Introduction
Glaucoma is the leading cause of blindness worldwide
and is one of the most common neurodegenerative diseases, which is
characterized by the irreversible and progressive loss of retinal
ganglion cells (RGCs) and damage to the optic nerve, usually in
response to abnormally increased intraocular pressure (1–4).
Müller cells are the principal glia of the retina,
and the predominant function of Müller cells is to regulate
extracellular glutamate levels (5). Glutamate, a normal constituent of the
retina, is taken up by Müller cells and is converted to glutamine,
which is taken up the neurons. The neurons use glutamine to
synthesize glutamate for neurotransmission (5). Müller cells are involved in glutamate
metabolism via the glutamate aspartate transporter (GLAST) and
glutamine synthetase (GS). The GLAST is responsible for the
transport of glutamate into Müller cells and GS is the enzyme,
which converts glutamate into glutamine inside the Müller cells
(6). Increased levels of
extracellular glutamate have been reported in a primate model of
glaucoma and in human patients with glaucoma (7). This increase in extracellular
glutamate levels is predominantly due to the downregulation of
GLAST (8). Excess glutamate
release is involved in glaucomatous neuropathy, which causes
excitotoxic damage to the RGCs through the activation of ionotropic
and metabotropic glutamate receptors (9,10).
Consequently, the efficient removal of glutamate from the
extracellular space is required for the maintenance of a healthy
retina.
Adenosine is a ubiquitous local modulator, which
regulates various physiological and pathological functions by
stimulating membrane receptors. Biochemical, pharmacological, and
molecular investigations have identified four adenosine receptor
subtypes, A1, A2A, A2B and
A3 (11). There is
increasing evidence that adenosine is an important intracellular
mediator in the retina and has considerable potential to protect
retinal neurons (12–14). Previously, an A2A
receptor (A2AR) antagonist has been suggested as an
attractive option to improve the treatment of neurological
disorders, including Parkinson's disease, Huntington's disease and
Alzheimer's disease (15,16). The function of the A2AR
antagonist may be to inhibit the release of glutamate and prevent
damage of the neuron (17). The
aim of the present study was to investigate whether the
A2AR antagonist, SCH442416, modulates the expression
levels of GS and GLAST, and the uptake of glutamate in retinal
Müller cells exposed to increased hydrostatic pressure.
Materials and methods
Pressure device
The pressure device used in the present study was
described in detail in our previous study (18). Briefly, a T75 culture flask
(Shanghai Jun Sheng Biological Technology Co., Ltd., Shanghai,
China) was equipped with a manometer (Fang Jun Instrument Co.,
Ltd., Shanghai, China) and placed in an incubator, maintained at
37°C, as the pressure device. An air mixture of 95% air and 5%
CO2 was pumped into the flasks to obtain pressure. The
pressure level of the model was 40 mmHg, as in our previous
investigation (18), which was
adjusted every 4 h. The total duration of the induced pressure was
24 h. In the experiments, several precautions were made to limit
artifacts from the experimental method. Laboratory film (Pechiney,
Stamford, CT, USA) was used to seal the interfaces and, to avoid
artifacts caused by 'on-off' changes in pressure, all operations
involving the refreshment of medium or adjustment of pressure were
performed within a 5 min period.
Müller cell culture
All investigations involving animals in the present
study were performed in strict accordance with the Association for
Research in Vision and Ophthalmology Statement for the Use of
Animals in Ophthalmic and Vision Research (19). The present study was approved by
the Ethics Committee of Ruijin Hospital, Shanghai Jiaotong
University (Shanghai, China). The primary culture of retinal Müller
cells was generated, as previously described (18). Briefly, the retinas of 80 newborn
(2–5 days old, male and females) Sprague-Dawley rats, obtained from
Shanghai Slack Laboratory Animal Co., Ltd. (Shanghai, China) were
collected following sacrifice by intraperitoneal of 30% chloral
hydrate (500 mg/kg; Chemical Reagent Co., Ltd., Shanghai, China)..
For each experiment, the retinas (n=20) were dissected and stored
on ice in D-Hank's solution (Anresco LLC, Solon, OH, USA). The
tissue was dissociated by centrifugation at room temperature for 5
min at 600 × g and was incubated for 15 min at 37°C in
phosphate-buffered saline (PBS), containing 0.125% trypsin (Anresco
LLC). Finally, the cell suspension was cultured in T75 culture
flasks at 37°C in humidified air containing 5% CO2.
