1. Ultrasound imaging and therapeutic
applications of UCA microbubbles as well as bioeffects
Ultrasound (US) imaging has been an important option
for diagnosis and the evaluation of the efficacy of particular
therapies in a variety of diseases. It is a safe, low-cost and
portable mode of imaging that provides real-time information for
clinicians and researchers. The wide-spread use of US contrast
agents (UCAs) has improved the resolution and sensitivity of US
images. UCAs may augment the acoustic impedance (Z) between lesions
and surrounding tissues by producing highly efficient scattering of
waves (1). Furthermore, when
treated with high acoustic energies, UCA microbubbles (MBs) undergo
non-linear oscillations leading to the emission of harmonics as a
source of sound, rather than simply using passive reflection.
Biological tissue does not display this degree of harmonic
generation, thus the contrast generated signal is able to improve
the signal-to-noise ratio, which can be exploited to produce images
of superior quality (2). UCAs in
current use are 2–10 µm in diameter and are stable MBs, composed of
inert gases, such as perfluorocarbon, stabilized by a coating of
biodegradable material, such as albumin or phospholipids (3).
Diagnostic applications of UCAs
Recently, a study into three varieties of UCAs
stabilized by phospholipid that are used in clinical practice,
Definity, Sonovue and MicroMarker, demonstrated that the acoustic
attenuation and contrast-to-tissue ratio of these molecules are
comparable at frequencies <30 MHz at the same concentration,
although their particle size distributions, encapsulated gases and
shells differ. However, at frequencies >30 MHz, MicroMarker has
higher attenuation values and contrast-to-tissue ratios than either
Definity or Sonovue. Furthermore, decantation was found to be an
effective method by which to alter the size distribution and
concentration of native clinical microbubble populations, enabling
further contrast enhancement for specific pre-clinical applications
(4).
Sonovue is the most commonly used form of lipid UCA.
It is approved by the food and drug administration and is nontoxic
and harmless in humans. In addition, it produced high quality
contrast imaging in a number of tissues and organs, such as the
liver, kidney, thyroid, breast, heart and blood vessels (5). It is particularly valuable in the
diagnosis and evaluation of tumors, the assessment of myocardial
motion and the evaluation of lung consolidation. Recently, Liu
et al (6) showed that the
peripheral enhancement pattern of breast cancer on
contrast-enhanced US may be valuable in the evaluation of
peripheral and central tumor angiogenesis, and of vascular
endothelial growth factor (VEGF) expression. Likewise, Sonovue
contrast doppler US gave a greater definition of the margins of
lesions in cervical cancer as the UCA increased the sensitivity of
detection of parametrial invasion and lymph node metastases
(7). US contrast enhancement
imaging has also been shown to improve the detection of regional
wall motion abnormalities in 2-dimensional and 3-dimensional
echocardiography (8). Sartori
et al (9) evaluated the
diagnostic accuracy of contrast-enhanced ultrasonography in
differentiating between neoplastic and non-neoplastic peripheral
pleuro-pulmonary lesions, observing that 40/42 non-neoplastic
lesions exhibited absence of enhancement, compared with 3/53
neoplastic lesions. Other researchers have conducted real-time US
contrast imaging at varying doses of contrast agent and have
demonstrated kinetic features that are indicative of invasive
breast tumors, with an injection of Sonovue of either 2.4 ml or 4.8
ml (10).
Recently, a novel tool used to monitor Sonovue
micro-bubble signals that also automatically measures contrast flow
intensity in atherosclerotic carotid plaque neovascularization has
been developed by Lisowska et al (11). The results showed that patients
with preserved contrast flow through the plaque were more likely to
have a history of cerebral stroke. Massive calcification of
atherosclerotic plaques correlated with a history of myocardial
infarction and the degree of coronary artery disease, but not with
a previous cerebral stroke.
Targeted imaging and therapy with
MBs
Targeted imaging of tissue-specific MBs has been
explored for a number of years. These were conjugated with ligands
for a variety of vascular biomarkers, including integrins that are
expressed during angiogenesis. However, it has been shown that
targeted ligands, which have a solid structure tethered to the MB
surface, are able to limit the MB oscillation and reduce their
harmonic signals, thus weakening US contrast imaging (12).
Therapeutic applications using MBs as vehicles for
drug or gene delivery to tissues have been investigated, as well as
the use of US for sonoporation, which may facilitate the entrance
of drug-loaded MBs into cells. Drugs may be attached to the
external surface of thin lipid monolayer bubbles by covalent or
noncovalent bonds, or incorporated into liposomes that are then
associated with the bubble surfaces (2).
Previous studies have also demonstrated that UCA MBs
were destroyed during needle injection using a variety of syringe
and needle combinations. The majority of protein-shelled MBs were
destroyed above a critical pressure drop of 109 ± 7 kPa.
