Introduction
Diabetic nephropathy (DN) is one of the most common
micro-vascular complications of diabetes, and is the leading cause
of end stage renal diseases worldwide (1). The majority of past studies on DN
have concentrated on the glomerular lesions (2). Conversely, the mechanism underlying
the development of renal tubule interstitial lesions has been
neglected; although the tubule interstitium occupies the majority
of the total kidney volume. The significance of renal interstitial
fibrosis (RIF) in the progression of DN has gained increasing
attention. Numerous in vivo and in vitro studies have
verified that the epithelial mesenchymal transition (EMT) of renal
tubular epithelial cells is a key mechanism underlying RIF
(3,4). EMT has been identified as a key
contributor to the loss of renal function throughout the nephron in
DN (5). Transforming growth factor
(TGF)-β1 is a pro-sclerotic cytokine, which is
associated with the EMT (6). The
upregulation of TGF-β1 expression in diabetes identifies
this pro-fibrotic cytokine as a potential candidate for mediation
of the development of such fibrotic complications (5). TGF-β1-induced EMT is
characterized by the loss of E-cadherin, cell adhesion and
connexin-mediated cell communication in the proximal tubule under
diabetic conditions; changes which occur prior to the development
of overt signs of renal damage (7).
It has previously been demonstrated that the
immunosuppressant mycophenolate mofetil (MMF) has certain
protective effects in experimental DN, and that its mechanism may
be associated with the inhibition of renal infiltrating
inflammatory cells (8,9). Angiotensin converting enzyme
inhibitors (ACEI) or angiotensin receptor antagonists may protect
the kidney by reducing diabetic glomerular hypertension, as well as
decreasing urinary protein and non-hemodynamic mechanisms. Renin
angiotensin system (RAS) blocking, combined with MMF treatment,
exerted markedly improved renal protection in a residual renal
kidney model compared with MMF or RAS blocking monotherapy
(10). However, whether MMF has
specific protective effects against diabetic nephropathy and its
underlying mechanism of action have remained to be elucidated.
In the present study, diabetic rats were treated
with benazepril (a representative ACEI) and/or MMF and the role of
MMF in DN was investigated using a streptozocin-induced diabetic
Sprague-Dawley rat model.
Materials and methods
Experimental animals
Forty adult male Sprague-Dawley rats (180–200 g)
were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd (Beijing, China) and maintained in the animal
facility of Qilu Hospital of Shandong University (Jinan, China).
All rats were housed under controlled temperature (22±1°C),
humidity (65–70%) and lighting (12/12 h circadian cycle), with
ad libitum access to standard rat diet and sterile water
throughout the study. All animal experimental procedures were
performed in accordance with the Guidelines for Animal Experiments
of Qilu Hospital of Shandong University and were approved by the
Institutional Ethics Committee for Laboratory Animal Care of Qilu
Hospital, Shandong University.
Determination of diabetes and diabetic
nephropathy
Following one week of feeding, eight rats were
randomly selected to be the normal control group (group N), and the
remaining 32 rats were administered a single intraperitoneal
injection of streptozotocin (STZ; 60 mg/kg) (Sigma-Aldrich, St.
Louis, MO, USA) with 0.1 mmol/l citric acid as buffer (Fuzhou
Maixin Biotech. Co., Ltd., Fuzhou, China). The control rats
received an injection of an identical volume of citric acid buffer
alone. Seventy-two hours following injection, blood glucose (BG)
was detected each day, for the subsequent three days. Diabetic rats
were administered a sub-therapeutic dose of insulin (Eli Lilly,
Indianapolis, IN, USA) following diagnosis, in order to maintain
the animals in a hyperglycemic state, ranging between 13.9 and 22.2
mmol/l, however in relatively good general health. Diabetes
induction was confirmed by three consecutive readings of BG>16.7
mmol/l (300 mg/dl).
Experimental groups and treatment
The diabetic rats were randomly divided into four
groups: The diabetes mellitus (DM) group, received no treatment
(n=8); B group, treated with benazepril (Beijing Nuohua China
Pharmaceutical Co., Beijing, China; n=8); M group, treated with MMF
(Roche Pharmaceutical Co., Basel, Switzerland; n=8) and the BM
group (n=8) treated with benazepril and MMF. Rats were treated with
drugs (10 mg/kg) daily by gavage administration for eight weeks,
beginning four weeks following successful confirmation of the
diabetic model, and the corresponding normal controls were given
identical volumes of distilled water. BG and proteinuria were
monitored weekly. Rats were anesthetized by intraperitoneal
injection of 10% chloral hydrate (3 ml/kg) on week 12. Blood was
obtained through the inferior vena cava following anesthesia and
centrifuged at 1,300 × g for 10 min. Serum was stored at −80°C for
biochemical indices measurement. Pre-cooled normal saline was
injected into the rat left ventricle and the right atrial was
incised for drainage. Kidneys were repeatedly lavaged until the
entire kidney turned pale. Kidneys were removed, weighed following
suck dry of filter paper. One kidney was cut along through the
hilum in the coronal plane. One half of the kidney was fixed in 10%
neutral formalin and reserved for pathological examination and
immunohistochemical specimens; the other half was immersed in fresh
fixatives for electron microscopy. The remaining kidney was snap
frozen in liquid nitrogen and stored at −80°C for western blot
analysis.