Following the initial outgrowth, the cell culture medium was
replaced every 48 h and maintained in Dulbecco's modified Eagle's
medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA),
supplemented with 2 mM glutamine, 100 U/ml penicillin, 100
µg/ml streptomycin and 10% fetal bovine serum (Sijiqing,
Zhejiang, China).
Following culture for 5–8 days, the flasks were
agitated at 37°C for 1 h at 100 rpm and the cell culture medium was
refreshed. By agitating the plates, other types of cell, including
microgilal cells and RGCs, which were initially adhered to the
surface of the Müller cells, were rinsed off with DMEM to obtain a
purified cell population. For passage, the cell cultures were
incubated at 37°C with PBS, containing 0.125% trypsin. The Müller
cells were identified via GS and glial fibrillary acidic protein
(GFAP) staining using indirect immunofluorescence. The cells were
fixed with 4% paraformaldehyde at room temperature for 10 min and
were incubated with 0.3% Triton X-100 at 37°C for 10 min. The cells
were washed three times (10 min/wash) with PBS, blocked with 10%
goat serum in PBS and subsequently incubated with the rabbit
anti-rat polyclonal antibody against GS (1:5,000; ab49873; Abcam,
Cambridge, MA, USA) and the mouse anti-rat monoclonal antibody
against GFAP (1:200; ab4648, Abcam) as an identity marker for
Müller cells. The cells were then incubated overnight at 4°C. The
following day, the cells were incubated with the secondary donkey
anti-rabbit IgG-Cy3 polyclonal antibody (1:200; 406402; BioLegend,
Inc., San Diego, CA, USA) at 37°C in darkness for 1 h. Following
three washes with PBS, the cells on the coverslips were mounted on
glass slides with Histomount (Invitrogen Life Technologies,
Carlsbad, CA, USA). The cells were viewed under an Axio micro scope
(Zeiss, Oberkochen, Germany), and images were acquired with a
digital camera (Canon, Tokyo, Japan).
Drug treatment
The A2A receptor antagonist,
2-(2-Furanyl)-7-[3-(4-methoxyphernyl)propyl]-7H-pyrazolo[4,3-e]
(1,2,4)
triazolo[1,5-c]pyrimidin-5-amine (SCH442416), was purchased from
Tocris Bioscience (Ellisville,. MO, USA). The experiments were
performed following the second passage, when cell confluence was
80-90%. The cells were cultured in serum-free medium and divided
into the following three groups: Normal culture group; 40 mmHg
pressure culture group; 40 mmHg pressure + 100 nM SCH442416 culture
group. The Müller cells in the three groups were continually
cultured at 37°C for another 24 h. The concentration of SCH442416
used in the present study was selected, according to preliminary
experiments (Data not shown).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
The cells were collected and used for total RNA
preparations. The total RNA was reverse-transcribed into cDNA using
a previously described method (20) and the Invitrogen Reverse
Transcription kit (Invitrogen Life Technologies). The PCR solution
contained 2 µl cDNA, specific primers (1 µM each) and
10 µl QuantiTect SYBR Green PCR kit reagent (Qiagen, Hilden,
Germany) in a final volume of 20 µl. The following primer
pairs from Sangon Biotech Co., Ltd. (Shanghai, China) were used:
GS, sense 5′-CCGCTCTTCGTCTCGTTC-3′ and anti-sense
5′-CTGCCTGATGCCTTTGTT-3′; GLAST, sense 5′-CCTATGTGGCAGTCGTTT-3′ and
anti-sense 5′-CTGTGATGGGCTGGCTAA-3′; and β-actin, sense
5′-GCGCTCGTCGTCGACAACGG-3′ and anti-sense
5′-GTGTGGTGCCAAATCTTCTCC-3′. The PCR parameters were as follows:
Initial denaturation at 94°C for 5 min; amplification and
quantification, 40 cycles at 94°C for 30 sec, 55°C for 30 sec, and
72°C for 30 sec; melting curve, 55°C with the temperature gradually
increased up to 95°C. The mRNA expression levels were normalized
against the levels of β-actin, as described previously (20).