Lipid-shelled MBs were found to have a pressure drop threshold
above which >50% were destroyed.
The commercial lipid-shelled agent Definity was
found to have a critical pressure drop of 230 ± 10 kPa. Therefore,
it may be that the use of a formula is able to preclude the
unnecessary destruction of microbubble contrast agent during in
vivo injections. This approach may also prevent the undesirable
release of drug or gene payloads in targeted MBs therapies
(13).
A higher local tissue concentration of drugs or
genes may be obtained by releasing these agents from bubbles by US
or US-targeted microbubble destruction (UTMD) in the immediate
proximity to permeabilized cells and tissues. This approach may
thus augment the effect of the drug or the efficacy of the gene
transfection. Chen et al (14) demonstrated that a UTMD-based short
hairpin RNA delivery system effectively induces apoptosis and
inhibits proliferation of cervical cancer cells and may thus be a
promising option with which to treat this disease. Li et al
(15) demonstrated that the
efficiency of non-augmented transfer of rAAV2 into renal carcinoma
cells was low (17.28 ± 2.44%); however, the use of UTMD enhanced
viral transfer efficiency by 2–3-fold, and enhanced viral genomic
DNA > 9 fold, without decreasing cell viability. Liu et
al (16) investigated the
effects of this treatment in ovarian cancer cells. They
demonstrated that PTX-loaded and LHRHa-targeted MBs (TPLMBs) in
combination with US (300 kHz, 0.5 W/cm2, 30 sec) led to
apoptosis in 41.30±3.93, 67.76±2.45 and 75.93±2.81% of cells at 24,
48 and 72 h following this treatment, respectively. UTMD
predominantly promoted the effect of targeted and PTX-loaded MB
chemotherapy in ovarian cancer cells. Pu et al (17) showed that UTMD may be developed as
a tool with which to facilitate the delivery of LHRH
receptor-targeted and paclitaxel-loaded lipid MB chemotherapeutics
in the treatment of intraperitoneal ovarian cancer xenografts. Yan
et al (18) found that
UTMD-mediated delivery of the Timp3 gene significantly increased
Timp3 protein levels in the infarct scar and border zone of an area
of myocardial ischemia at three days following the administration
of UTMD compared with delivery by the non-conjugated cationic
microbubble. Deng et al (19) showed that UTMD significantly
increased the cytoplasmic intake of pDNA and also maintained high
cell viability. The nuclear import and gene expression of
phstromal-derived growth factor (phSDF)-1α-NFκB-transfected cells
were significantly higher than those transfected with phSDF-1α.
Compared with the NFκB-free plasmids, the quantity of NFκB plasmids
in the nucleus increased 6.5-fold and the expression of SDF-1α was
4.4-fold greater. Ling et al (20) demonstrated that SDF-1 and VEGF
expression in the 1.5 W/cm2and 1 W/cm2 groups
was significantly increased compared with the 0.5W/cm2
or the control groups (3.8 to 4.7-fold; P<0.01). In addition,
the expression of interleukin-1β (IL-1β) in the 1.5
W/cm2 group was increased two fold compared with that in
the 1.0 W/cm2 group, whereas no significant change was
observed in the 0.5 W/cm2 group. Coronary angiography
and 99mTc-tetrofosmin scin-tigraphy revealed that myocardial
perfusion was markedly improved following treatment with UTMD +
mesenchymal stem cells (MSCs). The therapeutic effects were
markedly enhanced by MSC transplantation following the myocardial
microenvironmental changes induced by administration of UTMD. A
study also reported that UTMD effectively trans-fected the human
growth factor gene into target tissues and had a significant effect
on the recovery of injured facial nerves (21). Yang et al (22) found that cationic liposomes were
conjugated with MBs using a biotin-avidin system. Plasmids carrying
the most effective artificial miRNA sequences were delivered to the
livers of rats with hepatic fibrosis using a US-targeted cationic
liposome-bearing microbubble destruction gene delivery system. The
results showed that this method effectively transported the
plasmids to the rat liver. The artificial miRNA was shown to reduce
the pathological changes associated with hepatic fibrosis as well
as the protein and mRNA expression of CTGF and transforming growth
factor-β1.
There are a number of mechanisms by which to
increase cell membrane permeability that are in current use. These
include using micro-circumflex and micro-fluid to punch transient
holes in the surfaces of cell membranes (23–25),
increasing the level of oxyradicals in cells (26) and promoting endocytosis (27) and altering the liquidity of the
membrane phospholipid bilayer using US or UTMD (28). However, these mechanisms remain to
be fully elucidated. Jin et al (29) suggested a further mechanism,
hypothesizing that the initial stimulation of cellular endocytosis
by UTMD may be mediated by grid proteins, due to the consistent
peaks of endocytosis with UTMD, and those of clathrin-dependent
endocytosis.