Urine albumin and creatinine
measurements
Twenty-four hour urine collection measurements were
obtained using metabolic cages, and urine albumin was quantified
using a protein assay kit purchased from Bio-Rad Laboratories, Inc.
(Hercules, CA, USA). Urine creatinine was measured using a Cayman
creatinine assay kit (Ann Arbor, MI, USA).
Histology
Kidneys were perfused with ice-cold
phosphate-buffered saline (PBS), fixed in 10% buffered formaldehyde
for two days, embedded in paraffin (Fuzhou Maixin Biotech. Co.,
Ltd.) and processed for sectioning. The sections were 5 µm
thick and ~30 sections were obtained from each kidney. These
sections were used for hematoxylin and eosin, periodic acid/Schiff
(PAS), Masson, α-SMA and TGF-β1 immunohistochemical staining.
Pathological changes were detected by hematoxylin and eosin
staining (Fuzhou Maixin Biotech. Co., Ltd.), observed using light
microscopy (Nikon 80i; Nikon, Tokyo, Japan). Extracellular matrix
deposition (mesangial matrix expansion) in the glomeruli was
assessed with PAS staining (Fuzhou Maixin Biotech. Co., Ltd.).
Collagen fibers were stained by Masson staining (Fuzhou Maixin
Biotech. Co., Ltd.).
PAS staining
Paraffin sections were deparaffinized, oxidated in
1% aqueous periodate solution for 15 min and washed three times in
distilled water. Sections were then soaked in Schiff fluid for
10–30 min (control time under a microscope), washed and dyed with
hematoxylin for 2 min. Sections were differentiated by 1%
hydrochloric acid alcohol, rinsed with water until the nucleus
turned blue and dehydrated by gradient alcohol and mounted with
resinous mounting medium. The PAS positive reaction was red and the
nuclei were blue under the microscope (Nikon 80i; Nikon, Tokyo,
Japan).
Masson staining
Paraffin sections were deparaffinized and dyed with
hematoxylin for 10 min. Sections were rinsed in warm tap water for
10 min, washed in distilled water and stained in Biebrich
scarlet-acid fuchsin solution for 10–15 min. Following washing in
distilled water, sections were differentiated in
phosphomolybdic-phosphotungstic acid solution for 10–15 min or
until the collagen was not red. Sections were directly transferred
to aniline blue solution and stained for 5–10 min. Following
rinsing briefly in distilled water, sections were differentiated in
1% acetic acid solution for 2–5 min, washed in distilled water and
dehydrated quickly through 95% ethyl alcohol, absolute ethyl
alcohol until they were mounted with resinous mounting medium. The
collagen was blue, nuclei were black and the cytoplasm was red
under the microscope (Nikon 80i; Nikon, Tokyo, Japan).
Determination of tubulointerstitial
injury index
Twenty high-power fields (magnification, x200) per
section for each sample with PAS staining were randomly selected
and evaluated by three pathologists. The tubulointerstitial injury
index was scored as the percentage of tubulointerstitial injury
area within the total area: 0, normal; 1, <25%; 2, 25–50%; 3,
>50% (11).
Immunohistochemistry
Specimens were deparaffinized and rehydrated with
xylene and 80, 90, 95 and 100% ethanol. Following washing three
times with PBS, sections were placed in 0.01 mol/l citrate buffer
(pH 6.0), heated in a microwave (92–95°C, 15 min) for antigen
retrieval and cooled to room temperature. Following antigen
retrieval, primary antibodies, including mouse anti-rat α-smooth
muscle actin (α-SMA) monoclonal antibody (Abcam, Cambridge, UK;
cat. no. ab7817) and mouse anti rat TGF-β1 monoclonal
antibody (Abcam; cat. no. ab64715) were used at dilutions of 1:75
and 1:50, respectively, and incubated overnight at 4°C.