Western blot analysis
The cultured cells in the samples from the different
groups were washed twice with PBS. The total protein was extracted
with the EpiQuik Whole Cell Extraction kit (Epigentek, Farmingdale,
NY, USA) according the manufacturer's instructions. Protein
concentration was determined by the radioimmunoprecipitation buffer
assay (Cell Signaling Technology, Inc., Danvers, MA, USA) and lysed
in 2X Laemmli buffer (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The protein extracts (40 µg) were boiled for 10 min
and centrifuged at 14,000 × g. The proteins were separated on 12%
SDS-PAGE gels (Sigma-Aldrich, St. Louis, MO, USA) and were
transferred onto polyvinylidine fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were soaked in Tris-buffered
saline (Sigma-Aldrich), containing 20 mmol/l Tris-Cl, 140 mmol/l
NaCl (pH 7.5), with 5% non-fat milk and 0.1% Tween-20
(Sigma-Aldrich) for 1 h at room temperature. The membranes were
incubated with primary rabbit anti-rat polyclonal antibodies
against GS (ab49873; 1:10,000; Abcam) and GLAST (ab416; 1:200;
Abcam) overnight at 4°C. Rabbit anti-rat polyclonal anti-GAPDH
antibody (ab37168; 1:10,000; Abcam) was used as a reference to
normalize the intensities of the immunoreactions with different
antibodies. Following several washes with PBS, the membranes were
incubated with horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G (A20019; 1:2,000; Invitrogen Life Technologies)
for 1 h at room temperature and visualized using enhanced
chemofluorescence reagent (Beyotime Institute of Biotechnology,