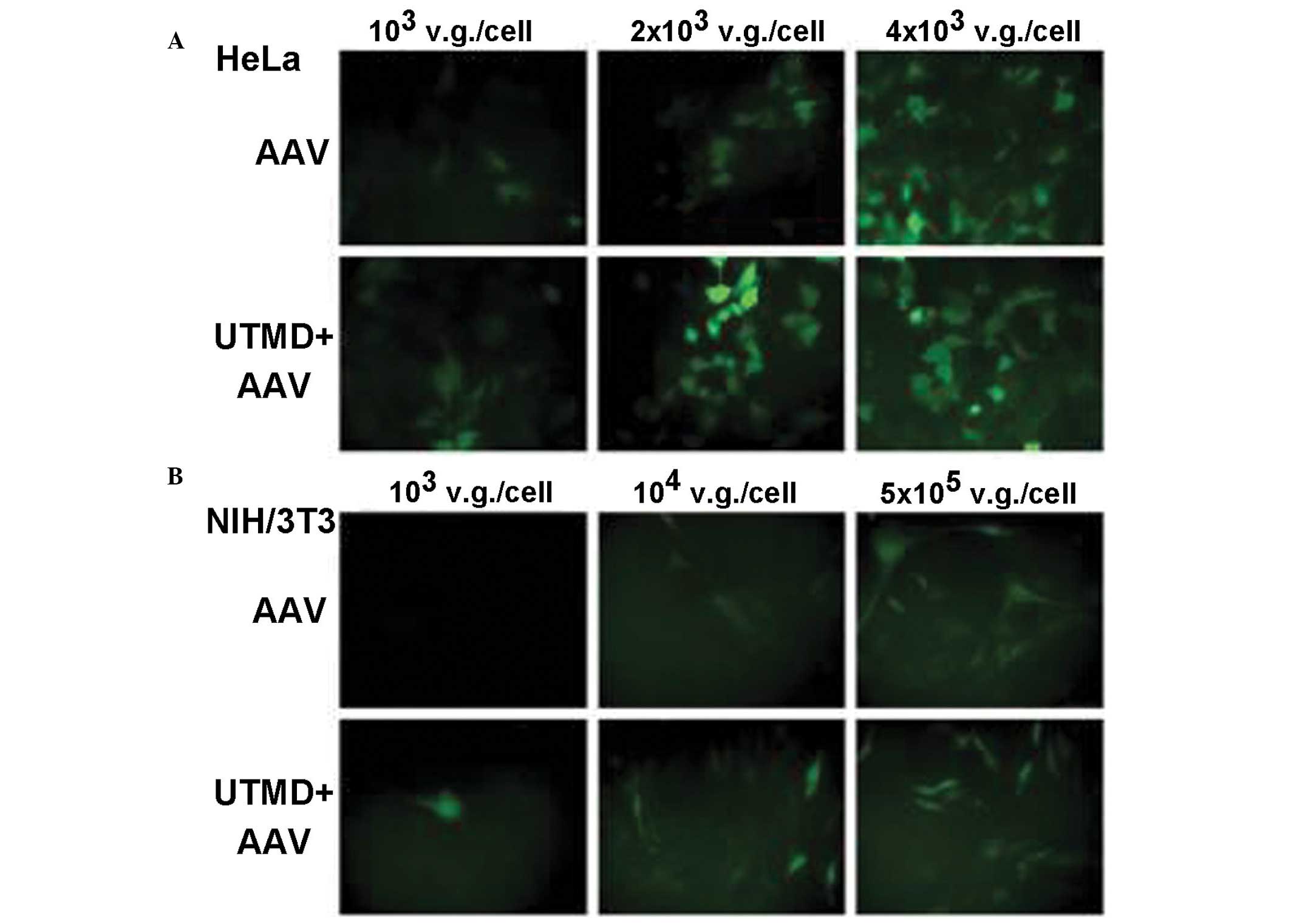
Du et al also conducted trials examining the
safety of UTMD. This group attempted to identify the optimal US or
UTMD settings for use in different types of tumor cells and those
normal cells and tissues may be well incubated under the action of
such optimal conditions (Fig. 1,
taken from reference 29) (15,30–36).
Zhang et al (37)
demonstrated that the use of the noninvasive UTMD technique
improved the localization of MSCs to the kidneys and promoted renal
repair in rats with diabetic nepro-pathy. Other studies have been
conducted in human participants into the safety of UTMD. For
example, Li et al (38)
found that UTMD specifically and reversibly enhanced interstitial
permeability whilst having no effect on the glomerulus.
