Introduction
Gastric cancer is one of the most prevalent types of
human cancer, and is the second most common cause of
cancer-associated mortality (1).
Previously, surgical resection combined with chemotherapy has
helped save the lives of patients with gastric cancer at an early
stage (2,3). However, the prognosis of patients
with gastric cancer at a late stage remains poor, predominantly due
to metastasis and recurrence (2,3).
Therefore, studies into effective molecular targets for gastric
cancer metastasis are necessary and important.
Roundabout homolog 1 (Robo1), a member of the Robo
family, has been observed to be expressed in various types of
cells. It has been reported that Robo1 acts as a critical regulator
in multiple biological processes, including proliferation,
differentiation and migration (4,5).
Robo1 has mononucleotide repeats in the coding exons, which may be
mutation targets in cases of cancer with microsatellite instability
(6). Je et al (7) reported five frameshift mutations in
the repeats, and co-occurrences of mutation and loss of expression
of the Robo1 gene were also observed in gastric cancer tissues,
suggesting that Robo1 may serve an important role in gastric
cancer. In addition, the protein expression levels of Robo1 were
observed to be negatively regulated by microRNA (miR)-218, and
miR-218 was able to inhibit invasion and metastasis of gastric
cancer by inhibiting Robo1, indicating that Robo1 is associated
with the invasion and metastasis of gastric cancer (7). It has been well established that one
gene can be targeted by numerous miRs and that one miR has various
target genes (8). Accordingly,
additional miRs targeting Robo1 may also exist in gastric cancer,
and serve crucial roles in the regulation of migration and invasion
in gastric cancer cells.
Previously, deregulation of miR-29a has been
demonstrated to participate in the initiation and development of
several types of human cancer, including neuroblastoma, glioma,
breast cancer and gastric cancer (9–12).
Chen et al (11) revealed
that miR-29a had an inhibitory effect on growth and invasion of
gastric cancer cells via targeting vascular endothelial growth
factor A (VEGF-A), suggesting that miR-29a acts as a tumor
suppressor in gastric cancer. However, whether there is an
association between miR-29a and Robo1 in gastric cancer remains to
be fully elucidated.
In the current study, the expression levels of Robo1
and miR-29a were investigated in gastric cancer tissues and cell
lines. Subsequently, the roles of Robo1 and miR-29a in migration
and invasion of the AGS and SGC-7901 gastric cancer cell lines were
further investigated.
Materials and methods
Clinical tissue collection
Written informed consent was collected from all
patients with gastric cancer and the present study was approved by
the ethics committee of The First Affiliated Hospital of Anhui
Medical University (Hefei, China). The patients were aged between
43 and 68 and included 10 males and 7 females. The samples were
collected between June and October 2012 by tumor resection. A total
of 17 gastric cancer tissues and their matched adjacent normal
tissues were obtained from the Department of General Surgery, The
First Affiliated Hospital of Anhui Medical University (Hefei,
China).
Cell culture
Human AGS and SGC-7901 gastric cancer cells, in
addition to the human normal GES-1 gastric mucosal epithelial cell
line were obtained from the Cell Bank of Central South University
(Changsha, China). Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Invitrogen Life Technologies, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen Life
Technologies), 100 IU/ml penicillin (Sigma-Aldrich, St. Louis, MO,
USA) and 100 µg/ml streptomycin sulfate (Sigma-Aldrich) at 37°C
with 5% CO2.
Transient transfection
The Robo1 small interfering RNA (siRNA), Robo1
plasmid, miR-29a mimic, miR-29a inhibitor and scramble miRNA were
purchased from Nlunbio (Changsha, China). Cells in the logarithmic
growth phase were seeded into 6-well plates. Transfection of cells
with these oligonucleotides was performed using Lipofectamine 2000
(Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells with TRIzol
reagent (Life Technologies) according to the manufacturer's
instructions. The relative expression of miR-29a was determined by
RT-qPCR using the mirVana™ qRT-PCR microRNA Detection kit (Life
Technologies) following the manufacturer's instructions. Specific
primer sets for miR-29a and U6 (internal reference) were obtained
from Life Technologies. Expression of Robo1 mRNA was determined by
RT-qPCR using the standard SYBR Green RT-PCR kit (Takara Bio, Inc.,
Otsu, Japan) following the manufacturer's instructions. The
specific primer pairs used were as follows: Robo1, sense
5′-CTTACACCCGTAAAAGTGACGC-3′ and antisense
5′-TGGTCTCTCTAAGACAGTCAGC-3′; GAPDH as an internal control, sense
5′-CTGGGCTACACTGAGCACC-3′ and antisense
5′-AAGTGGTCGTTGAGGGCAATG-3′. The following cycling conditions were
used: 95°C for 10 min and 40 cycles of denaturation at 95°C for 15
sec and an annealing/elongation step at 60°C for 60 sec. Reverse
transcription was performed at 16°C for 30 min, followed by an
incubation step at 42°C for 30 min and enzyme inactivation at 85°C
for 5 min. An ABI 7500 Fast Real-Time PCR System (Applied
Biosystems, Foster City, CA, USA) was used. The relative expression
of Robo1 mRNA or miR-29a was quantified using GraphPad Prism
software, version 4.0 (GraphPad Software, Inc., La Jolla, CA, USA)
and the 2−ΔΔCt method.