Haimen, China). Images were captured using ImageQuant Las 4000 mini
(GE Healthcare Life Sciences, Kochi, Japan) and the protein bands
were quantitatively analyzed using ImagePro Plus image analysis
software v.7.0 (Zeiss).
Glutamate uptake assay
The cultured Müller cells in the treatment groups
were washed in PBS and pre-incubated in Kreb's solution
(Sigma-Aldrich), containing 119 mM NaCl, 4.7 mM KCl, 1.2 mM
KH2PO4, 25 mM NaHCO3, 2.5 mM
CaCl2 and 1 mM MgCl2, for 30 min at 37°C. The
Müller cells were then exposed to 0.5 µCi/ml L-[2,3-3H]
glutamate (New England Nuclear, Boston, MA, USA) and 10 mmol/l
unlabeled glutamate for 60 min at 37°C. The reaction was terminated
by washing the cells three times with ice-cold PBS. The Müller
cells were subsequently lysed in PBS and small aliquots (20
µl) were removed from each well for the determination of
protein content. The L-[2,3-3H] glutamate content of the lysates
were determined by scintillation counting (Triathler Scintillator;
Beijing Huaruison Science and Technology Development Co., Ltd.,
Beijing, China). All experiments were performed in triplicate for
each of the four separate cell preparations.
Statistical analysis
The data are expressed as the mean ± standard
deviation. All analyses were performed using SPSS 19.0 statistical
software (IBM SPSS, Chicago, IL, USA). The data were analyzed using
one-way analysis of variance, followed by a least significant
difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
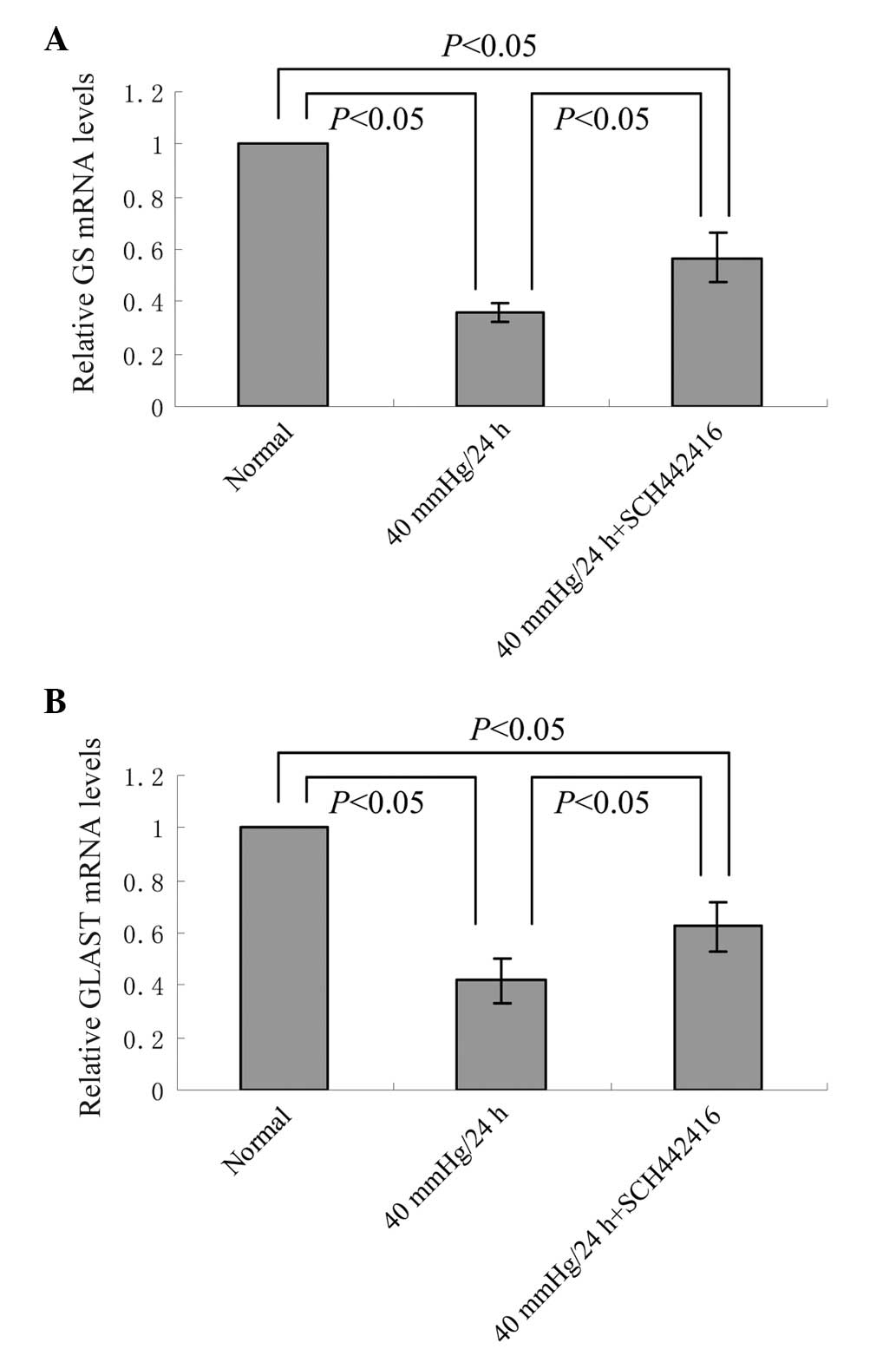
