Introduction
Esophageal cancer (EC) is one of the most common
types of cancer worldwide with a variable geographic distribution
(1). It ranks eighth in order of
occurrence and is the sixth leading cause of cancer-related
mortality worldwide, with a higher incidence in males (1). It consists of two histological types,
esophageal squamous cell carcinoma (ESCC) and esophageal
adenocarcinoma (EAC), each with distinct etiologic and pathologic
characteristics (2). ESCC is the
major histological type of esophageal cancer in developing
countries (3). Although advances
have been made in the treatment of ESCC, including surgery,
chemotherapy, radiation or a combination of these options, the
prognosis of patients with ESCC remains poor, with the overall
5-year survival rate of patient after surgery is only 14–22%
(3,4). To improve the overall outcome for
patients with ESCC, it is important to understand the molecular
mechanisms underlying these processes, which may contribute to the
indentification of useful biomarkers and novel therapeutic
agents.
MicroRNAs (miRNAs), a class of small non-coding RNAs
19–25 nucleotides in size, are involved in multiple biological
processes, such as cell cycle, metabolism, cell differentiation,
proliferation, oncogenesis, angiogenesis and cell invasion
(5–8). miRNAs are novel posttranscriptional
regulators of gene expression that target the 3′ untranslated
region (3′-UTR) of mRNAs in a sequence-specific manner for
translational repression or degradation (9,10).
MicroRNAs (miRNAs) have been recognized as critical regulators of
cancer invasion and metastasis, either as promoters or as
suppressors in recent years (11–13).
In view of the close correlation between miRNAs and the biological
progression of multiple cancers, miRNAs are presently considered to
be potential novel targets for the treatment of various types of
cancer (5,6,14,15).
miR-338-3p is a recently identified miRNA and is
involved in cell proliferation, differentiation and invasion
(16–18). Previous investigation using miRNA
microarrays has revealed that miR-338-3p is consistently
downregulated in ESCC (19).
However, knowledge regarding the exact roles of miR-338-3p in ESCC
and the underlying molecular mechanisms remain relatively unclear.
Therefore, the aim of this study was to identify its role in ESCC
cells in vitro and in vivo to determine its utility
in ESCC diagnosis and therapy.
Materials and methods
Clinical samples
A total of 48 patients with ESCC were enrolled in
the present study and had undergone routine surgery at the Second
Hospital of Jilin University (Changchun, China) between July 2011
and August 2013. ESCC samples and the corresponding adjacent
esophageal tissues taken from the 48 patients were collected,
immediately snap frozen in liquid nitrogen, and stored at −80°C
until RNA extraction. This study was approved by the ethical
committee of the Affiliated Hospital of Jilin University, and all
patients provided written informed consent.
Cell lines and cell culture
EC9706 human ESCC cell lines were purchased from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China), were maintained in RPMI-1640 medium supplemented
with 10% fetal bovine serum (FBS; Gibco BRL, Gaithersburg, MD, USA)
and incubated at 37°C and 5% CO2.
miRNA transfection
Cells were transfected with miR-338-3p mimics or
corresponding negative control (GenePharma Co. Ltd., Shanghai,
China) using Lipofectamine 2000 (Invitrogen Life Technologies,
Carlbad, CA, USA) at a final concentration of 50 nM, according to
the manufacturer's instructions. Transfection efficiencies were
evaluated in every experiment by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) 48 h posttransfection. Cells
were subdivided into the following three groups: Untransfected
blank group (Blank), transfected negative control group (NC) and
miR-338-3p mimic transfection group (miR-338-3p).
RNA preparation and RT-qPCR
Isolation of total RNA from cells was performed
using Qiazol reagent and the miRNeasy mini kit (Qiagen, Valencia,
CA, USA) at 48 h posttransfection according to the manufacturer's
instructions. MicroRNA was reverse transcribed using the One Step
Primescript miRNA cDNA Synthesis kit (Qiagen). Then miR-338-3p was
quantified as described previously (20), using an Applied Biosystems 7900
Real-Time PCR system (Applied Biosystems Life Technologies, Foster
City, CA, USA) and SYBR® Premix Ex Taq™ kits (Takara,
Otsu, Japan) according to the manufacturer's instructions. The
cycling conditions were as follows: Denaturation at 94°C for 5 min,
followed by 40 cycles of the following for amplification, 94°C for
30 sec, 58°C for 60 sec and 72°C for 60 sec. U6 snRNA was used as
an endogenous control. The comparative 2−ΔΔCt method was
used for relative quantification and statistical analysis. The
above experiment was repeated at least three times.
Cell proliferation and colony formation
assays
The status of cell proliferation was determined by a
3-(4,5-dimethylthia-zole-2-yl)-2,5-biphenyl tetrazolium bromide
(MTT; Amresco, Solon, OH, USA) assay. Exponentially growing ESCC
cells were adjusted to 2.5×104 cells/ml with RPMI-1640,
and then plated in 96-well plates (Corning, Corning, NY, USA) at
200 µl/well and then incubated for 12 h according to routine
procedure. After transfection with miR-338-3p mimics or negative
control for 48 h, 20 µl MTT (5 g/l) was added to each well.
The medium was then removed after 4 h incubation and 100
µl/well dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis,
MO, USA) was added to dissolve the reduced formazan product.
Finally, the plate was read in an enzyme-linked immunosorbent
microplate reader (Bio-Rad 2550, Bio-Rad, Hercules, CA, USA) at 490
nm. The cellular proliferation inhibition rate was calculated as
described previously (21).