Cell migration and invasion assays
Cell migration and invasive capabilities were
determined by performing a Transwell assay (Chemicon International,
Inc., Temecula, CA, USA). For the migration assay, cells were
plated in the upper chamber with the non-coated membrane. For the
invasion assay, the chamber was coated with Matrigel (Becton
Dickinson, Franklin Lakes, NJ, USA) and dried overnight, then cells
were plated in the upper chamber with the Matrigel-coated membrane.
For the two assays, DMEM supplemented with 10% FBS was used as a
chemoattractant in the lower chamber. Following 24 h of incubation
at 37°C, cells on the lower face of the membrane were stained with
0.4% crystal violet for 20 min and observed under a microscope
(CX22; Olympus, Tokyo, Japan).
Western blotting
Total protein was extracted using
radioimmunoprecipitation assay solution (Sigma-Aldrich). The
protein concentration was determined by the Bradford DC protein
assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). For
determination of the protein level, proteins were separated with
10% SDS-PAGE and were blotted onto polyvinylidene difluoride (PVDF;
Invitrogen Life Technologies) membranes, which were then incubated
in Tris-buffered saline with Tween-20 (Sigma-Aldrich) with 50 g/l
skimmed milk at room temperature for 4 h. Subsequently, the PVDF
membranes were incubated with mouse anti-Robo1 (cat. no. ab201632;
1:50 dilution; Abcam, Cambridge, MA, USA) and mouse anti-GAPDH
antibodies (cat. no. sc-365062; 1:50 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), respectively, at room
temperature for 1 h. Following washing with PBST three times, the
PVDF membranes were incubated with peroxidase-conjugated rabbit
anti-mouse secondary antibody (cat. no. ab175743; 1:20,000
dilution; Abcam) for 1 h at room temperature. Chemiluminescent
detection was performed with an ECL kit (Pierce Chemical Company,
Rockford, IL, USA).
Bioinformatical analysis
TargetScan (Release 6.2, www.targetscan.org) was used to predict the putative
target genes of miR-29a.
Dual luciferase reporter assay
SGC-7901 cells were cotransfected using
Lipofectamine 2000 with the reporter constructs Robo1-3′
untranslated region (UTR)-psi-CHECK2 (containing the 3′-UTR of
Robo1 including the miRNA-29a binding sites) or
mutant-Robo1-3′UTR-psi-CHECK2 (containing the corresponding mutated
sequence of 3′-UTR of Robo1), while miR-29a mimics or scramble
miRNA were used as the negative control. Luciferase activity was
determined after 48 h using the Dual-Glo Substrate system (Promega
Corporation, Madison, WI, USA) and the LD400 Luminometer (Beckman
Coulter, Inc., Brea, CA, USA). Data are presented as the ratio of
Renilla luciferase to Firefly luciferase.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Differences between two groups were determined using Student's
t-test. Analysis was performed using SPSS software, version 15.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-29a is downregulated in
gastric cancer tissues and cell lines
The endogenous expression of miR-29a was determined
in human gastric cancer tissues and their adjacent normal tissues
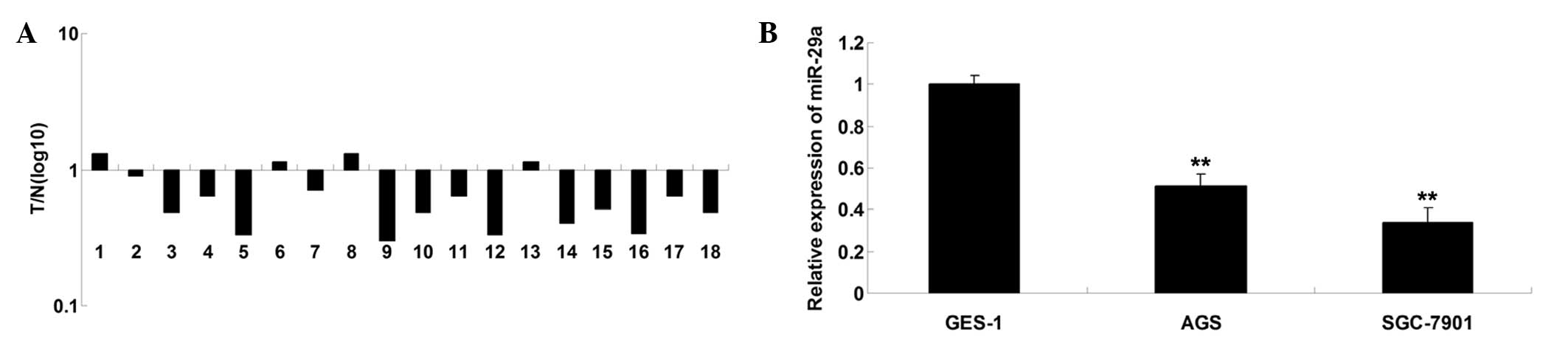
using RT-qPCR. As demonstrated in Fig.