Immunoglobulin G-conjugated horseradish peroxidase (HRP) goat
anti-mouse polyclonal antibody (1:500; cat. no. ZB-2305; Beijing
ZSGB Biotech. Co., Ltd., Beijing, China) and 3,3-diaminobenzidine
tetrahydrochloride (Vector Laboratories, Burlingame, CA, USA) were
employed to visualize antibody binding (control time under a
microscope at room temperature). Positive staining was defined as
the presence of tan-yellow color granular staining. Optical density
values of positive areas in each slice were calculated using
Image-Pro Plus 6.0 analysis software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Electron microscopy
Following perfusion, the kidneys were excised and
immersed in fresh fixative comprised of 2.5% glutaraldehyde in 0.1
M Na cacodylate buffer (pH 7.4; Fuzhou Maixin Biotech. Co., Ltd.)
for ~16 h at 4°C. The tissue blocks were post-fixed with 1% osmium
tetroxide/0.8% potassium ferricyanide in 0.1 M cacodylate buffer,
treated with aqueous 1% uranyl acetate, dehydrated in graded
ethanol solutions (Fuzhou Maixin Biotech. Co., Ltd.) and embedded
in Polybed epoxy resin for morphological analysis (Polysciences,
Warrington, PA, USA). Sections were cut (70 nm), placed on 200-mesh
copper/rhodium grids and stained with uranyl acetate and lead
citrate (Fuzhou Maixin Biotech. Co., Ltd.), prior to observation at
60 kV on a Philips CM10 transmission electron microscope (Philips,
Eindhoven, The Netherlands). The thickness of the basal membrane
was measured using a validated simplified method (12).
Western blot analysis
Kidney tissues were resuspended in ice-cold
radioimmunoprecipitation assay buffer (Bio-Rad Laboratories, Inc.)
for lysis. For the protein assay, a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology, Haimen, China) was
used. Approximately 30 µg protein samples were loaded,
separated on 9.0% Tris-Glycine-SDS-PAGE and electroblotted onto
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.).
The membranes were then incubated for 1 h in a blocking solution
containing 5% non-fat milk in a PBS 0.05% Tween solution. Following
blocking, membranes were incubated with the mouse anti-rat α-SMA
monoclonal antibody (1:250) or the mouse anti rat TGF-β1
monoclonal antibody (1:500; Abcam) overnight at 4°C. Membranes were
subsequently incubated with polyclonal goat anti-mouse
HRP-conjugated secondary antibody (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 1 h at 37°C. Immunoreactive bands were
detected using a chemiluminescence detection kit (Amersham
Pharmacia Biotech, Piscataway, NJ, USA). Band intensities were
quantified using Image J 1.32 software (National Institutes of
Health, Bethesda, MD, USA). β-actin was used as an internal loading
control.
Statistical analysis
Values are presented as the mean ± standard
deviation. Statistical analysis was performed using GraphPad Prism
software version 4.0 (GraphPad Software, Inc., San Diego, CA, USA).
One-way analysis of variance was performed where appropriate.
Post-hoc Bonferroni pair-wise comparisons were used to assess
significant differences between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Kidney hypertrophy, urinary albumin
excretion and renal function are improved following MMF treatment
in diabetic rats
Following the successful induction of diabetes in
the rat model, rats gradually developed polydipsia, polyuria,
polyphagia, weight loss, grey hair and slow reactions in the DM
group. Mean BG levels remained unchanged in the DM group with
sub-therapeutic doses of insulin over the 12-week experimental
period, although the BG levels were significantly higher than those
in the non-diabetic control rats (Fig.
1A). There was no significant difference in the normalized
kidney weight between groups at the commencement of the experiment;
however, the normalized kidney weight had significantly increased
in the DM group compared with that of the N group upon termination
of the experiments, suggesting the presence of kidney hypertrophy
(Fig. 1B). The normalized kidney
weight increased in the benazepril, MMF and combination regimen
groups; however, the kidney weight was significantly lower than
that of the DM group. Notably, the increase in kidney weight was
markedly smaller in the combination regimen group than that in the
benazepril or MMF groups (Fig.
1B).
 | Figure 1Changes in blood glucose, normalized
kidney weight, urine protein and serum creatitine levels amongst
the five groups. (A) Average blood glucose levels in diabetic rats
(DM, B, M, BM) compared with non-diabetic rats (N). (B) Normalized
kidney weight. (C) Urinary albumin excretion over 24 h. (D) Serum
creatitine levels. Values are presented as the mean ± standard
deviation. *P<0.05, vs. N; #P<0.05, vs.
DM; ΔP<0.05, vs. B or M; n=8 per group. N,
non-diabetic control; DM, diabetic rats; B, diabetic rats treated
with benazepril; M, diabetic rats treated with mycophenolate
mofetil; BM, diabetic rats treated with benazepril and
mycophenolate mofetil. |
As expected, urinary albumin excretion and serum
creatitine (Scr) revealed a similar trend to that observed with the
normalized kidney weight amongst the groups (Fig. 1C and D). Twenty-four hour urinary
protein and Scr levels were significantly increased in the DM group
compared with those of the control group; however this increase was
attenuated following benazepril or MMF treatment. A greater
improvement in these two indices was observed in the combination
regimen group compared with the single-drug treatment groups
(Fig. 1C and D).