2. Diagnosis and therapeutic application of
UCA nanobubbles (NBs)
Due to the rapid development in the field of
nanotechnology, a number of NB contrast agents have been produced
using liposomes, inorganic materials, metals and polymers (39). During the synthesis of NBs, the
organic solvent is removed by evaporation or extraction, and the
internal water-phase is eliminated during lyophilization or spray
drying. NBs may possess a porous internal structure with multiple
voids that are amenable to analysis with scanning or transmission
electron microscopy. When they are collectively deposited on the
surfaces of tissues or cells in a layering effect, these particles
create a local acoustic impedance mismatch that produces a strong
US signal without a concomitant increase in the background level.
Unlike MB formulations that are naturally echogenic, these NBs do
not have good inherent acoustic reflectivity (40,41).
Furthermore, polymer-shelled nano-sized MBs have less elastic
shells, which may generate less non-linear harmonic waves.
Sciallero et al (42) found
that for polymeric nano-contrast agent, the optimum
contrast-to-tissue ratio was obtained at an excitation pressure
amplitude of 230 kPa. Although this pressure amplitude is higher
than that which is conventionally used for lipid MBs, it does not
cause the rupture of the polymeric nanobubble contrast agent.
Targeted imaging with NBs
Due to their small size, NB-attached ligands are
important in targeted-molecular imaging (43–45).
Lin et al (46)
demonstrated a simple ultrasonic approach with which to produce
protein-caged nanomaterials coated with MBs for use as bimodal
contrast agents. Two types of protein-caged nanomaterials may be
rapidly transformed into MBs with the use of high-intensity
US-induced emulsification and cross-linking of protein-caged
nanomaterial with bovine serum albumin in aqueous solutions. These
are prepared either by a self-assembled protein corona around
polymer-coated nanoparticles (referred to here as protein-caged
NPs) or by protein-caged fluorescent gold nanoclusters (referred to
here as protein-caged NCs). The sonochemical route produces MBs
with a mean diameter of 1–3 µm, which are able to safely pass
through the microvasculature without diffusing across the
endothelium. This transformation of versatile nanomaterials into
MBs, which comprise a gas core surrounded by a biocompatible
protein/NP shell, forms nanomaterials with the added ability to
scatter sound waves, offering further potential in clinical
application as UCAs.
Photoacoustic molecular imaging
Recently, the use of photo-acoustic molecular
imaging has developed rapidly (47–50).
Kim et al (51) have
developed novel dual-modal contrast agent encapsulated-ink poly
actic-co-glycolic acid (PLGA) MBs and NBs for photoacoustic and US
imaging. Soft gelatin phantoms with embedded tumor simulators of
encapsulated-ink PLGA MBs and NBs in various concentrations are
visible in photoacoustic (PA) and US images. By treating the
surface of the bubbles in order to target specific molecules and
using in combination with PA and US imaging, these contrast agents
can be used to investigate intracranial tumor boundary mapping as
well as for molecular imaging of primary and metastatic tumors
(52). In addition, pulsed
magneto-motive US imaging (pMMUS) has been developed as a
contrast-agent-assisted US-based imaging modality that is able to
capture biological events at the cellular and molecular levels.
During pMMUS imaging, a high intensity pulsed magnetic field is
used to excite cells or tissue, which have been labeled with
magnetic nanoparticles. US imaging is then used to monitor the
mechanical response of the tissue to an externally applied magnetic
field (53). The use of gold
nanostructures in PA imaging and photothermal therapy has been
extensively investigated. However, the structure of nonspherical
gold nanoparticles is easily damaged following laser irradiation,
and may thus lose a degree of efficacy in this context. A novel
class of exogenous PA contrast agents, palladium nanosheets (PNSs)
with strong optical absorption in the near-infrared (NIR) region
has also been applied (54).