Effect of SCH442416 on the mRNA
expression levels of GS and GLAST in the cultured retinal Müller
cells under pressure conditions
The mRNA expression levels of GS and GLAST of the
retinal Müller cells incubated in serum-free medium, in the
presence or absence of SCH442416, under 40 mmHg pressure for 24 h
was analyzed using RT-qPCR. Compared with the normal culture group,
the mRNA expression levels of GS and GLAST were significantly
decreased in the Müller cells cultured with or without SCH442416
under 40 mmHg pressure conditions (P<0.05; Fig. 1). However, the mRNA expres sion
levels of GS and GLAST in the 40 mmHg pressure + 100 nM SCH442416
culture group were significantly higher, compared with those in the
40 mmHg pressure culture group (P<0.05; Fig. 1).
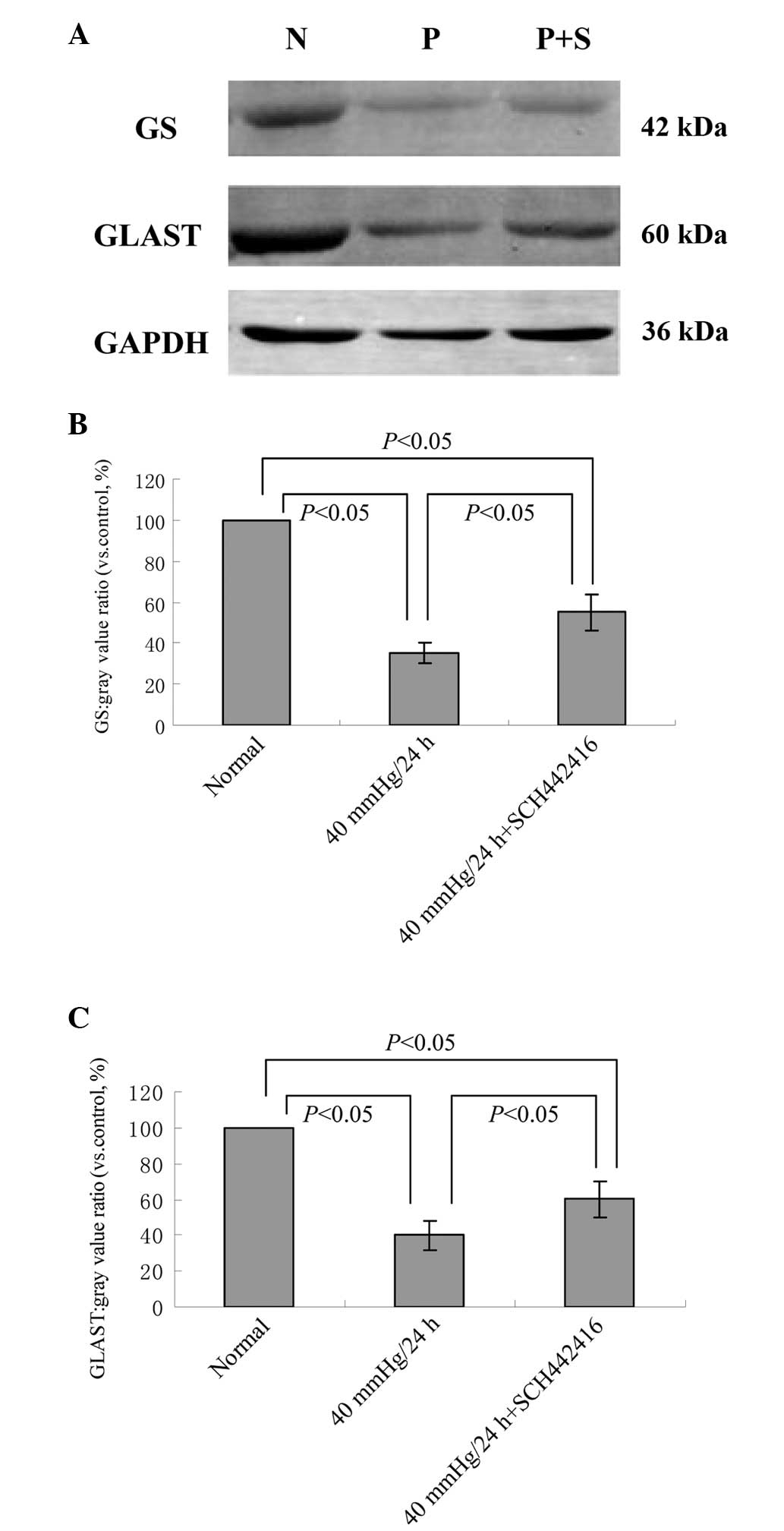
Effect of SCH442416 on the protein
expression levels of GS and GLAST in the cultured retinal Müller
cells under pressure conditions
The protein expression levels of GS and GLAST in
retinal Müller cells were compared between the normal control group
and the groups under 40 mmHg pressure for 24 h, in the presence or
absence of SCH442416. Western blotting revealed that the expression
levels of GS and GLAST were significantly decreased in the Müller
cells cultured with or without SCH442416 under 40 mmHg pressure,
compared with the normal culture (P<0.05; Fig. 2). However, the protein expression
levels of GS and GLAST in the 40 mmHg pressure + 100 nM SCH442416
group were significantly higher, compared with the 40 mmHg pressure
group (P<0.05; Fig. 2).