MMF treatment decreases glomerular
hypertrophy and mesangial matrix expansion in diabetic rats
Mesangial matrix expansion is one of the hallmarks
of diabetic nephropathy, and was therefore investigated in diabetic
rats in the present study via analysis of kidney sections obtained
from the various groups. As indicated in Fig. 2, glomerular hypertrophy and
accelerated mesangial matrix expansion, characterized by increased
areas positive for PAS staining, were identified in the DM group
compared with that of the non-diabetic controls. Glomerular
hypertrophy and mesangial matrix expansion were attenuated
following benazepril or MMF treatment. Notably, lesions in the BM
group were markedly fewer than those in the single-drug groups.
Masson staining additionally indicated increased levels of collagen
in the DM group compared with those of the control group. However,
collagen generation was ameliorated in the treatment groups
(Fig. 2).
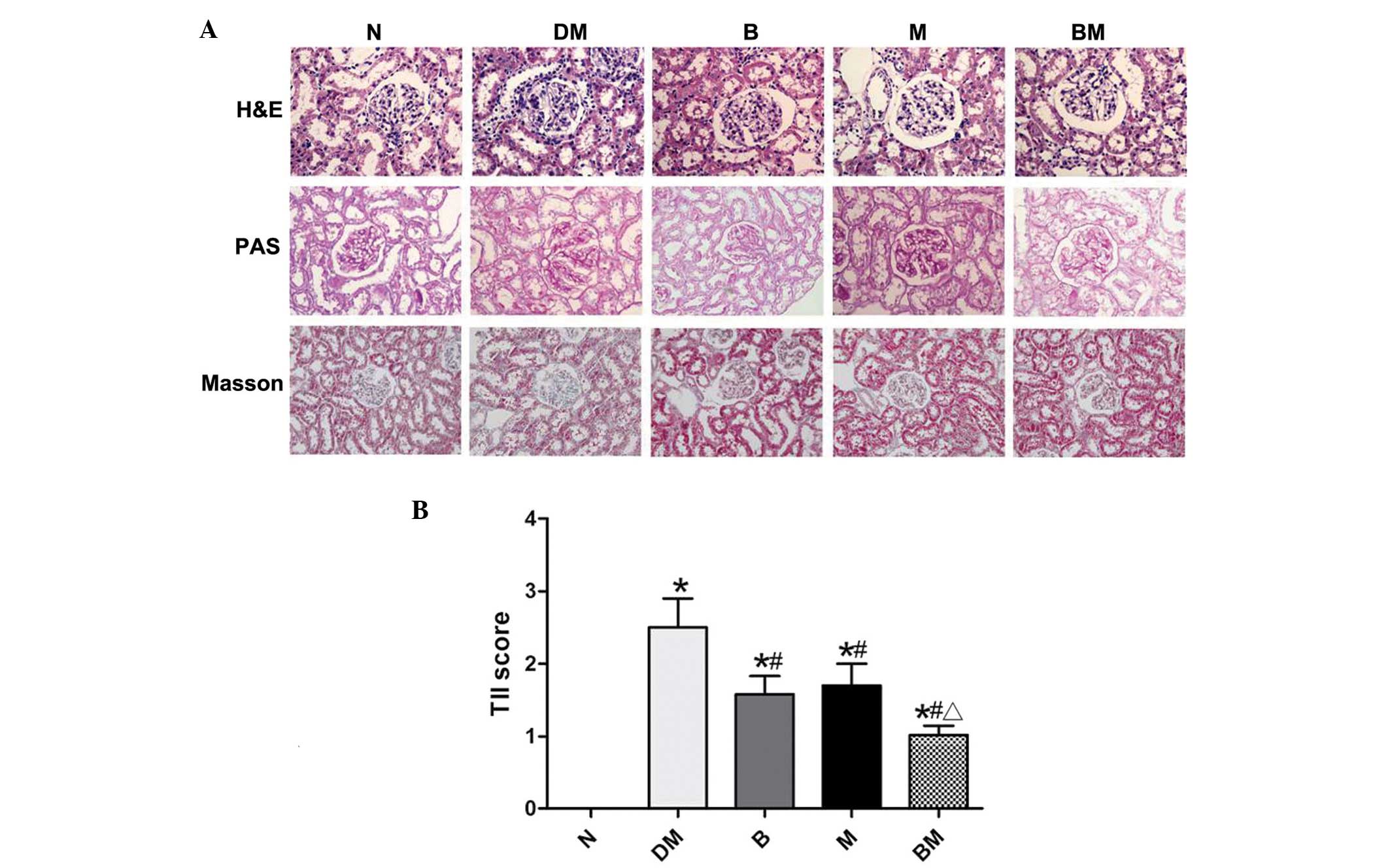 | Figure 2(A) Representative histology of renal
sections stained with H&E, PAS and Masson in the various groups
(magnification, x40). (B) TII scores. Values are presented as the
mean ± standard deviation. *P<0.05, vs. N;
#P<0.05, vs. DM; ΔP<0.05, vs. B or M;
n=8 per group. N, non-diabetic control; DM, diabetic rats; B,
diabetic rats treated with benazepril; M, diabetic rats treated
with mycophenolate mofetil; BM, diabetic rats treated with
benazepril and mycophenolate mofetil; H&E, hematoxylin and
eosin; PAS, periodic acid/Schiff; TII, tubular injury index. |
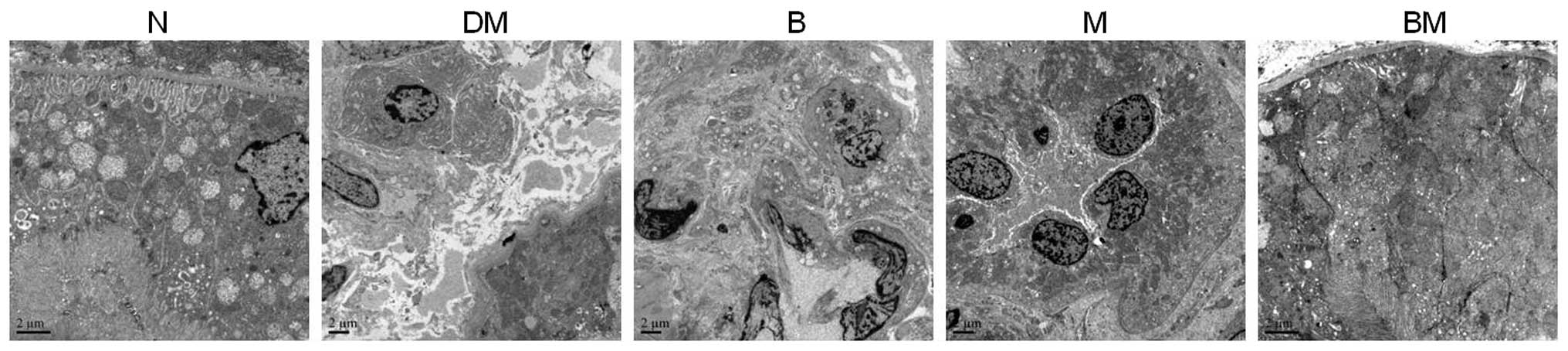
In order to further investigate the structural
alterations of the glomeruli, particularly those of the glomerular
basement membrane (GBM), samples were examined using transmission
electron microscopy. The GBM was found to be thicker in diabetic
rats compared with that of the non-diabetic controls; however,