Multimodal imaging
Multimodal imaging has become increasingly important
in the diagnosis and prognosis of a number of diseases. Currently,
certain noninvasive, quantitative and functional imaging techniques
are used in standard clinical practice. These include, US imaging,
magnetic resonance imaging (MRI) and optical imaging (fluorescence
imaging). Each of these modalities has particular advantages and
disadvantages. US is a real-time, low-cost, non-ionizing and widely
available imaging tool, but its resolution is low compared with
other techniques and it is highly operator-dependent. MRI is useful
for imaging soft tissue. It has a high spatial resolution and
possesses multi-planar imaging capacities. However, the cost
remains relatively high and it takes longer to obtain images than
US, with relatively low sensitivity. Fluorescence imaging has high
sensitivity and produces multicolor images. However, it is
nonquantitative and does not penetrate tissues well. These
different modalities can be mediated by nanoparticles to produce
complementary methods of imaging (55). Barnett et al (56) demonstrated that perfluorocarbon
nanoparticles, including rhodamine perfluorooctylbromide (PFOB)
nanoparticles and rhodamine perfluoropolyether nanoparticles are
multimodal cellular contrast agents, which may be amenable to
development for use in real-time targeted delivery and imaging of
transplanted human pancreatic islets or other cells, using MRI, US
or computed tomography (CT) imaging. Furthermore, Anayama et
al (57) used a nano-sized
liposome-based contrast agent in ultrasonic bronchoscopies, and CT
and fluorescence optical imaging to delineate features of a rabbit
lung VX2 tumor. Contrast agents have been added to NBs so that they
can be visualized using 1H MRI, 19FMRI, XR/CT and US imaging. This
has led to a novel generation of imaging biomaterials that render
cells visible with multiple imaging modalities (58). Similarly, Cheng et al
(59) investigated a multimodal
imaging contrast agent in rat and mouse models, demonstrating via
MTT and hemolysis studies that the nanodroplets used were
biocompatible and safe, and exhibited significant
ultrasound-triggered phase transition properties under clinical
diagnostic ultrasound irradiation.
Rapoport et al (60) demonstrated that at physiological
temperatures, nanodroplets converted into nanobubbles/MBs.
Doxorubicin (Dox) was localized in the MB walls, which were formed
by the block copolymer. Dox-loaded micelles and NBs extravasated
selectively into the tumor interstitium when injected into mice.
Here, the NBs coalesced to produce MBs with a strong, durable US
contrast. Dox was shown to be strongly retained in the MBs but was
released in response to therapeutic US. When direct US was applied,
the MBs cavitated. This process significantly enhanced
intracellular Dox uptake by tumor cells in vitro compared
with that observed in unsonicated MBs and unsonicated micelles, and
resulted in tumor regression in the mouse model.
In 2011 Ke et al (61) synthesized a new drug-loaded gold
nano-microcapsule, which may be used in diagnostic imaging as well
as photothermal therapy, triggered by UTMD. In 2013, Ke et
al (62) also demonstrated
that the multifunctional nanocapsules were synthesized through
loading PFOB and superparamagnetic iron oxide nanoparticles into
polylactic acid nanocapsules (NCs), followed by the formation of
PEGylated gold nanoshell on the surface. The resulting
multi-component NCs were shown to be able to act as nanotheranostic
agents. These were used to achieve successful noninvasive bimodal
US/MRI guided photothermal ablation in human tumor xenograft
models. Such a single theranostic agent used in combination with
real-time US and high-resolution MR imaging would be of great value
in providing comprehensive diagnostic information and identifying
the dynamics of disease progression for the accurate and timely
application of localized therapy. These molecules show great
potential as an effective nanoplatform for contrast imaging guided
photo-thermal therapy (Figs. 2 and
3, taken from reference 61).
 | Figure 3(A) Contrast-enhanced ultrasonograms
before, during and after the intratumoral injection of the agent
(0.2 ml, 2 mg/ml) into mice for visualization of the agent
distribution to guide the subsequent therapy (tumors indicated by
T). (B) T2-weighted MR images of the tumors at 0, 0.5, 1, 2, 4 and
24 h following intravenous injection of the agent (0.15 ml, 2
mg/ml) for visualization of tumor areas to guide the subsequent
photothermal ablation (tumors are indicated by red circles). (C) MR
intensity profile of the tumors from tumor-bearing mice at 0, 5,
10, 15, 20 and 25 h following intravenous administration of the
nanocapsules. MR, magnetic resonance. |
3. Conclusion
Multifunctional US MB/NB formulations have been
rapidly developed for use in combining ultrasonic contrast enhanced
imaging and passive-active targeted therapy. Their application has
shown promise in the diagnosis and treatment of a variety of
diseases, in particular, malignancy.
Acknowledgments
This review was supported by the National Natural
Science Foundation of China (grant nos. 81171352 and 81271596).
References
|
1
|
Xing ZW, Wang J, Ke H, et al: The
fabrication of novel nano-bubble ultrasound contrast agent for
potential tumor imaging. Nanotechnology. 21:1456072010. View Article : Google Scholar
|
|
2
|
Tran TD, Caruthers SD, Hughes M, et al:
Clinical applications of perfluorocarbon nanoparticles for
molecular imaging and targeted therapeutics. Int J Nanomedicine.
2:515–526. 2007.
|
|
3
|
Quaia E: Microbubble ultrasound contrast
agents: an update. Eur Radiol. 17:1995–2008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun C, Sboros V, Butler MB and Moran CM:
In vitro acoustic characterization of three phospholipid ultrasound
contrast agents from 12 to 43 MHz. Ultrasound Med Biol. 40:541–550.