1A, the relative expression of miR-29a was frequently
downregulated in 77.8% (14/18) of gastric cancer tissues compared
with the corresponding adjacent normal tissues. Similarly, it was
identified that expression of miR-29a was significantly reduced in
AGS and SGC-7901 gastric cancer cell lines, compared with that of
the GES-1 human gastric mucosal epithelial cell line (Fig. 1B). It is suggested that
downregulation of miR-29a may be associated with the development
and progression of gastric cancer.
Overexpression of miR-29a inhibits the
migration and invasion of gastric cancer cell lines
Based on the results obtained, whether miR-29a
serves a role in the regulation of migration and invasion of
gastric cancer cells was further investigated. AGS human gastric
cancer cells were transfected with miR-29a mimics or the negative
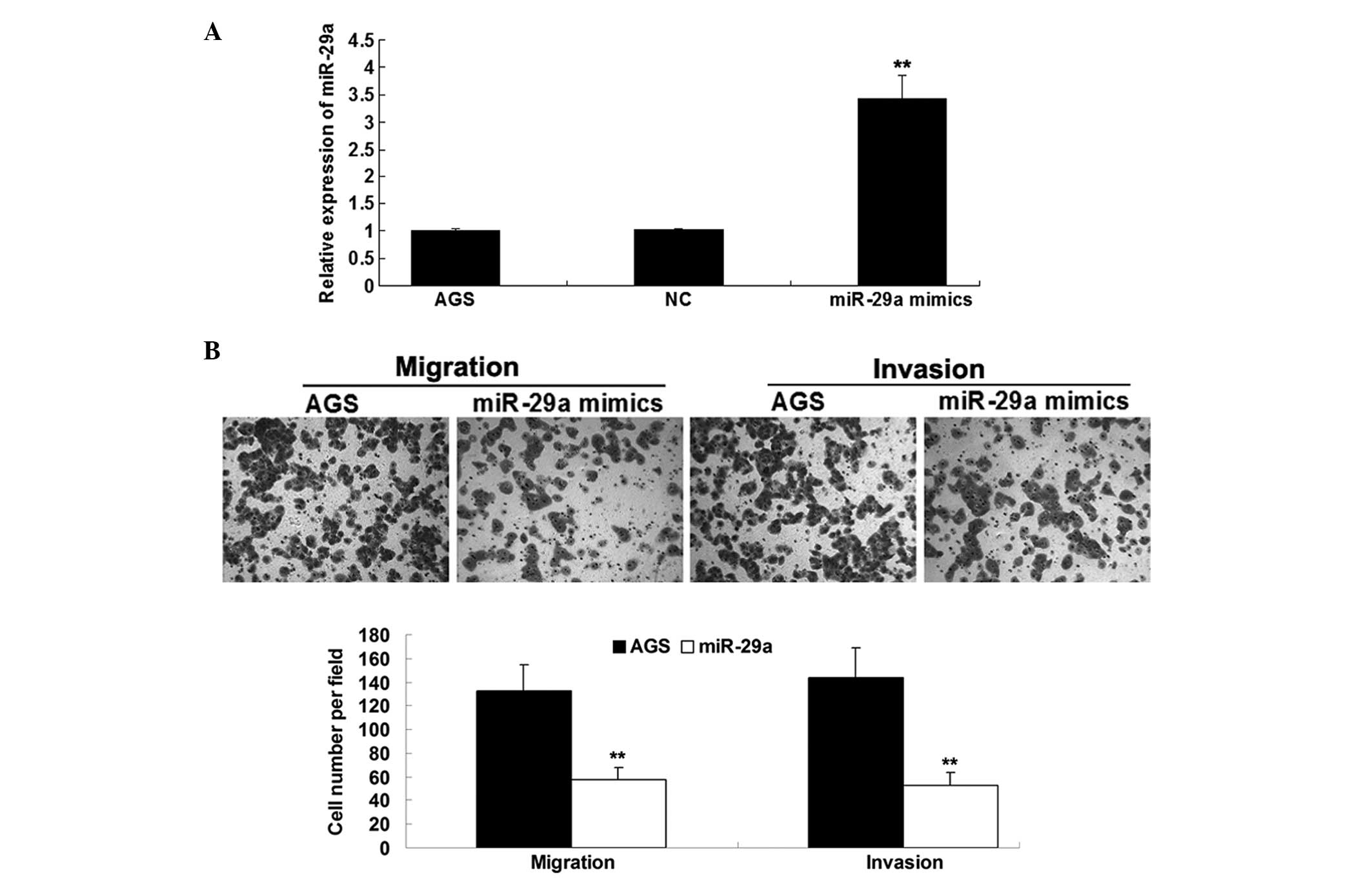
control, respectively. As indicated in Fig. 2A, transfection of miR-29a mimics
resulted in a significant increase in miR-29a expression, when
compared with the negative control transfected with scramble miRNA.