these alterations in thickness were attenuated in the benazepril,
MMF and combined treatment groups (Fig. 3). Extracellular matrix accumulation
was also detected in the diabetic kidneys under electron
microscopy, and this effect was also attenuated in the single and
combined treatment groups (Fig.
3).
Renal tubule and interstitial lesions were also
found to be more severe in the DM group than those present in the
normal control and medicated groups. Renal tubular epithelial cell
particle vacuole degeneration, renal tubular expansion and renal
interstitial inflammatory cell infiltration were more marked in the
DM group compared with those of the control group; while these
lesions were significantly reduced in the benazepril, MMF and
combined treatment groups (Fig.
4).
EMT is involved in DN and is ameliorated
following MMF treatment
To further evaluate whether EMT was involved in the
development of DN, markers of EMT, including α-SMA and
TGF-β1, were detected. As shown in Fig. 5A and B, α-SMA expression was only
detected in the smooth muscle cells of the blood vessel walls in
the normal control group. However, α-SMA expression was detected
mainly in the renal tubular epithelial cell cytoplasm and tubule
interstitium as well as in the walls of the blood vessels and
surrounding the glomerulus in the DM group. Significantly increased
α-SMA expression was detected in the DM group compared with that in
the control group. Furthermore, α-SMA expression was significantly
decreased in benazepril and MMF treatment groups compared with that
in the DM group. In addition, α-SMA expression levels were markedly
lower in the combined treatment group than those in the single-drug
treatment groups. The results of western blot analysis of α-SMA
protein expression were highly consistent with those observed by
immunohistochemistry (Fig. 5C and
D).