For the colony formation assay, after cells were
transfected with miR-338-3p mimics or negative control for 48 h,
they were seeded in a 6-well plate at a low density (1,000
cells/per well), and cultured for 7 days. Then cells were fixed
with 4% paraformaldehyde for 20 min and counted after staining with
1% crystal violet. The experiments were conducted in triplicate
wells at least three times.
Cell cycle and cell apoptosis assay
The effects of miR-338-3p on ESCC cell cycle and
apoptosis were examined by flow cytometry. In brief, cells were
transfected with either miR-338-3p mimics or negative control miRNA
for 48 h, then cells were harvested and washed twice with
phosphate-buffered saline (PBS), fixed with 70% ethanol at −20°C
for 30 min, and stored at 4°C overnight, then washed with PBS
again, treated with 100 ml of 100 mg/l RNase at 37°C for 30 min,
and stained with 100 ml of 50 mg/l propidium iodide at 4°C for 30
min in the dark. The multiplication cycle and apoptotic rate were
assayed using flow cytometry (FACSCalibur; BD Biosciences,
Mansfield, MA, USA), and the data were analyzed using CellQuest 2.0
software (BD Biosciences San Jose, CA, USA). The percentages of
cells in the G0/G1 phase and S phase, and the apoptotic rate were
measured by calculating the ratio of the number of corresponding
cells to the number of total cells. In addition, Bcl-2 and survivin
protein expression were determined by western blot analysis using
specific antibodies as an additional indicator of apoptosis.
Wound-healing assays
Cells were treated with miR-338-3p mimics or
corresponding negative controls when cells were grown to 80–90%
confluence in 24-well plates. After 24 h of transfection, linear
scratch wounds were created on the confluent cell monolayers with a
pipette tip. To stop cells from entering the cell cycle prior to
wounding, cells were maintained in serum-free medium. To visualize
migrating cells and wound healing, images were captured at 0 and 24
h under an inverted microscope (IX51; Olympus Corporation, Tokyo,
Japan). More than five field areas were selected randomly from each
well and the cells in three wells of each group were
determined.
Transwell assay
Cell invasion was measured using an 8-µm-pore
polycarbonate membrane Boyden chamber insert in a Transwell
apparatus (Millipore, MA, USA). In brief, the concentration of
cells in each group was adjusted to 2.5×105 cells/ml at
48 h post-transfection. The upper chamber of a 24-well transwell
permeable support with an 8-µm pore size was loaded with 200
µl cell suspension, and the lower chamber was filled with
500 µl RPMI-1640 medium containing 10% FBS, and then
cultured for 48 h. Five wells were used for each group. After
incubation, the media was removed from the upper chamber, and cells
were scraped out of the upper chamber with a cotton swab. Cells
that had migrated to the other side of the membrane were fixed with
methanol, stained with hematoxylin, mounted and dried at 80°C for
30 min. The number of cells invading the Matrigel was counted in
three randomly selected fields using an inverted microscope (IX51;
Olympus Corporation). Experiments were performed in triplicate.
Western blot analysis
After 48 h of transfection, total proteins were
prepared from the cells, quantities using a bicinchoninic acid
protein assay (Beyotime Institute of Biotechnology, Haimen, China).
Proteins were fractionated by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis transferred to a polyvinylidene fluoride
membrane (Invitrogen Life Technologies), blocked in 5% dry milk at
room temperature for 1 h and immunostained with antibodies at 4°C
overnight using anti-MMP-2 (1:1,000; cat. no. 13132; Cell Signaling
Technology, Inc., Danvers, MA, USA); and anti-MMP-9 (1:2,000; cat.
no. sc-12759; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
anti-survivin (1:5,000; cat. no. 2802s; Cell Signaling Technology,
Inc.); and anti-Bcl-2 (1:3,000; cat. no. sc-7382; Santa Cruz
Biotechnology, Inc). Anti-GAPDH (1:5,000; cat. no. sc-47724; Santa
Cruz Biotechnology, Inc.) was used as a loading control. The
membranes were incubated with the goat anti-mouse horseradish
peroxidase (HRP)-conjugated IgG (1:10,000; cat. no. sc-2005; Santa
Cruz Biotechnology, Inc.) or goat anti-rabbit HRP-conjugated IgG
(1:10,000; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) for 2
h at room temperature. All results were visualized through a
chemiluminescent detection system (Pierce, Pittsburgh, PA, USA) and
then exposed in Molecular Imager ChemiDoc XRS system (Bio-Rad). The
integrated density of the band was quantified by Quantity One v4.62
software (Bio-Rad).
Tumor growth in vivo
Thirty male BALB mice 5–6 weeks old, were purchased
from Jilin Institute of Experimental Animals (Changchun, China).
The research protocol was approved and mice were maintained in
accordance with the Institutional Guidelines of the Experimental
Animals of Jilin University.
Then, 2×106 untreated EC9706 cells,
stably expressing miR-338-3p or corresponding negative control
suspended in 50 µl PBS were injected into the flanks of mice
(n=10), respectively. Mice were monitored weekly for tumor growth.
Tumor volume was measured every week and calculated as 0.5236 ×
width2 × length. Three weeks after inoculation, mice
were sacrificed by cervical dislocation, and tumors were resected
and weighed, and part of tumors was used to measure the miR-338-3p
level.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Comparisons between the groups were analyzed with
one-way analysis of variance or two-tailed Student's t-test from
SPSS version 19.0 software (SPSS Inc., Chicago, IL, USA) and
GraphPad Prism version 5.01 (GraphPad Software, San Diego, CA, USA)
for Windows®. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-338-3p was decreased in human ESCC
tissues
The expression of miR-338-3p was analyzed in 48 EC
samples and corresponding adjacent tissues by RT-qPCR.