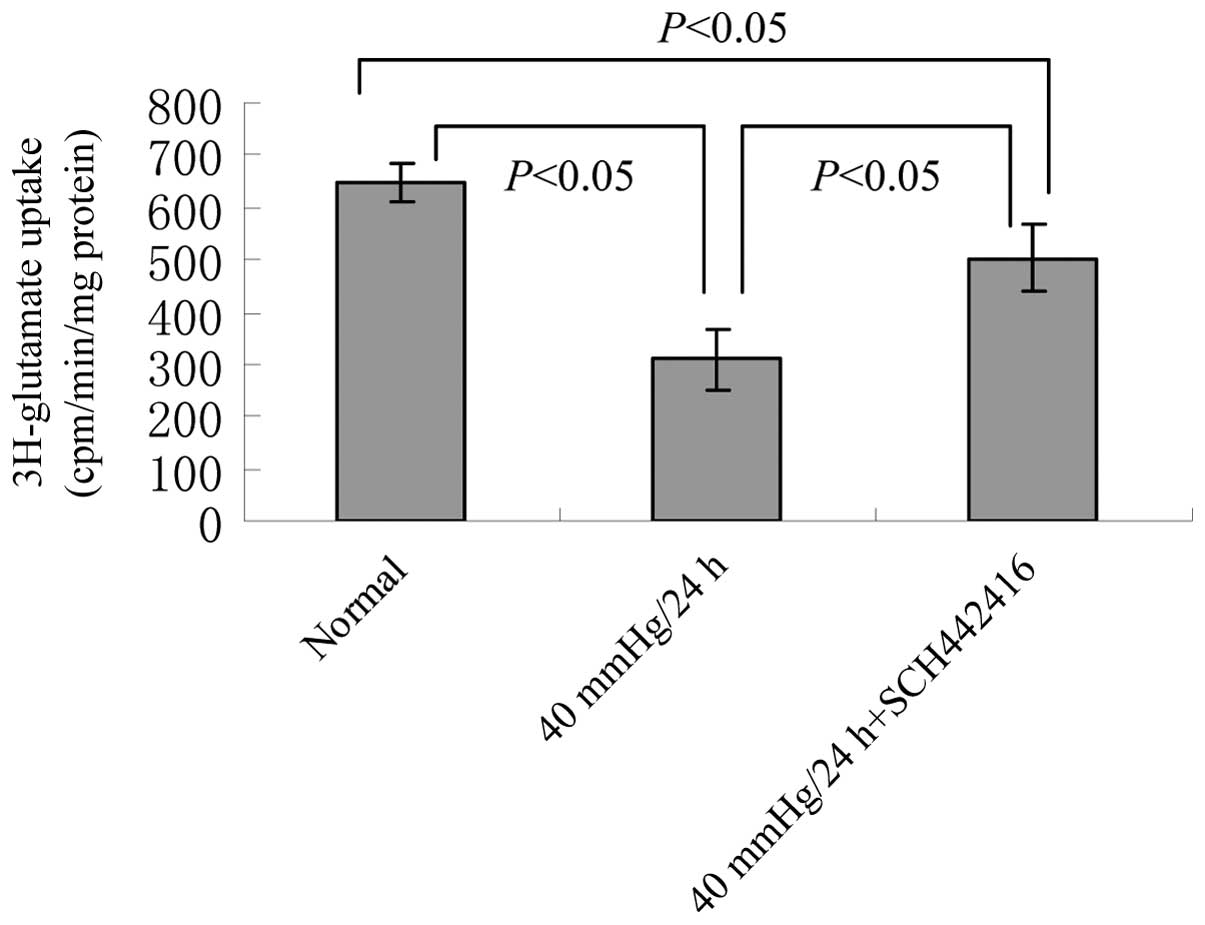
Effect of SCH442416 on glutamate uptake
activity in the cultured retinal Müller cells under pressure
conditions
A glutamate uptake assay was performed using a
scintillation counting method to determine the 3H-glutamate content
in the lysates. Compared with the normal culture group, the
glutamate uptake activity was significantly decreased in the Müller
cells cultured with or without SCH442416 under 40 mmHg pressure
(P<0.05; Fig. 3). However, the
glutamate uptake activity in the 40 mmHg pressure + 100 nM
SCH442416 culture group was significantly higher, compared with
that in the 40 mmHg pressure culture group (P<0.05; Fig. 3).
Discussion
The present study used a novel pressure model, which
involved the culture of retinal Müller cells under hydrostatic
pressure. The hydrostatic pressure used in this model was adjusted
to 40 mmHg, a moderately elevated pressure, which often occurs in
chronic glaucoma models (21). In
the present study, several precautions and design considerations
were made to limit artifacts from the experimental procedure.
Laboratory film was used to seal interfaces and, to avoid artifacts
from 'on-off' changes in pressure, replacements of media or
adjustments of pressure were completed without delay. Our previous
study also revealed that this pressure model was effective
(18).
Glutamate acts as a neurotransmitter in the normal
retina. However, excessive stimulation of glutamate receptors can
result in excitotoxicity (22).
Intraocular glutamate can cause severe degeneration of the inner
retinal layers, particularly the RGC layer (23). These findings support the
hypothesis that increased extracellular glutamate concentration or
decreased glutamate clearance results in excitotoxic damage and may
contribute to the pathogenesis of glaucoma (24–26).
Müller cells maintain an close association with retinal neurons and
are important in regulating extracellular glutamate levels.
Glutamate is transported into the Müller cells via GLAST and is
catalyzed by GS to the non-toxic amino acid, glutamine. Glutamate
transport is the only mechanism for removing glutamate from the
extracellular fluid (27). It las
been suggested that functional impairment of glutamate transporters
may be involved in excitotoxicity and contribute to the
pathogenesis of glaucoma (28,29).