2014. View Article : Google Scholar :
|
|
5
|
Pfister K, Kasprzak PM, Apfelbeck H, et
al: The significance of contrast-enhanced ultrasound in vascular
surgery. Zentralbl Chi. Dec 10–2013.(Epub ahead of print) (In
German).
|
|
6
|
Liu H, Jiang Y, Dai Q, et al: Peripheral
enhancement of breast cancers on contrast-enhanced ultrasound:
correlation with microvessel density and vascular endothelial
growth factor expression. Ultrasound Med Biol. 40:293–299. 2014.
View Article : Google Scholar
|
|
7
|
Malinova M: Preoperative sonovue contrast
color Doppler in patients with cervical cancer. Preliminary report.
Akush Ginekol (Sofiia). 52(Suppl 1): 11–16. 2013.In Bulgarian.
|
|
8
|
Hoffmann R, von Bardeleben S, Barletta G,
et al: Comparison of two- and three-dimensional unenhanced and
contrast-enhanced echocardiographies versus cineventriculography
versus cardiac magnetic resonance for determination of left
ventricular function. Am J Cardiol. 113:395–401. 2014. View Article : Google Scholar
|
|
9
|
Sartori S, Postorivo S, Vece FD, et al:
Contrast-enhanced ultrasonography in peripheral lung
consolidations: What's its actual role? World J Radiol. 5:372–380.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saracco A, Szabó BK, Aspelin P, et al:
Contrast-enhanced ultrasound using real-time contrast harmonic
imaging in invasive breast cancer: comparison of enhancement
dynamics with three different doses of contrast agent. Acta Radiol.
Jan 20–2014.Epub ahead of print. PubMed/NCBI
|
|
11
|
Lisowska A, Knapp M, Tycinska A, et al:
Usefulness of automatic measurement of contrast flow intensity: an
innovative tool in contrast-enhanced ultrasound imaging of
atherosclerotic carotid plaque neovascularization. A pilot study.
Int Angiol. 33:50–57. 2014.PubMed/NCBI
|
|
12
|
Nagesha D, Laevsky GS, Lampton P, et al:
In vitro imaging of embryonic stem cells using multiphoton
luminescence of gold nanoparticles. Int J Nanomedicine. 2:813–819.
2007.
|
|
13
|
Threlfall G, Wu HJ, Li K, et al:
Quantitative guidelines for the prediction of ultrasound contrast
agent destruction during injection. Ultrasound Med Biol.
39:1838–1847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen ZY, Liang K, Lin Y and Yang F: Study
of the UTMD-based delivery system to induce cervical cancer cell
apoptosis and inhibit proliferation with shRNA targeting Survivin.
Int J Mol Sci. 14:1763–1777. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li F, Jin L, Wang H, et al: The dual
effect of ultrasound-targeted microbubble destruction in mediating
recombinant adeno-associated virus delivery in renal cell
carcinoma: transfection enhancement and tumor inhibition. J Gene
Med. 16:28–39. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu H, Chang S, Sun J, et al:
Ultrasound-mediated destruction of LHRHa-targeted and
paclitaxel-loaded lipid microbubbles induces proliferation
inhibition and apoptosis in ovarian cancer cells. Mol Pharm.
11:40–48. 2014. View Article : Google Scholar :
|
|
17
|
Pu C, Chang S, Sun J, et al:
Ultrasound-mediated destruction of LHRHa-targeted and
paclitaxel-loaded lipid microbubbles for the treatment of
intraperitoneal ovarian cancer xenografts. Mol Pharm. 11:49–58.
2014. View Article : Google Scholar :
|
|
18
|
Yan P, Chen KJ, Wu J, et al: The use of
MMP2 antibody-conjugated cationic microbubble to target the
ischemic myocardium, enhance Timp3 gene transfection and improve
cardiac function. Biomaterials. 35:1063–1073. 2014. View Article : Google Scholar
|
|
19
|
Deng Q, Chen JL, Zhou Q, et al: Ultrasound
microbubbles combined with the NFκB binding motif increase
transfection efficiency by enhancing the cytoplasmic and nuclear
import of plasmid DNA. Mol Med Rep. 8:1439–1445. 2013.PubMed/NCBI
|
|
20
|
Ling ZY, Shu SY, Zhong SG, et al:
Ultrasound targeted micro-bubble destruction promotes angiogenesis
and heart function by inducing myocardial microenvironment change.