 | Figure 5α-SMA expression in the five groups
was analyzed. (A) Representative immunohistochemical staining of
α-SMA expression in renal tissues of the five groups
(magnification, x40). (B) OD values of α-SMA in the renal tubular
interstitium were quantified. (C) Representative western blot
analysis of α-SMA protein expression in renal tissues. (D) Relative
quantitation of α-SMA protein. Data were obtained from three
independent experiments in each condition. *P<0.05, vs. N;
#P<0.05, vs. DM; ΔP<0.05, vs. B or M;
n=8 for each group. N, non-diabetic control; DM, diabetic rats; B,
diabetic rats treated with benazepril; M, diabetic rats treated
with mycophenolate mofetil; BM, diabetic rats treated with
benazepril and mycophenolate mofetil; α-SMA, α-smooth muscle
actin. |
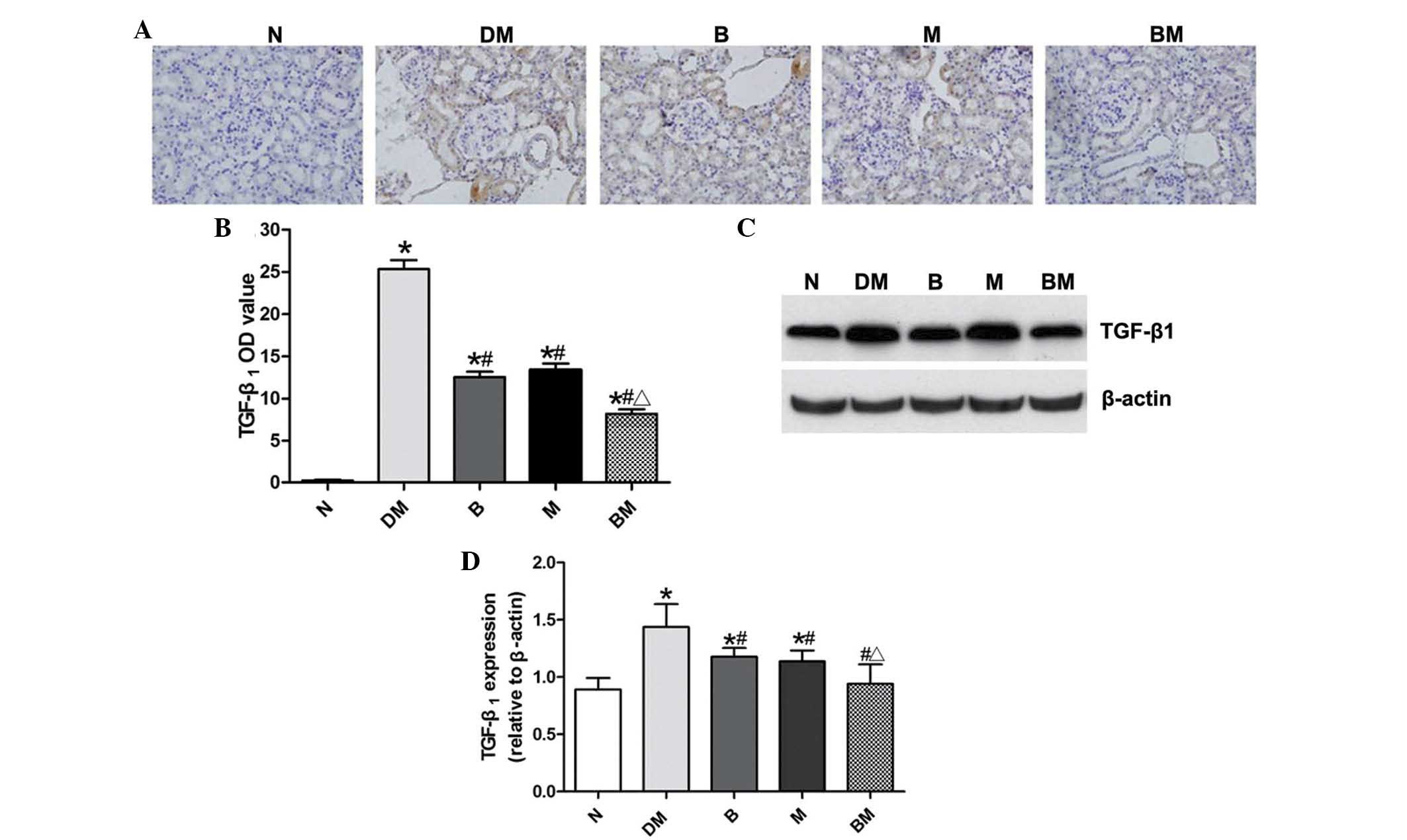
In analogy with the trend observed in α-SMA
expression, there was almost no TGF-β1 expression
detected in the normal control group; whereas, TGF-β1
expression was significantly increased and mainly expressed in the
renal tubular epithelial cell cytoplasm in the DM group (Fig. 6A and B). However, TGF-β1
expression was significantly decreased in the benazepril and MMF
groups, compared with that of the DM group. In addition,
TGF-β1 expression was significantly reduced in the
combined treatment group, compared with the single drug treatment
groups. Western blot analyses revealed analogous results to those
identified by immunohistochemistry (Fig. 6C and D).