In addition, it was further identified that restoration of miR-29a
led to reduced migration and invasion of gastric cancer cells, when
compared with the control cells (Fig.
2B). Accordingly, it is suggested that miR-29a acts as a tumor
suppressor and contributes to inhibition of migration and invasion
of gastric cancer cells.
miR-29a negatively regulates Robo1 gene
expression
Targetscan prediction software was further used to
identify potential targets for miR-29a. Robo1 was identified as a
putative target gene for miR-29a, which has been reported to
participate in the regulation of cell migration and invasion
(13). To further confirm this
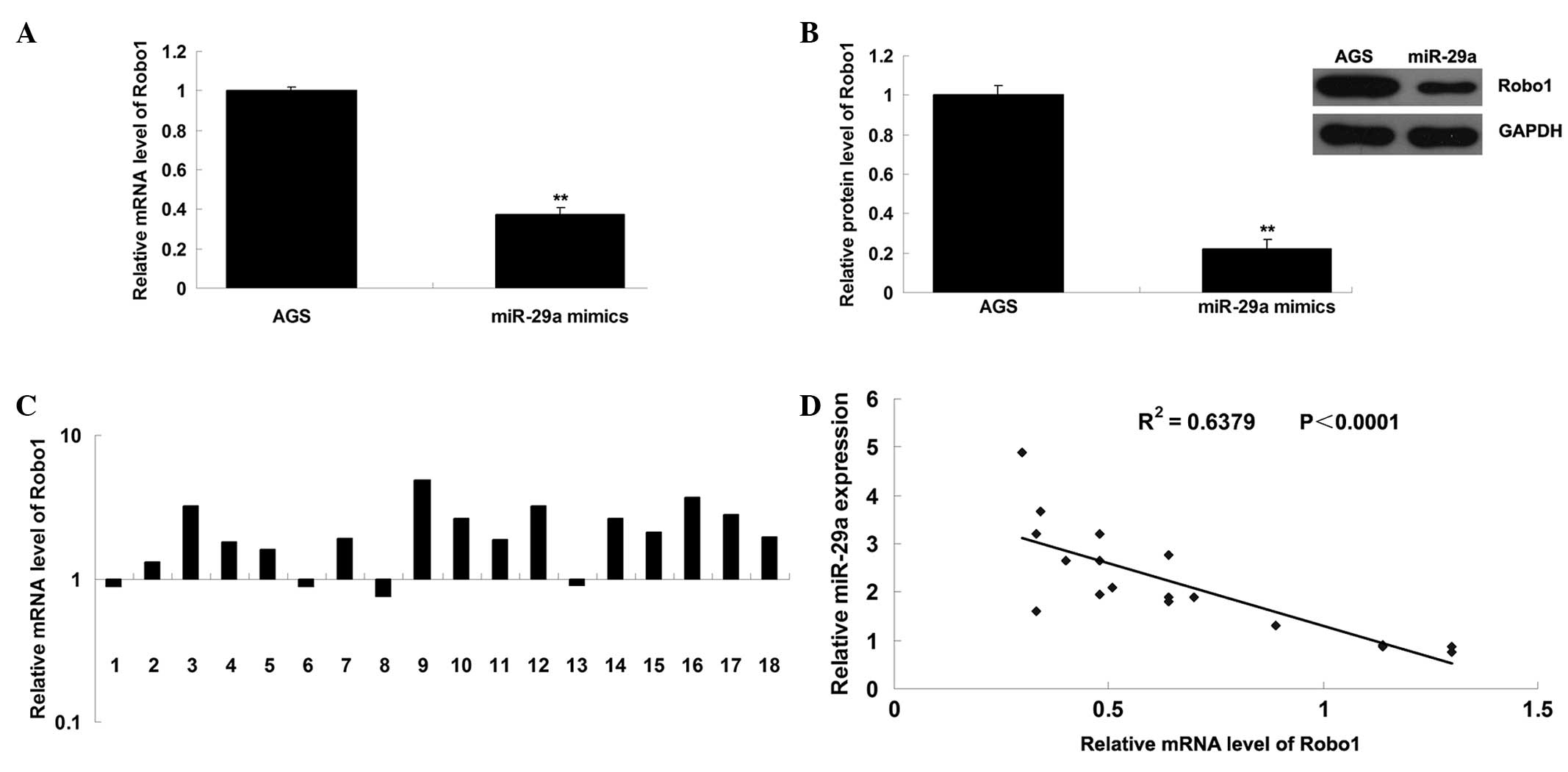
prediction, the mRNA and protein expression levels of Robo1 were
determined in AGS cells transfected with miR-29a mimic. It was
observed that the mRNA and protein expression of Robo1 was
significantly downregulated following overexpression of miR-29a,
compared with the negative control (Fig. 3A and B). Furthermore, the mRNA
levels of Robo1 in gastric cancer and adjacent normal tissues were
determined with RT-qPCR, then the significance of the miR-29a/Robo1
correlation was assessed in gastric cancer. As presented in
Fig. 3C, the mRNA expression of
Robo1 was frequently increased in gastric cancer tissues, compared
with the matched adjacent normal tissues. Furthermore, as
demonstrated in Fig. 3D, a
significant inverse correlation was observed between miR-29a and
Robo1 mRNA expression. Accordingly, the data suggest that miR-29a
negatively regulates Robo1 gene expression and Robo1 is a potential
target gene of miR-29a.