The present study indicated that Müller cells treated with 40 mmHg
pressure decreased the expression levels of GS and GLAST, and
reduced the L-[2,3–3H] glutamate uptake activity, which was
consistent with the results of previous studies (30,31).
A2AR is expressed in the inner nuclear
layer, RGC layer and, less prominently, in the outer nuclear layer
(32–34). Previous studies have demonstrated
that A2AR antagonists can enhance the recovery of
retinal function following ischemia attack (35,36).
The present study demonstrated that the A2AR antagonist,
SCH442416, increased the expression levels of GS and GLAST, and
increased the L-[2,3–3H] glutamate uptake activity in Müller cells
subjected to 40 mmHg pressure. This suggested that the
A2AR antagonist may protect RGCs by accelerating the
clearance of extracellular glutamate in retina.
Collectively, the data of the present study
suggested that Müller cells treated with 40 mmHg pressure decreased
the expression levels of GS and GLAST, and reduced glutamate uptake
activity. By contrast, SCH442416 increased the expression levels of
GS and GLAST, and increased glutamate uptake activity in the Müller
cells under pressure, therefore, the SCH442416 A2AR
antagonist may be a potential candidate as a neuroprotective agent
for the treatment of glaucoma by accelerating the clearance of
extracellular glutamate. Further investigations are required to
confirm these effects in animal experiments.
Acknowledgments
This study was funded by the National Natural
Science Foundation of China (grant no. 81371014) and the Shanghai
'Science and Technology Innovation Actio§n Plan' Basic Research Key
Project (grant nos. 11JC1407700 and 11JC1407701).
References
|
1
|
Foster PJ, Buhrmann R, Quigley HA and
Johnson GJ: The definition and classification of glaucoma in
prevalence surveys. Br J Ophthalmol. 86:238–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kwon YH, Fingert JH, Kuehn MH and Alward
WL: Primary open angle glaucoma. N Engl J Med. 360:1113–1124. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quigley HA: Glaucoma. Lancet.
377:1367–1377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Som mer A: Intraocula r pressure and
glaucoma. Am J Ophthalmol. 107:186–188. 1989. View Article : Google Scholar
|
|
5
|
Newman E and Reichenbach A: The Müller
cell: A functional element of the retina. Trends Neurosci.
19:307–312. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pow DV and Crook DK: Direct
immunocytochemical evidence for the transfer of glutamine from
glial cells to neurons: Use of specific antibodies directed against
the D-steroisomers of glutamate and glutamine. Neuroscience.
70:295–302. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dreyer EB: A proposed role for
excitotoxicity in glaucoma. J Glaucoma. 7:62–67. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naskar R, Vorwerk CK and Dreyer EB:
Concurrent downregulation of a glutamate transporter and receptor
in glaucoma. Invest Ophthamol Vis Sic. 41:1940–1944. 2000.
|
|
9
|
Zhong YS, Leung CK and Pang CP: Glial
cells and glaucomatous neuropathy. Chin Med J. 120:326–335.
2007.PubMed/NCBI
|
|
10
|
Kawasaki A, Otori Y and Barnstable CJ:
Müller cell pretection of rat retinal ganglion cells from glutamate
and nitric oxide neurotoxicity. Invest Ophthamol Vis Sic.
41:3444–3450. 2000.
|
|
11
|
Fredholm BB, IJzerman AP, Jacobson KA,
Klotz KN and Linden J: Nomenclature and classification of adenosine
receptors. Pharmacol Rev. 53:527–552. 2001.PubMed/NCBI
|
|
12
|
Ghiardi GJ, Gidday JM and Roth S: The
purine nucleoside adenosine in retinal ischemia-reperfusion injury.
Vision Res. 39:2519–2535. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Larsen AK and Osborne NN: Involvement of
adenosine in retinal ischemia. Studies on the rat. Invest
Ophthalmol Vis Sci. 37:2603–2611. 1996.PubMed/NCBI
|
|
14
|
Li B and Roth S: Retinal ischemic
preconditioning in the rat: Requirement for adenosine and
repetitive induction. Invest Ophthalmol Vis Sci. 40:1200–1216.
1999.PubMed/NCBI
|
|
15
|
Wang Z, Che PL, Du J, Ha B and Yarema KJ:
Static magnetic field exposure reproduces cellular effects of the
Parkinson's disease drug candidate ZM241385. PLoS One.