Discussion
The results of the present study revealed that
normalized kidney weight, 24 h urinary protein, renal function and
renal pathological changes were markedly improved in the benazepril
and MMF groups compared with those of the diabetic group at the end
of the experimental period. Additionally, the improvement of these
indices was greater in the combined benazepril and MMF treatment
group compared with those in the single-drug treatment groups.
Furthermore, there was no statistical difference in BG between the
medicated groups and the DM group (Fig. 1A). All these results suggested that
MMF has a protective role in DN, and that benazepril combined with
MMF exerts coordinated protective effects against DN. Additionally,
the protective effect of MMF was not dependent on hypoglycemic
action.
Previous studies have demonstrated that
tubulointerstitium pathological alterations occur at the same time
or even prior to the appearance of alterations in the glomerular
filtration membrane in DN (13),
indicating that the development of renal tubular interstitial
lesions is not entirely dependent on glomerular lesions, but is an
independent factor associated with DN. It has been confirmed that
the severity of tubulointerstitial lesions is closely associated
with urinary protein excretion and the progressive decline in renal
function, as well as directly influencing the prognosis of DN under
high glucose conditions (14).
Glomerular hypertrophy, mesangial matrix hyperplasia, vacuoles and
granular degeneration of renal tubular epithelial cells, renal
tubular expansion, irregular thickening of basement membrane and
small focal mononuclear macrophage infiltration in the tubular
interstitium were observed in the STZ-induced diabetic rats in the
present study. Twenty-four hour urinary protein increases and
impaired renal function were also identified in these rats. The
aforementioned results confirmed that the experimental model of DN
was successful.
The phenotype of renal tubular epithelial cells
changes as a result of a variety of pathological factors in the
development of DN. The expression of epithelial marker antigens,
including cytokeratin and cadherin, is lost (15). Mesenchymal cell marker, including
α-SMA, vimentin and fibroblast specific protein, expression is
initiated and extracellular matrix (ECM) is produced and secreted,
indicating EMT (16).
TGF-β1 is hypothesized to have a key role in this
process (6,17). α-SMA expression is widely used for
the detection of EMT, and its expression is restricted to renal
vascular smooth muscle cells since there are almost no
myofibroblasts in the normal kidney (18). When α-SMA expression is detected in
the glomerular mesangial cells, renal tubular epithelial cells,
renal interstitial fibroblasts and/or other inherent renal cells,
their cell phenotype begins to transform from static to
proliferative or secretory type cells, initiating ECM synthesis and
secretion and inducing the development of myofibroblast properties
(19). The results of the present
study indicated that α-SMA expression confined to the renal tissue
vascular smooth muscle cells in the normal control group; whereas
α-SMA expression was significantly increased in the renal tubular
interstitium, as well as on the walls of the blood vessels in the
DM group. α-SMA expression was lower in the renal tubular
interstitium of all the medicated groups compared with that of the
DM group. Furthermore, expression was significantly lower in the
combined therapy group than the single-drug treatment groups,
suggesting that the EMT of renal tubule epithelium occurring
following 12 weeks in the DM group, was partially inhibited by
benazepril or MMF treatment alone, and combined treatment had
synergistic inhibitory effects on EMT in diabetes.
ACEIs are known to improve diabetic glomerular
hemodynamics, reduce glomerular hypertension and proteinuria by
blocking the renin-angiotensin aldosterone system, as well as
exerting renal protective effects independent of its
anti-hypertensive effect (20).
Previous studies have shown that angiotensin II (Ang II) has a
significant role in the process of renal tubular interstitial
fibrosis repair. Ang II stimulates renal tubular epithelial cell
hypertrophy, induces TGF-β1 production and induces the
differentiation of fibroblasts and renal tubular epithelial cells
into myofibroblasts. ECM production is increased and degradation is
decreased via the RAS system and Ang II type I receptors, which
results in tubular interstitial fibrosis (21). Furthermore, Ang II, as an
inflammatory factor, induces the activation and translocation of
inflammation-initiated nuclear factor-κB in the early stages of DN
(22), leading to the expression
of cytokines and adhesion molecules, including intercellular
adhesion molecule 1, monocyte chemotactic factor (MCP-1) and
osteopontin, and the recruitment of large numbers of inflammatory
cells infiltrating into the glomerular and renal tubular
interstitium. In the present study, benazepril was used to block
Ang II production, and renal hypertrophy, matrix accumulation,
glomerular and renal interstitial fibrosis were alleviated
following treatment, consistent with previous findings.