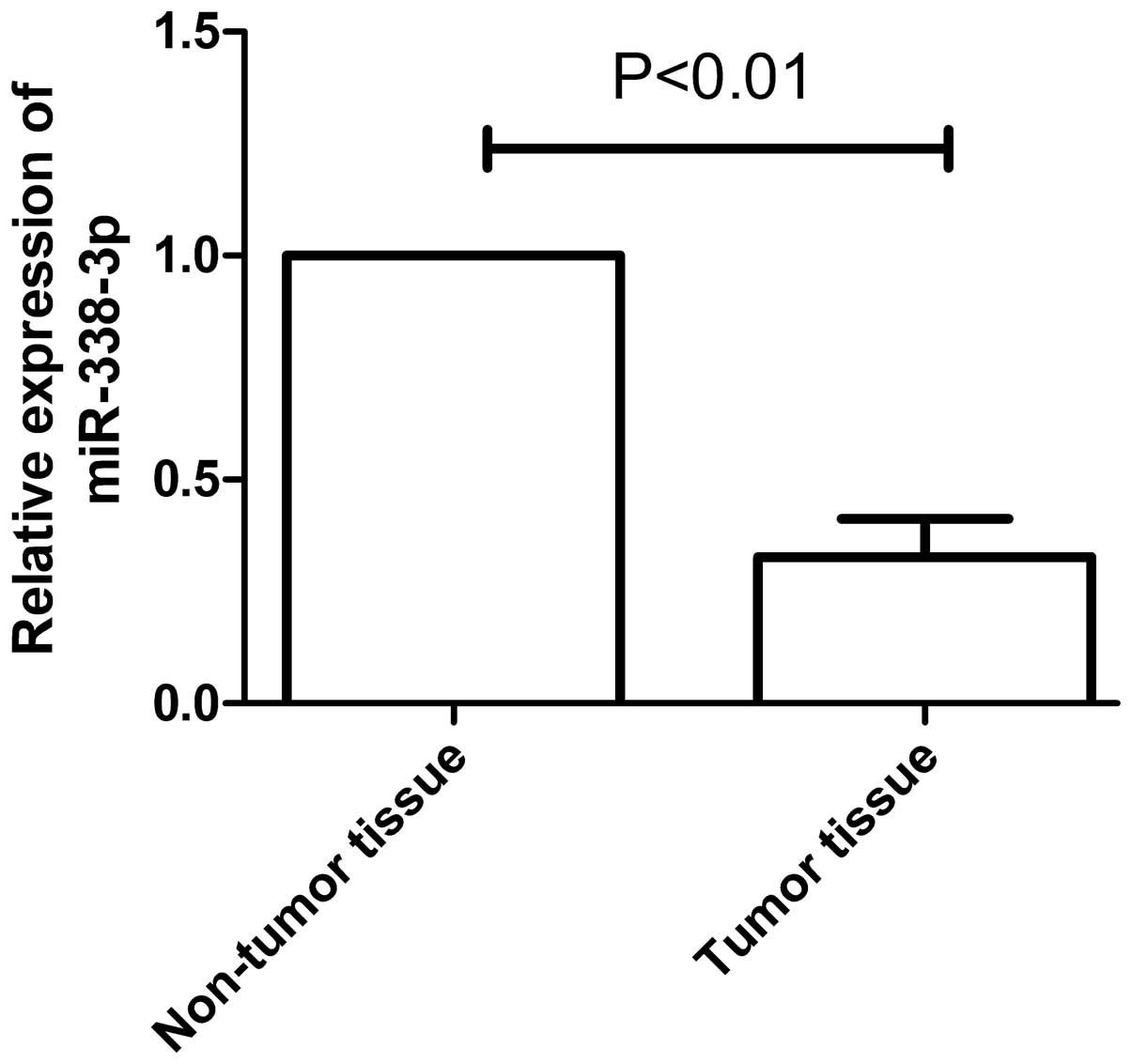
Significantly, miR-338-3p expression was lower in EC tissues than
corresponding adjacent non-tumor tissues (P<0.01, Fig. 1). The correlation between
miR-338-3p expression and clinicopathological characteristics is
shown Table I. No positive
correlation with gender, age or tumor location was observed;
however, there was a significant correlation with tumor stage
(P<0.01) and metastasis (P<0.01). The aberrant expression
level of miR-338-3p implied that miR-338-3p may be key in ESCC
development.
 | Table ICorrelation between relative level of
miR-338-3p in ESCC tissue, and clinicopathological features in
patients with ESCC. |
Table I
Correlation between relative level of
miR-338-3p in ESCC tissue, and clinicopathological features in
patients with ESCC.
