
outgoing messengers meet. Proc Natl Acad Sci USA. 90:10909–10913.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Young E: The anti-inflammatory effects of
heparin and related compounds. Thromb Res. 122:743–752. 2008.
View Article : Google Scholar
|
|
21
|
Yong HY, Koh MS and Moon A: The p38 MAPK
inhibitors for the treatment of inflammatory diseases and cancer.
Expert Opin Investig Drugs. 18:1893–1905. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kyriakis JM and Avruch J: Mammalian MAPK
signal transduction pathways activated by stress and inflammation:
A 10-year update. Physiol Rev. 92:689–737. 2012. View Article : Google Scholar : PubMed/NCBI
|