Ultrasound Med Biol. 39:2001–2010. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hao YN, Luo WL, Wang D and Wang ZG:
Experimental research on treatment of injured facial nerves induced
by hepatocyte growth factor mediated by ultrasound-targeted
microbubble destruction. J Craniofac Surg. 24:421–424. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang D, Gao YH, Tan KB, et al: Inhibition
of hepatic fibrosis with artificial microRNA using ultrasound and
cationic liposome-bearing microbubbles. Gene Ther. 20:1140–1148.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prentice P, Cushierp A, Dholakiak, et al:
Membrane disruption by optically controlled microbubble
cavitiation. Nat Phys. 1:107–110. 2005. View Article : Google Scholar
|
|
24
|
Tachibana K, Uchida T, Ogawa K, et al:
Induction of cell-membrane porosity by ultrasound. Lancet.
353:14091999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Wamel A, Kooiman K, Harteveld M, et
al: Vibrating micro-bubbles poking individual cells: drug transfer
into cells via sonoporation. J Control Release. 112:149–155. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Juffermans LJ, Dijkmans PA, Musters RJ, et
al: Transient permeabilization of cell membranes by
ultrasound-exposed microbubbles is related to formation of hydrogen
peroxide. Am J Physiol Heart Circ Physiol. 291:H1595–H1601. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miller DL and Gies RA: The interaction of
ultrasonic heating and cavitation in vascular bioeffects on mouse
intestine. Ultrasound Med Biol. 24:123–128. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schlicher RK, Radhakrishna H, Tolentino
TP, et al: Mechanism of intracellular delivery by acoustic
cavitation. Ultrasound Med Biol. 32:915–924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin LF, Li F, Wang HP, et al: Ultrasound
targeted microbubble destruction stimulates cellular endocytosis in
facilitating adeno-associated virus delivery. Int J Mol Sci.
14:9737–9750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du J, Shi QS, Sun Y, et al: Enhanced
delivery of monomethoxypoly(ethylene
glycol)-poly(lactic-co-glycolic acid)-poly l-lysine nanoparticles
loading platelet-derived growth factor BB small interfering RNA by
ultrasound and/or micro-bubbles to rat retinal pigment epithelium
cells. J Gene Med. 13:312–323. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du J, Sun Y, Shi QS, et al: Biodegradable
nanoparticles of mPEG-PLGA-PLL triblock copolymers as novel
non-viral vectors for improving siRNA delivery and gene silencing.
Int J Mol Sci. 13:516–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi Q, Liu P, Sun Y, et al: siRNA delivery
mediated by copolymer nanoparticles, phospholipid stabilized
sulphur hexa-fluoride microbubbles and ultrasound. J Biomed
Nanotechnol. 10:436–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jin L, Li F, Wang H, et al:
Ultrasound-targeted microbubble destruction enhances gene
transduction of adeno-associated virus in a less-permissive cell
type, NIH/3T3. Mol Med Rep. 8:320–326. 2013.PubMed/NCBI
|
|
34
|
Li HL, Zheng XZ, Wang HP, et al:
Ultrasound-targeted micro-bubble destruction enhances AAV-mediated
gene transfection in human RPE cells in vitro and rat retina in
vivo. Gene Ther. 16:1146–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng X, Du L, Wang H and Gu Q: A novel
approach to attenuate proliferative vitreoretinopathy using
ultrasound-targeted microbubble destruction and recombinant
adenoassociated virus-mediated RNA interference targeting
transforming growth factor-b2 and platelet-derived growth factor-B.
J Gene Med. 14:339–347. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li YH, Jin LF, Du LF, et al: Enhancing
HSP70-ShRNA transfection in 22RV1 prostate cancer cells by
combination of sonoporation, liposomes and HTERT/CMV chimeric
promoter. Int J Oncol. 43:151–158. 2013.PubMed/NCBI
|
|
37
|
Zhang Y, Ye C, Wang G, et al:
Kidney-targeted transplantation of mesenchymal stem cells by
ultrasound targeted microbubble destruction promotes kidney repair
in diabetic nephropathy rats. BioMed Res Int. 2013:5263672013.
View Article : Google Scholar
|
|
38
|
Li P, Gao Y, Zhang J, et al: Renal
interstitial permeability changes induced by microbubble enhanced
diagnostic ultrasound. J Drug Target. 21:507–514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jafari S, Diou O, Mamou J, Renault G, et
al: High-frequency (20 to 40 MHz) acoustic response of
liquid-filled nanocapsules. IEEE Trans Ultrason Ferroelectr Freq
Control. 61:5–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hughes GA: Nanostructure-mediated drug
delivery. Nanomedicine. 1:22–30. 2005. View Article : Google Scholar
|
|
41
|
Lanza GM, Trousil RL, Wallace KD, et al:
In vitro characterization of a novel, tissue-targeted ultrasonic
contrast system with acoustic microscopy. J Acoust Soc Am.
104:3665–3672. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sciallero C, Paradossi G and Trucco A: A
preliminary in vitro assessment of polymer-shelled microbubbles in
contrast-enhanced ultrasound imaging. Ultrasonics. 52:456–464.