| Feature | n | miR-338-3p
expression level | P-value |
|---|
| Age (years) | | | >0.05 |
| <60 | 22 | 0.3186±0.023 | |
| ≥60 | 26 | 0.3212±0.024 | |
| Gender | | | >0.05 |
| Male | 32 | 0.3210±0.022 | |
| Female | 16 | 0.3142±0.019 | |
| Tumor location | | | >0.05 |
| Middle | 30 | 0.3174±0.019 | |
| Lower | 18 | 0.3323±0.023 | |
| Metastasis | | | <0.05 |
| Yes | 24 | 0.2623±0.015 | |
| No | 24 | 0.3613±0.027 | |
| TNM stage | | | <0.05 |
| 0–I | 31 | 0.3831±0.026 | |
| II–IV | 17 | 0.2234±0.011 | |
miR-338-3p inhibits cell proliferation
and colony formation in EC9706 cells
As it was shown that miR-338-3p is significantly
downregulated in ESCC tissue, the present study aimed to determine
how miR-338-3p affects ESCC cell behavior. To this end, miR-338-3p
mimics and corresponding negative controls were transfected into
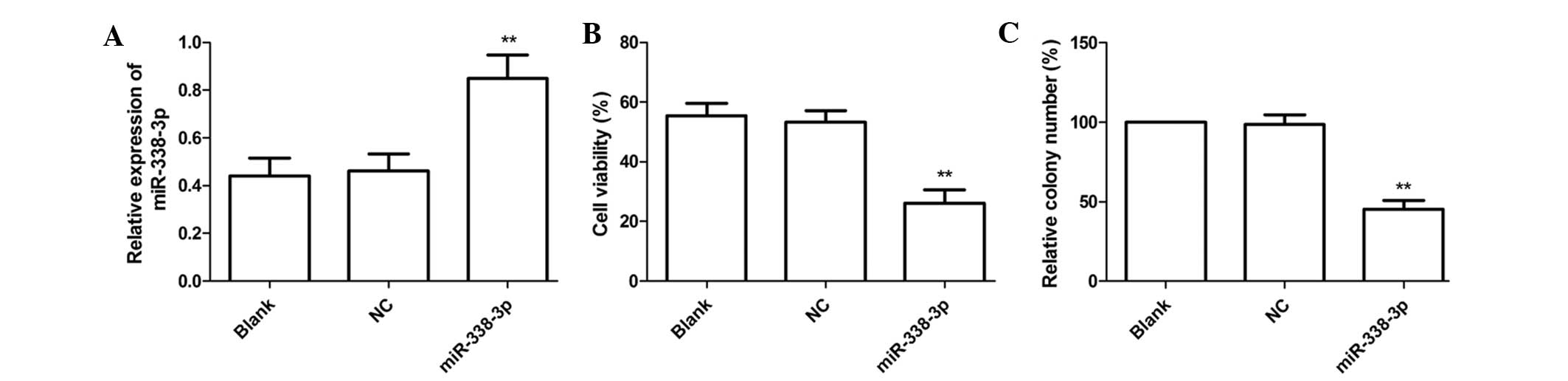
EC9706 cells, respectively. The results of RT-qPCR demonstrated
that expression of miR-338-3p in was increased in the miR-338-3p
group compared with the NC and blank groups (P<0.05, Fig. 2A). Then cell proliferation was
determined by an MTT assay. As shown in Fig. 2B, the viability of EC9706 cells was
markedly decreased in the miR-338-3p group compared with the NC and
blank groups (P<0.01).
The effect of miR-338-3p on cell colony formation in
EC9706 cells was also determined and it was revealed that
transfection with miR-338-3p mimics significantly inhibited cell
colony formation compared with cells transfected with negative
control and untransfected cells (P<0.05, Fig. 2C). These findings suggested that
the miR-338-3p expression greatly inhibited cell proliferation and