Robo1 3′-UTR is a target of miR-29a
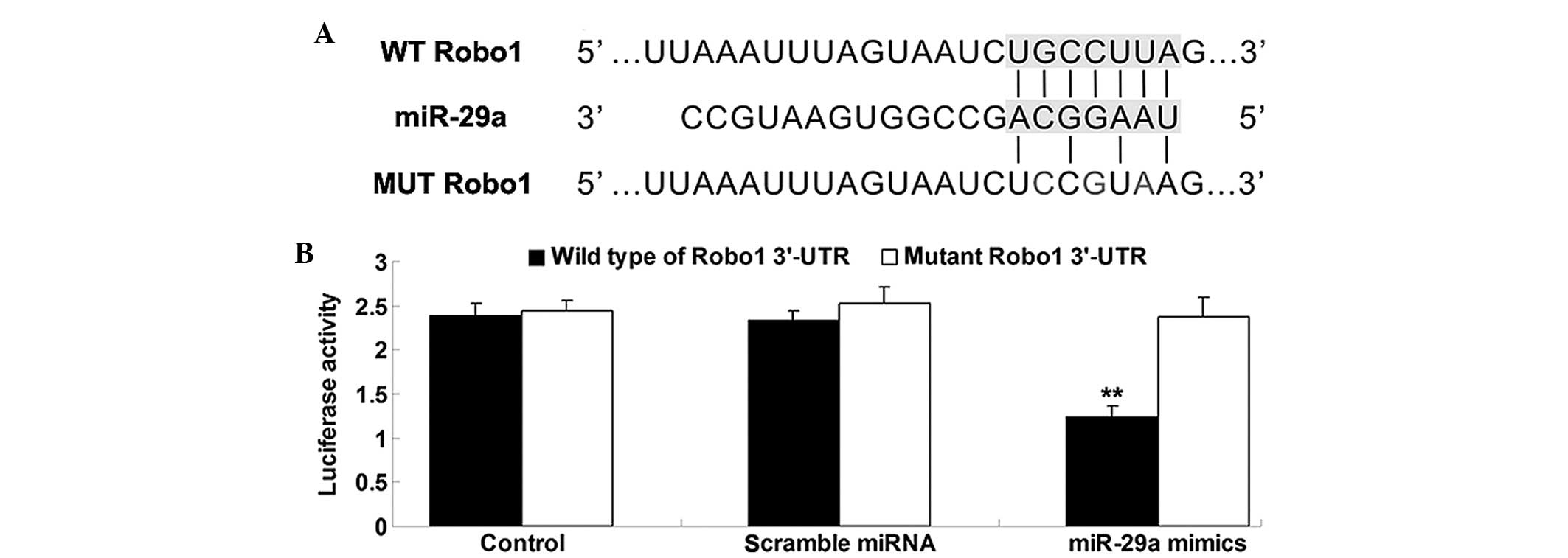
To verify whether Robo1 3′-UTR is a target of
miR-29a, a luciferase reporter assay was conducted. The vectors
containing the wide type of Robo1 3′UTR as well as the mutant type
of Robo1 3′UTR were constructed (Fig.
4A). As demonstrated in Fig.
4B, cotransfection of AGS cells with Robo1-3′-UTR/pmirGLO and
miR-29a mimics resulted in a significant reduction in the
luciferase activity, when compared with the negative control
(P<0.05). This inhibitory effect was abrogated by point
mutations in the core binding sites of the Robo1 3′-UTR. All of
these observations indicated that miR-29a exerts suppressive
effects on Robo1 expression via binding to the 3′ UTR of Robo1.