2012. View Article : Google Scholar
|
|
43
|
Nie L, Chen M, Sun X, et al: Palladium
nanosheets as highly stable and effective contrast agents for in
vivo photoacoustic molecular imaging. Nanoscale. 6:1271–1276. 2014.
View Article : Google Scholar
|
|
44
|
Trung, Tran TD, Caruthers SD, Hughes M, et
al: Clinical applications of perfluorocarbon nanoparticles for
molecular imaging and targeted therapeutics. Int J Nanomedicine.
2:515–526. 2007.
|
|
45
|
Milgroom A, Intrator M, Madhavan K, et al:
Mesoporous silica nanoparticles as a breast-cancer targeting
ultrasound contrast agent. Colloids Surf B Biointerfaces.
11:652–657. 2014. View Article : Google Scholar
|
|
46
|
Lin CA, Chuang WK, Huang ZY, et al: Rapid
transformation of protein-caged nanomaterials into microbubbles as
bimodal imaging agents. ACS Nano. 6:5111–5121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wilson KE, Wang TY and Willmann JK:
Acoustic and photo-acoustic molecular imaging of cancer. J Nucl
Med. 54:1851–1854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Homan KA, Souza M, Truby R, et al: Silver
nanoplate contrast agents for in vivo molecular photoacoustic
imaging. ACS Nano. 6:641–650. 2012. View Article : Google Scholar
|
|
49
|
Ku G, Zhou M, Song S, et al: Copper
sulfide nanoparticles as a new class of photoacoustic contrast
agent for deep tissue imaging at 1064 nm. ACS Nano. 6:7489–7496.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sciallero C and Trucco A: Ultrasound
assessment of polymer-shelled magnetic microbubbles used as dual
contrast agents. J Acoust Soc Am. 133:EL478–EL484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim C, Qin R, Xu JS, Wang LV and Xu R:
Microbubbles and nanobubbles for photoacoustic and ultrasound
imaging. J Biomed Opt. 15:0105102010. View Article : Google Scholar
|
|
52
|
Xu JS, Huang J, Qin R, et al: Synthesizing
and binding dual-mode poly (lactic-co-glycolicacid) (PLGA)
nanobubbles for cancer targeting and imaging. Biomaterials.
31:1716–1722. 2010. View Article : Google Scholar
|
|
53
|
Mehrmohammadi M, Shin TH, Qu M, et al: In
vivo pulsed magneto-motive ultrasound imaging using
high-performance magnetoactive contrast nanoagents. Nanoscale.
5:11179–11186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nie L, Chen M, Sun X, et al: Palladium
nanosheets as highly stable and effective contrast agents for in
vivo photoacoustic molecular imaging. Nanoscale. 6:1271–1276. 2014.
View Article : Google Scholar
|
|
55
|
Park J, Park D, Shin U, et al: Synthesis
of laboratory ultrasound contrast agents. Molecules.
18:13078–13095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Barnett BP, Ruiz-Cabello J, Hota P, et al:
Use of perfluorocarbon nanoparticles for non-invasive multimodal
cell tracking of human pancreatic islets. Contrast Media Mol
Imaging. 6:251–259. 2011.PubMed/NCBI
|
|
57
|
Anayama T, Nakajima T, Dunne M, et al: A
novel minimally invasive technique to create a rabbit VX2 lung
tumor model for nano-sized image contrast and interventional
studies. PLoS One. 8:e673552013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Arifin DR, Kedziorek DA, Fu Y, et al:
Microencapsulated cell tracking. NMR Biomed. 26:850–859. 2013.
View Article : Google Scholar :
|
|
59
|
Cheng X, Li H, Chen Y, et al:
Ultrasound-triggered phase transition sensitive magnetic
fluorescent nanodroplets as a multimodal imaging contrast agent in
rat and mouse model. PLoS One. 8:e850032013. View Article : Google Scholar
|
|
60
|
Rapoport N, Gao Z and Kennedy A:
Multifunctional nanoparticles for combining ultrasonic tumor
imaging and targeted chemotherapy. J Natl Cancer Inst.
99:1095–1106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ke H, Wang J, Dai Z, Jin Y, Qu E, Xing Z,
Guo C, Yue X and Liu J: Gold-nanoshelled microcapsules: A
theranostic agent for ultrasound contrast imaging and photothermal
therapy. Angew Chem Int Ed Engl. 50:3017–3021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ke H, Wang J, Tong S, et al: Gold
nanoshelled liquid perfluoro-carbon magnetic nanocapsules: a
nanotheranostic platform for bimodal ultrasound/magnetic resonance
imaging guided photo-thermal tumor ablation. Theranostics. 4:12–23.
2013. View Article : Google Scholar
|