colony formation in EC9706 cells.
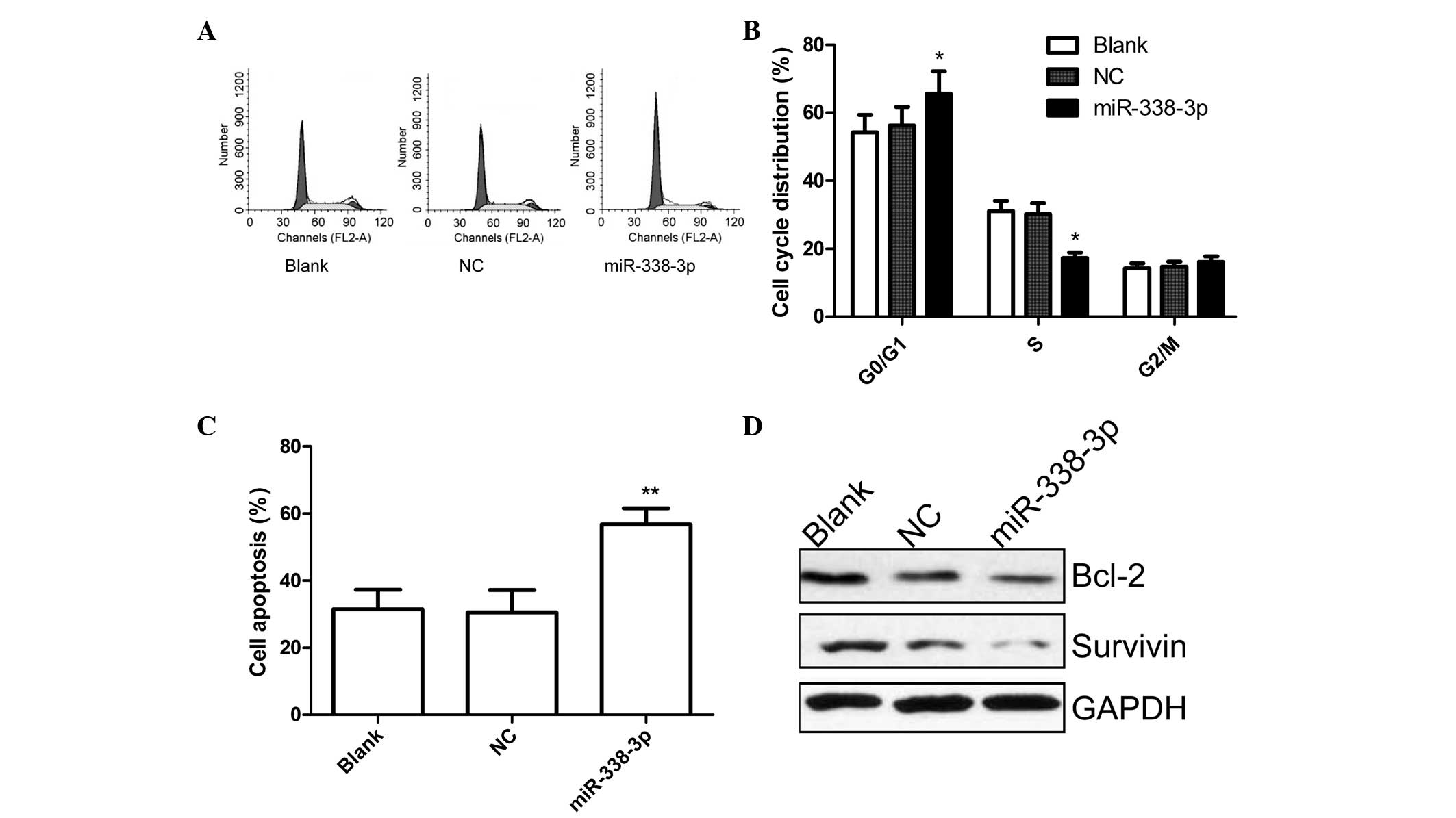
miR-338-3p induced cell cycle arrest and apoptosis
in EC9706 cells. To determine the effects on miR-338-3p cell cycle,
FACScan flow cytometry assays were performed. Flow cytometric
analysis revealed that the G1-phase cell population was increased
in the miR-338-3p group compared to blank group and NC group
(P<0.05, Fig. 3A and B). In
addition, miR-338-3p overexpression resulted in a lower percentage
of cells in S phase compared with those of blank group and NC group
(P<0.05, Fig. 3A and B).
In addition, flow cytometric analysis also showed
that cells transfected with miR-338-3p could significantly induce
cell apoptosis compared with untreated cells and cells transfected
with negative control (P<0.05, Fig.
3C).
To determine the potential mechanism underlying cell
apoptosis in vitro, survivin and Bcl-2 expression was
detected using western blot analysis. It was found that survivin
and Bcl-2 protein expression was significantly decreased in
miR-338-3p treatment groups compared with the blank and NC groups
(Fig. 3D).
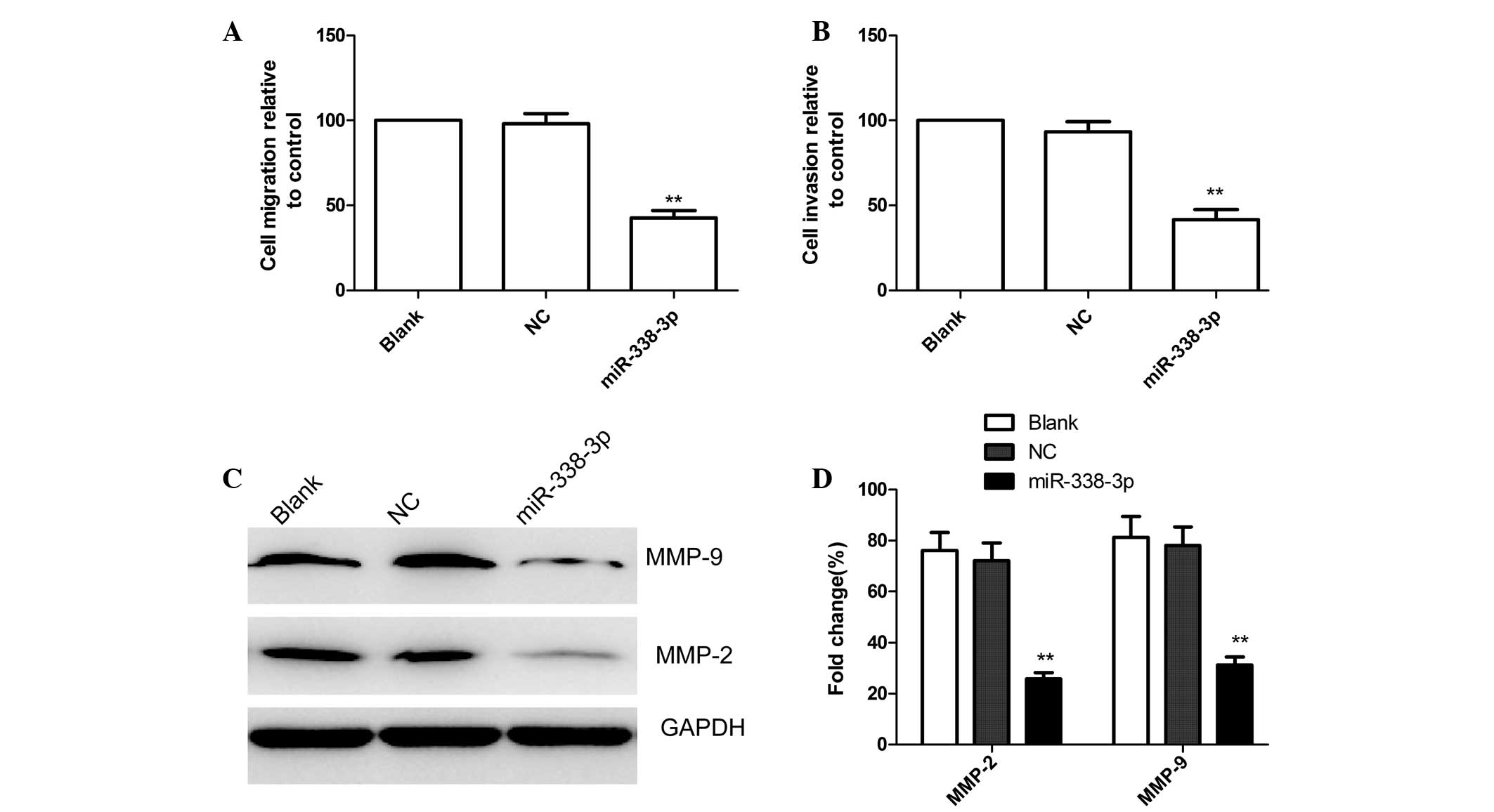
miR-338-3p inhibits cell migration and
invasion in EC9706 cells
To test whether miR-338-3p overexpressing cells
possessed a reduced propensity for migration and invasion,
wound-healing and Transwell assays were performed. For the
wound-healing assay, microscopic observations of the three groups
were recorded 24 h after scratching the cell surface. The capacity
for wound healing was lower for the miR-338-3p group than for the
blank and NC groups (P<0.05; Fig.