Knockdown of Robo1 reduces migration and
invasion in gastric cancer cells
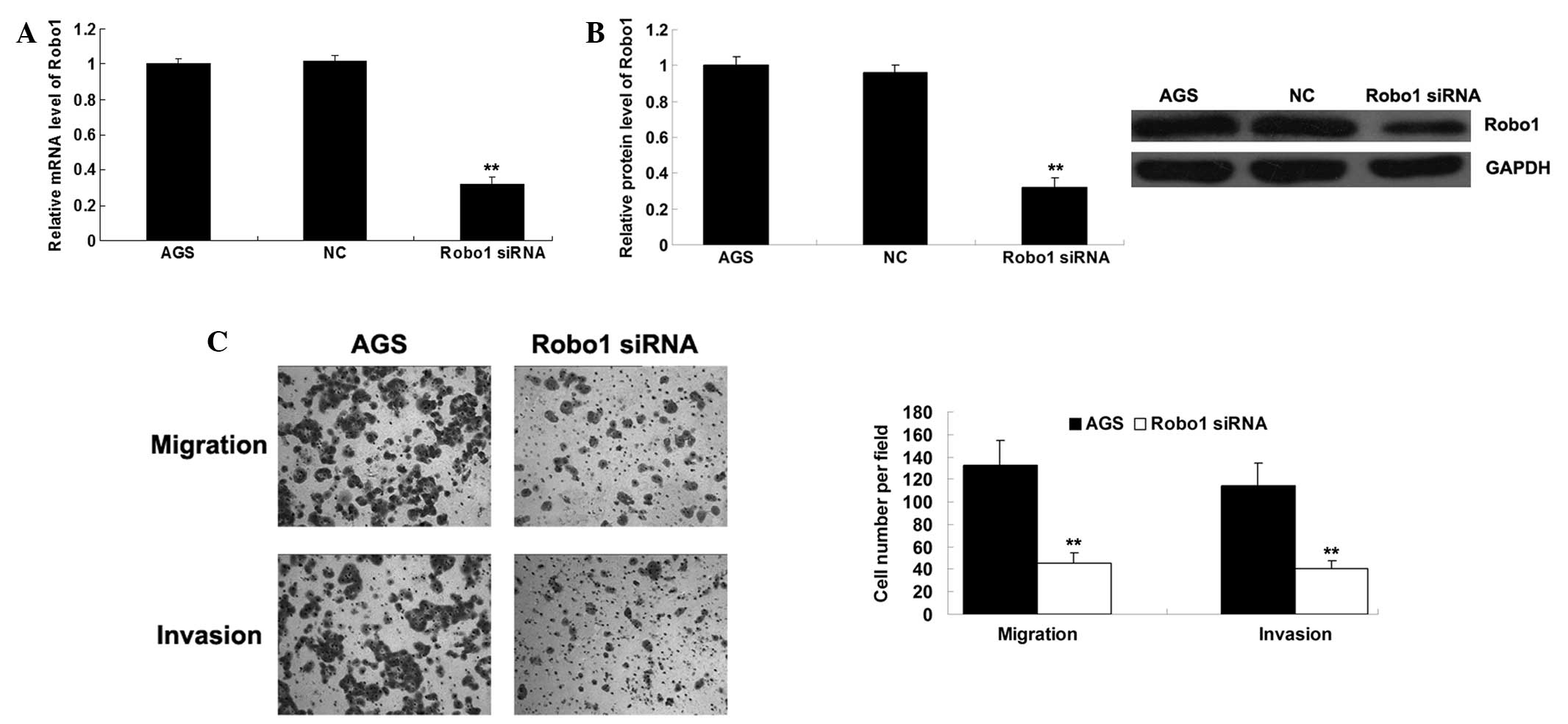
To verify the effect of Robo1 on gastric cancer cell
migration and invasion, the expression of Robo1 was observed to be
downregulated by Robo1 siRNA. As presented in Fig. 5A and B, the mRNA and protein
expression levels of Robo1 were notably downregulated following
knockdown of the Robo1 gene in AGS cells. Consistently,
downregulation of Robo1 markedly inhibited migration and invasion
in AGS cells (Fig. 5C), which
resembled the suppressive effects of miR-29a. These observations
suggest that miR-29a may inhibit gastric cancer cell migration and
invasion via targeting Robo1.
Discussion
Initiation and progression of cancer has been
demonstrated to involve deregulation of various genes, including
upregulation of oncogenes and downregulation of tumor suppressor
genes (14). Robo1 has been
reported to be involved in gastric cancer. Tie et al
(7) demonstrated that as a target
of miR-218, Robo1 contributed to gastric cancer metastasis
(7). In addition, dysfunction of
miR-29a has been reported to be involved in gastric cancer
(11). However, the downstream
target gene of miR-29a in gastric cancer remains largely unclear.