5:e138832010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tarazi FI, Sahli ZT, Wolny M and Mousa SA:
Emerging therapies for Parkinson's disease: From bench to bedside.
Pharmacol Ther. 144:123–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pepponi R, Ferrante A, Ferretti R, et al:
Region-specific neuroprotective effect of ZM 241385 towards
glutamate uptake inhibition in cultured neurons. Eur J Pharmacol.
617:28–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu J, Zhong Y, Cheng Y, et al: Effect of
high hydrostatic pressure on the expression of glutamine synthetase
in rat retinal Müller cells cultured in vitro. Exp Ther Med.
2:513–516. 2011.PubMed/NCBI
|
|
19
|
Statement for the Use of Animals in
Ophthalmic and Visual Research. The Association for Research in
Vision and Ophthalmology; Rockville: 2015
|
|
20
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldblum D and Mittag T: Prospects for
relevant glaucoma models with retinal GC damage in the rodent eye.
Vision Res. 42:471–478. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vizi ES, Kisfali M and Lőrincz T: Role of
nonsynaptic GluN2B-containing NMDA receptors in excitotoxicity:
evidence that fluoxetine selectively inhibits these receptors and
may have neuroprotective effects. Brain Res Bull. 93:32–38. 2013.
View Article : Google Scholar
|
|
23
|
Siliprandi R, Canella R, Carmignoto G,
Schiavo N, Zanellato A, Zanoni R and Vantini G:
N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina.
Vis Neurosci. 8:567–573. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dreyer EB, Zurakowski D, Schumer RA, Podos
SM and Lipton SA: Elevated glutamate in the vitreous body of humans
and monkeys with glaucoma. Arch Ophthalmol. 114:299–305. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dreyer EB and Grosskreutz CL: Excitatory
mechanisms in retinal ganglion cell death in primary open angle
glaucoma (POAG). Clin Neurosci. 4:270–273. 1997.PubMed/NCBI
|
|
26
|
Vorwerk CK, Gorla MS and Dreyer EB: An
experimental basis for implicating excitotoxicity in glaucomatous
optic neuropathy. Surv Ophthalmol. 43(Suppl 1): S142–S150. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Danbolt NC: Glutamate uptake. Prog
Neurobiol. 65:1–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Naskar R, Vorwerk CK and Dreyer EB:
Concurrent downregulation of a glutamate transporter and receptor
in glaucoma. Invest Ophthalmol Vis Sci. 41:1940–1944.
2000.PubMed/NCBI
|
|
29
|
Martin KR, Levkovitch-Verbin H, Valenta D,
Baumrind L, Pease ME and Quigley HA: Retinal glutamate transporter
changes in experimental glaucoma and after optic nerve transection
in the rat. Invest Ophthalmol Vis Sci. 43:2236–2243.
2002.PubMed/NCBI
|
|
30
|
Ishikawa M, Yoshitomi T, Zorumski CF and
Izumi Y: Effects of acutely elevated hydrostatic pressure in the
rat ex vivo retinal preparation. Invest Ophthalmol Vis Sci.
51:6414–6423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishikawa M, Yoshitomi T, Zorumski CF and
Izumi Y: Downregulation of glutamine synthetase via GLAST
suppression induces retinal axonal swelling in a rat ex vivo
hydrostatic pressure model. Invest Ophthalmol Vis Sci.
52:6604–6616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kvanta A, Seregard S, Sejersen S, Kull B
and Fredholm BB: Localization of adenosine receptor messenger RNAs
in the rat eye. Exp Eye Res. 65:595–602. 1997. View Article : Google Scholar
|
|
33
|
Blazynsk C: Discrete distributions of
adenosine receptors in mammalian retina. J Neurochem. 54:648–655.
1990. View Article : Google Scholar
|
|
34
|
Crooke A, Guzmán-Aranguez A, Peral A,
Abdurrahman MK and Pintor J: Nucleotides in ocular secretions:
Their role in ocular physiology. Pharmacol Ther. 119:55–73. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li B, Rosenbaum PS, Jennings NM, Maxwell
KM and Roth S: Differing roles of adenosine receptor subtypes in
retinal ischemia-reperfusion injury in the rat. Exp Eye Res.
68:9–17. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhong Y, Yang Z, Huang WC and Luo X:
Adenosine, adenosine receptors and glaucoma: An updated overview.
Biochim Biophys Acta. 1830:2882–2890. 2013. View Article : Google Scholar : PubMed/NCBI
|