4A).
Using a Transwell assay, it was demonstrated that
the mean number of cells penetrating the membrane was not
identified to be significantly different for the blank and NC
groups (P>0.05; Fig. 4B).
However, the mean number of cells penetrating the Transwell
membrane was significantly lower in the miR-338-3p group compared
with the blank and NC groups (P<0.05; Fig. 4B). Based on these results, it was
concluded that exogenous overexpression of miR-338-3p decreases the
invasive ability of EC9706 cells.
Furthermore, the effects of miR-338-3p on the
expression of cell invasion relevant proteins, MMP-2 and MMP-9 were
analyzed by western blot analysis. As shown in Fig. 4C and D, MMP-2 and MMP-9 protein
expression significantly decreased in the miR-338-3p treatment
group compared with the blank and NC groups (P<0.05).
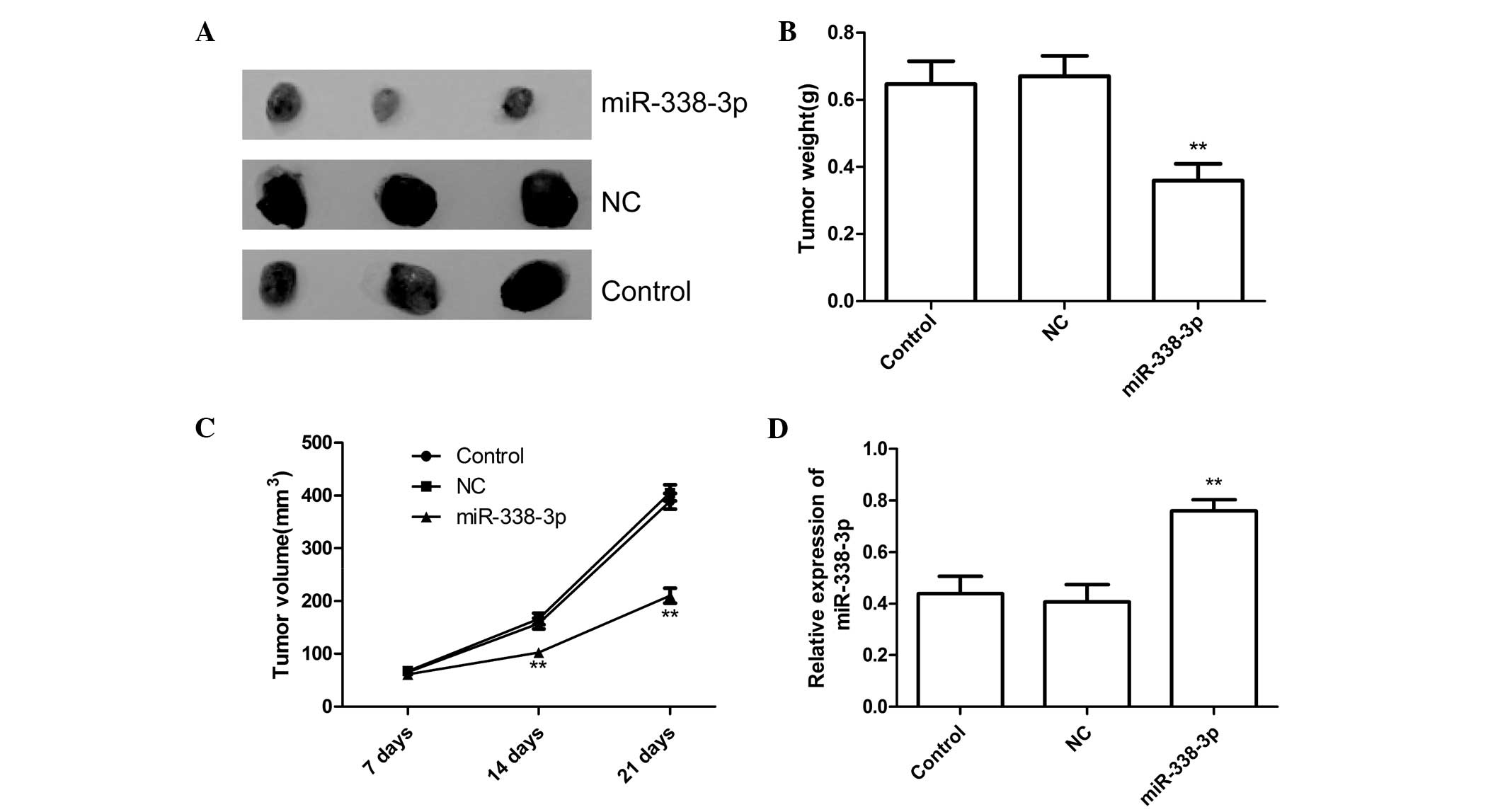
miR-338-3p suppresses tumor growth in a
mouse xenograft model
It was investigated whether miR-338-3p could
function as a tumor suppressor (as it does in other malignancies)
using a mouse xenograft model. miR-338-3p mimic transfected EC9706
cells produced tumors with significantly reduced weight and volume
21 days after injection compared with tumors initiated by cells
treated with negative control miRNA or untreated cells (P<0.05,
Fig. 5A–C). To clarify miR-338-3p
transfection activity, miR-338-3p expression was examined in
xenograft tumors 21 days after injection. The results showed that
miR-338-3p expression was upregulated in the xenograft tumors of
miR-338-3p group compared with the xenograft tumors of the NC or
control group (untreated group). These results may suggest that
overexpression of miR-338-3p could inhibit tumor growth of ESCC
in vivo.