In the current study, it was observed that miR-29a/Robo1 signaling
serves a key role in the regulation of migration and invasion in
gastric cancer cells. The expression levels of miR-29a were
frequently reduced in gastric cancer tissues, when compared with
matched adjacent normal tissues. Furthermore, miR-29a was
additionally downregulated in gastric cancer cell lines compared
with the normal gastric mucosal epithelial cell line. These
observations suggest that miR-29a may serve a role in the malignant
progression of gastric cancer. It was further demonstrated that
restoration of miR-29a and knockdown of Robo1 may significantly
inhibit the migratory and invasive capabilities of gastric cancer
cells, and Robo1 was identified as a novel target of miR-29a. These
observations suggest that Robo1 may act as an oncogene while
miR-29a is hypothesized to be a tumor suppressor in gastric cancer.
Thus, it is suggested that their deregulation may promote gastric
cancer metastasis.
It is widely accepted that deregulated expression
levels of certain miRNAs directly participate in the development
and progression of numerous types of human cancer. In addition to
its role in cancer, miR-29a has also been demonstrated to be
associated with osteoblastic differentiation (15), myogenesis (16), sclerosis (17), fibrosis (18), HIV-1 replication (19), diabetes (20) and Alzheimer's disease (21). In the current study, it was
identified that overexpression of miR-29a markedly suppressed the
migratory and invasive capacities of gastric cancer cells. Chen
et al (11) reported that
miR-29a was notably downregulated in gastric cancer, consistent
with the results of the current study. In addition, Chen et
al (11) demonstrated that
miR-29a had an inhibitory effect on cell proliferation and invasion
in gastric cancer cells, at least in part via targeting VEGF-A. As
VEGF-A acts as a key regulator in angiogenesis, it was further
identified that miR-29a was able to suppress the tumor microvessel
density in gastric cancer (11).
Cui et al observed that miR-29a inhibited cell proliferation
and induced cell cycle arrest through the downregulation of p42.3
in gastric cancer (22). Taking
these previous observations together with the results of the
current study, it is suggested that miR-29a is able to suppress
malignant phenotypes of gastric cancer cells, including cell
proliferation, cell cycle progression, migration, invasion and
tumor angiogenesis, highlighting the importance of miR-29a in
gastric cancer.
Furthermore, the molecular mechanisms underlying the
inhibitory effect of miR-29a on gastric cancer were investigated.
As one miR has multiple target genes, other miR-29a targets are
likely to exist, which are involved in the development of gastric
cancer. Putative targets of miR-29a were searched using a
bioinformatics approach, and Robo1 was predicted to be a novel
target of miR-29a. To verify this prediction, western blotting and
a luciferase activity assay were performed, and it was identified
that miR-29a was capable of inhibiting the protein expression
levels of Robo1, by directly binding to the 3′-UTR of Robo1 mRNA in
gastric cancer cells. In addition, as Robo1 has been demonstrated
to act as a key migration and invasion factor in cancer cells, it
is suggested that downregulation of Robo1 induced by miR-29a
overexpression may contribute to inhibition of migration and
invasion in gastric cancer cells. In addition, it has been
previously reported that extracellular signal-regulated kinase
(ERK) signaling and matrix metalloproteinase 9 (MMP-9) were
downstream effectors of Robo1 (13). Upregulation of ERK signaling has
been identified to contribute to the development and progression of
various human malignancies including gastric cancer (23–26).
MMP-9 additionally serves a critical role in the regulation of
cancer cell invasion through degradation of the extracellular
matrix and promotion of cancer metastasis (27–29).
In conclusion, to the best of our knowledge the
present study suggests for the first time, that miR-29a inhibits
gastric cancer cell migration and invasion via inhibition of Robo1.
Therefore, miR-29a and Robo1 may serve as diagnostic or therapeutic
targets for gastric cancer.
Acknowledgments
This study was supported by the Natural Science
Foundation of China (grant no. 30871207 and 81270454), the Key
Project of Education Department of Anhui Province (grant no.
KJ2008A154) and the Key Project of Anhui Science and Technology
(grant no. 12070403086).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishiguro H, Kimura M and Takeyama H: Role
of microRNAs in gastric cancer. World J Gastroenterol.
20:5694–5699. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piazuelo MB and Correa P: Gastric cancer:
Overview. Colomb Med (Cali). 44:192–201. 2013.
|
|
4
|
Cornide-Petronio ME and Barreiro-Iglesias
A: Role of Slit and Robo proteins in the development of
dopaminergic neurons. Dev Neurosci. 35:285–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dickinson RE and Duncan WC: The SLIT-ROBO
pathway: A regulator of cell function with implications for the
reproductive system. Reproduction. 139:697–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Je EM, Gwak M, Oh H, et al: Frameshift
mutations of axon guidance genes ROBO1 and ROBO2 in gastric and
colorectal cancers with microsatellite instability. Pathology.
45:645–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tie J, Pan Y, Zhao L, et al: MiR-218
inhibits invasion and metastasis of gastric cancer by targeting the
Robo1 receptor. PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheung IY, Farazi TA, Ostrovnaya I, et al:
Deep microRNA sequencing reveals downregulation of miR-29a in
neuro-blastoma central nervous system metastasis. Genes Chromosomes
Cancer. 53:803–814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao D, Jiang X, Yao C, et al: Heat shock
protein 47 regulated by miR-29a to enhance glioma tumor growth and
invasion. J Neurooncol. 118:39–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen L, Xiao H, Wang ZH, et al: miR-29a
suppresses growth and invasion of gastric cancer cells in vitro by
targeting VEGF-A. BMB Rep. 47:39–44. 2014. View Article : Google Scholar :
|
|
12
|
Zhong S, Li W, Chen Z, Xu J and Zhao J:
MiR-222 and miR-29a contribute to the drug-resistance of breast
cancer cells. Gene. 531:8–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dontula R, Dinasarapu A, Chetty C, et al:
MicroRNA 203 modulates glioma cell migration via Robo1/ERK/MMP-9
Signaling. Genes Cancer. 4:285–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fabbri M, Calore F, Paone A, Galli R and
Calin GA: Epigenetic regulation of miRNAs in cancer. Adv Exp Med
Biol. 754:137–148. 2013. View Article : Google Scholar
|
|
15
|
Kapinas K, Kessler CB and Delany AM:
miR-29 suppression of osteonectin in osteoblasts: Regulation during
differentiation and by canonical Wnt signaling. J Cell Biochem.
108:216–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang XH, Hu Z, Klein JD, Zhang L, Fang F
and Mitch WE: Decreased miR-29 suppresses myogenesis in CKD. J Am
Soc Nephrol. 22:2068–2076. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maurer B, Stanczyk J, Jüngel A, et al:
MicroRNA-29, a key regulator of collagen expression in systemic
sclerosis. Arthritis Rheum. 62:1733–1743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roncarati R, Viviani Anselmi C, Losi MA,
et al: Circulating miR-29a, among other up-regulated microRNAs, is
the only biomarker for both hypertrophy and fibrosis in patients
with hypertrophic cardiomyopathy. J Am Coll Cardiol. 63:920–927.
2014. View Article : Google Scholar
|
|
19
|
Ahluwalia JK, Khan SZ, Soni K, et al:
Human cellular microRNA hsa-miR-29a interferes with viral nef
protein expression and HIV-1 replication. Retrovirology. 5:1172008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng H, Zhong M, Zhao W, et al: Urinary
miR-29 correlates with albuminuria and carotid intima-media
thickness in type 2 diabetes patients. PLoS One. 8:e826072013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shioya M, Obayashi S, Tabunoki H, et al:
Aberrant microRNA expression in the brains of neurodegenerative
diseases: miR-29a decreased in Alzheimer disease brains targets
neurone navigator 3. Neuropathol Appl Neurobiol. 36:320–330. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui Y, Su WY, Xing J, et al: MiR-29a
inhibits cell proliferation and induces cell cycle arrest through
the downregulation of p42.3 in human gastric cancer. PLoS One.
6:e258722011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukui H, Zhang X, Sun C, et al: IL-22
produced by cancer-associated fibroblasts promotes gastric cancer
cell invasion via STAT3 and ERK signaling. Br J Cancer.
111:763–771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Do MT, Na M, Kim HG, et al: Ilimaquinone
induces death receptor expression and sensitizes human colon cancer
cells to TRAIL-induced apoptosis through activation of ROS-ERK/p38
MAPK-CHOP signaling pathways. Food Chem Toxicol. 71:51–59. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Y, Zhao W, Xu QW, Wang XS, Zhang Y
and Zhang J: IQGAP3 promotes EGFR-ERK signaling and the growth and
metastasis of lung cancer cells. PLoS One. 9:e975782014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu G, Qin XQ, Guo JJ, Li TY and Chen JH:
AKT/ERK activation is associated with gastric cancer cell
resistance to paclitaxel. Int J Clin Exp Pathol. 7:1449–1458.
2014.PubMed/NCBI
|
|
27
|
Guo N, Liu F, Yang L, Huang J, Ding X and
Sun C: Chemokine receptor 7 enhances cell chemotaxis and migration
of metastatic squamous cell carcinoma of head and neck through
activation of matrix metalloproteinase-9. Oncol Rep. 32:794–800.
2014.PubMed/NCBI
|
|
28
|
Pal S, Moulik S, Dutta A and Chatterjee A:
Extracellular matrix protein laminin induces matrix
metalloproteinase-9 in human breast cancer cell line mcf-7. Cancer
Microenviron. 7:71–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Chen LJ, Zhou HC, et al:
Prognostic value of matrix metalloproteinase-9 in gastric cancer: a
meta-analysis. Hepatogastroenterology. 61:518–524. 2014.PubMed/NCBI
|