Discussion
Increasing evidence showed that miRNAs have critical
regulatory roles in cancer biology (22,23).
Specifically for ESCC, it has been reported that miRNA contributes
to proliferation, apoptosis, migration and invasion (24,25).
For example, Ma et al (26)
found that miR-21 was overexpressed in ESCC cell lines and ESCC
tissue, and overexpression of miR-21 promoted cell proliferation
and invasion of ESCC cells by targeting phosphatase and tensin
homolog. Zhang et al (27)
suggest that miR-518b may function as a tumor suppressor by
targeting Rap1b in the development of ESCC, and that overexpression
miR-518b inhibited ESCC cell proliferation and invasion. Wang et
al (28) demonstrated that
high expression of miR-625 inhibited proliferation and invasion in
ESCC cells via controlling Sox2 expression. Wang et al
(29) found that miR-655 is
expressed at low levels in primary ESCC tissues, and upregulation
of miR-655 inhibits ESCC cell invasiveness by targeting
tumor-transforming gene-1 (PTTG1). The present aimed to provide
evidence that the overexpression of miR-338-3p inhibited
proliferation, colony formation, migration and invasion in
ESCC.
The miR-338 gene is located on chromosome 17q25
within the eighth intron of the apoptosis-associated tyrosine
kinase (AATK) gene. There are two mature forms of miR-338:
miR-338-3p and miR-338-5p (30).
It has been shown that miR-338-3p is involved in a variety of
physiological and pathological processes, and was downregulated in
several malignancies. For instance, Huang et al (31) showed that miR-338-3p expression is
downregulated in hepatocellular carcinoma, in which miR-338-3p
downregulation was significantly associated with TNM stage,
vascular invasion, intrahepatic metastasis, tumor size and tumor
grade. Xue et al (16)
found that miR-338-3p downregulation in certain selective CRC
samples, and that the miR-338-3p expression was not only associated
with TNM stage but also with tumor metastasis. Peng et al
(32) found that the level of
miR-338 expression was significantly reduced in the tumor tissues
compared with the adjacent normal mucosa tissues. In line with
these results, our studies showed that miR-338-3p was frequently
downregulated in ESCC tissues, and that miR-338-3p expression was
significantly correlated with tumor stage and metastasis. These
findings indicate that miR-338-3p may be a novel tumor suppressor
miRNA for treatment of ESCC.
miR-338-3p has been shown to be involved in the
development of several types of cancer (16–18,30–32);
however, the underlying mechanism and its involvement in the
development in ESCC remains to be elucidated. To reveal the exact
role of miR-338-3p in ESCC, the effect of miR-338-3p on
proliferation, colony formation, cell cycle, apoptosis, migration
and invasion was analyzed by upregulating the expression level of
miR-338-3p. The results showed that upregulation of miR-338-3p
inhibited cell proliferation colony formation, migration and
invasion, and induced cell apoptosis and cell arrest G0/G1 stage.
In addition, it was demonstrated that overexpression of miR-338-3p
could inhibit tumor growth of ESCC in vivo. These results
suggest that miR-338-3p may be involved in the development of
ESCC.
The degradation of basement membrane (BM) and
extracellular matrix (ECM) is a critical event in tumour invasion
and metastasis (33). Matrix
metalloproteinases (MMPs), particularly MMP2 and MMP9, are a major
group of enzymes that regulate ECM and BM composition during normal
development and pathological responses (34,35).
The results showed that enforced expression of miR-338-3p in EC9706
cells inhibited migration and invasion, and decreased MMP-2 and
MMP-9 expression. These findings suggest that upregulation of
miR-338-3p suppresses ESCC cell migration and invasion probably
through inhibition of MMP-9 and MMP-2 expression.
In conclusion, the findings of the present study
demonstrate that miR-338-3p was downregulated in ESCC tissues
compared with corresponding adjacent tissues, which were associated
with tumor depth, stage and metastasis, and that enforced
expression of miR-338-3p significantly inhibited cell
proliferation, clonogenicity, migration and invasion, and induced
G1 arrest and cell apoptosis in vitro, as well as suppressed
tumor growth in a nude mouse model. These findings suggest that
miR-338-3p may be a potential therapeutic target in ESCC
treatment.
References
|
1
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCann J: Esophageal cancers: changing
character, increasing incidence. J Natl Cancer Inst. 91:497–498.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koshy M, Esiashvilli N, Landry JC, Thomas
CR Jr and Matthews RH: Multiple management modalities in esophageal
cancer: combined modality management approaches. Oncologist.
9:147–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin J, Huang S, Wu S, et al: MicroRNA-423
promotes cell growth and regulates G(1)/S transition by targeting
p21Cip1/Waf1 in hepatocellular carcinoma. Carcinogenesis.
32:1641–1647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jovanovic M and Hengartner MO: miRNAs and
apoptosis: RNAs to die for. Oncogene. 25:6176–6187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stevanato L and Sinden JD: The effects of
microRNAs on human neural stem cell differentiation in two- and
three-dimensional cultures. Stem Cell Res Ther. 5:492009.
View Article : Google Scholar
|
|
9
|
Djuranovic S, Nahvi A and Green R: A
parsimonious model for gene regulation by miRNAs. Science.
331:550–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasinski AL and Slack FJ: Epigenetics and
genetics. MicroRNAs en route to the clinic: progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valastyan S, Reinhardt F, Benaich N, et
al: A pleiotropically acting microRNA, miR-31, inhibits breast
cancer metastasis. Cell. 137:1032–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iorio MV, Visone R, Di Leva G, et al:
MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iorio MV and Croce CM: MicroRNAs in
cancer: small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho WC: OncomiRs: the discovery and
progress of microRNAs in cancers. Mol Cancer. 6:602007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue Q, Sun K, Deng HJ, Lei ST, Dong JQ and
Li GX: MicroRNA-338-3p inhibits colorectal carcinoma cell invasion
and migration by targeting smoothened. Jpn J Clin Oncol. 44:13–21.
2014. View Article : Google Scholar
|
|
17
|
Sun K, Guo C, Deng HJ, Dong JQ, Lei ST and
Li GX: Construction of lentivirus-based inhibitor of
hsamicroRNA-338-3p with specific secondary structure. Acta
Pharmacol Sin. 34:167–175. 2013. View Article : Google Scholar
|
|
18
|
Tsuchiya S, Oku M, Imanaka Y, et al:
MicroRNA-338-3p and microRNA-451 contribute to the formation of
basolateral polarity in epithelial cells. Nucleic Acids Res.
37:3821–3827. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang M, Liu R, Sheng J, et al:
Differential expression profiles of microRNAs as potential
biomarkers for the early diagnosis of esophageal squamous cell
carcinoma. Oncol Rep. 29:169–176. 2013.
|
|
20
|
Chen X, Pan M, Han L, Lu H, Hao X and Dong
Q: miR-338-3p suppresses neuroblastoma proliferation, invasion and
migration through targeting PREX2a. FEBS Lett. 587:3729–3737. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun K, Deng HJ, Lei ST, Dong JQ and Li GX:
miRNA-338-3p suppresses cell growth of human colorectal carcinoma
by targeting smoothened. World J Gastroenterol. 19:2197–2207. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akanuma N, Hoshino I, Akutsu Y, et al:
MicroRNA-133a regulates the mRNAs of two invadopodia-related
proteins, FSCN1 and MMP14, in esophageal cancer. Br J Cancer.
110:189–198. 2014. View Article : Google Scholar :
|
|
25
|
Lee KH, Goan YG, Hsiao M, et al:
MicroRNA-373 (miR-373) post-transcriptionally regulates large tumor
suppressor, homolog 2 (LATS2) and stimulates proliferation in human
esophageal cancer. Exp Cell Res. 315:2529–2538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma WJ, Lv GD, Tuersun A, et al: Role of
microRNA-21 and effect on PTEN in Kazakh's esophageal squamous cell
carcinoma. Mol Biol Rep. 38:3253–3260. 2011. View Article : Google Scholar
|
|
27
|
Zhang M, Zhou S, Zhang L, et al: miR-518b
is downregulated, and involved in cell proliferation and invasion
by targeting Rap1b in esophageal squamous cell carcinoma. FEBS
Lett. 586:3508–3521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Qiao Q, Chen M, et al: miR-625
downregulation promotes proliferation and invasion in esophageal
cancer by targeting Sox2. FEBS Lett. 588:915–921. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Zang W, Du Y, et al: Mir-655
upregulation suppresses cell invasion by targeting pituitary
tumor-transforming gene-1 in esophageal squamous cell carcinoma. J
Transl Med. 11:3012013. View Article : Google Scholar
|
|
30
|
Kos A, Olde Loohuis NF, Wieczorek ML, et
al: A potential regulatory role for intronic microRNA-338-3p for
its host gene encoding apoptosis-associated tyrosine kinase. PLoS
One. 7:e310222012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang XH, Wang Q, Chen JS, et al:
Bead-based microarray analysis of microRNA expression in
hepatocellular carcinoma: miR-338 is downregulated. Hepatol Res.
39:786–794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peng Y, Liu YM, Li LC, Wang LL and Wu XL:
MicroRNA-338 inhibits growth, invasion and metastasis of gastric
cancer by targeting NRP1 expression. PLoS One. 9:e944222014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rowe RG and Weiss SJ: Navigating ECM
barriers at the invasive front: the cancer cell-stroma interface.
Annu Rev Cell Dev Biol. 25:567–595. 2009. View Article : Google Scholar
|
|
34
|
Buchheit CL, Weigel KJ and Schafer ZT:
Cancer cell survival during detachment from the ECM: multiple
barriers to tumour progression. Nat Rev Cancer. 14:632–641. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
MacDougall JR and Matrisian LM:
Contributions of tumor and stromal matrix metalloproteinases to
tumor progression, invasion and metastasis. Cancer Metastasis Rev.
14:351–362. 1995. View Article : Google Scholar : PubMed/NCBI
|