MMF, as a novel immune inhibitor, inhibits T and B
lymphocyte proliferation, monocyte and mesangial cell
proliferation, tubular interstitial mononuclear macrophage
infiltration and the synthesis of multiple cytokines, including
TGF-β1, MCP-1 and TNF-α (23). MMF has therefore been widely used
in the treatment and prevention of transplant rejection, autoimmune
diseases and primary glomerular disease. Roos et al
(24) revealed that MMF was able
to reduce the generation of α-SMA and type I collagen in cultured
rat lung fibroblasts in a systemic sclerosis model, suggesting that
MMF had direct anti-fibrotic effects. Whether MMF also has a direct
anti-fibrosis role in DN remains to be elucidated.
In conclusion, the results of the present study
demonstrated that MMF and benazepril have protective effects in a
rat model of DN. Furthermore, combined treatment with MMF and
benazepril had significantly greater protective effects than single
drug treatments. The underlying mechanism may be associated with
the inhibition of renal tubular epithelial cell
transdifferentiation, but this hypothesis requires further
investigation.
Acknowledgments
The present study was supported by the China
National Natural Science Foundation Projects for Young Scientists
(no. 81100520), the General Financial Grant from China Postdoctoral
Science Foundation (no. 2012M511517) and the Foundation for
Outstanding Young Scientists in Shand ong Province (no.
BS2011SW014).
References
|
1
|
Ziyadeh FN and Sharma K: Overview:
combating diabetic nephropathy. J Am Soc Nephrol. 14:1355–1357.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anil Kumar P, Welsh GI, Saleem MA and
Menon RK: Molecular and cellular events mediating glomerular
podocyte dysfunction and depletion in diabetes mellitus. Front
Endocrinol (Lausanne). 5:1512014.
|
|
3
|
Lan HY: Tubular epithelial-myofibroblast
transdifferentiation mechanisms in proximal tubule cells. Curr Opin
Nephrol Hypertens. 12:25–29. 2003. View Article : Google Scholar
|
|
4
|
Loeffler I, Liebisch M and Wolf G:
Collagen VIII influences epithelial phenotypic changes in
experimental diabetic nephropathy. Am J Physiol Renal Physiol.
303:F733–F745. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hills CE and Squires PE: TGF-beta1-induced
epithelial-to-mesenchymal transition and therapeutic intervention
in diabetic nephropathy. Am J Nephrol. 31:68–74. 2010. View Article : Google Scholar
|
|
6
|
Hills CE and Squires PE: The role of TGF-β
and epithelial-to mesenchymal transition in diabetic nephropathy.
Cytokine Growth Factor Rev. 22:131–139. 2011.PubMed/NCBI
|
|
7
|
Hills CE, Siamantouras E, Smith SW,
Cockwell P, Liu KK and Squires PE: TGFβ modulates cell-to-cell
communication in early epithelial-to-mesenchymal transition.
Diabetologia. 55:812–824. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu YG, Lin H, Qian H, et al:
Renoprotective effects of combination of angiotensin converting
enzyme inhibitor with mycophenolate mofetil in diabetic rats.
Inflamm Res. 55:192–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Utimura R, Fujihara CK, Mattar AL,
Malheiros DM, Noronha IL and Zatz R: Mycophenolate mofetil prevents
the development of glomerular injury in experimental diabetes.
Kidney Int. 63:209–216. 2003. View Article : Google Scholar
|
|
10
|
Fujihara CK, Noronha IL, Malheiros,
Antunes GR, de Oliveira IB and Zatz R: Combined mycophenolate
mofetil and losartan therapy arrests established injury in the
remnant kidney. J Am Soc Nephrol. 11:283–290. 2000.PubMed/NCBI
|
|
11
|
Wesson DE, Nathan T, Rose T, Simoni J and
Tran RM: Dietary protein induces endothelin-mediated kidney injury
through enhanced intrinsic acid production. Kidney Int. 71:210–217.
2007. View Article : Google Scholar
|
|
12
|
Marquez B, Zouvani I, Karagrigoriou A,
Anastasiades E, Pierides A and Kyriacou K: A simplified method for
measuring the thickness of glomerular basement membranes.
Ultrastruct Pathol. 27:409–416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Najafian B, Alpers CE and Fogo AB:
Pathology of human diabetic nephropathy. Contrib Nephrol.
170:36–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bonventre JV: Can we target tubular damage
to prevent renal function decline in diabetes? Semin Nephrol.
32:452–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Habib SL: Alterations in tubular
epithelial cells in diabetic nephropathy. J Nephrol. 26:865–869.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y: Epithelial to mesenchymal
transition in renal fibrogenesis: pathologic significance,
molecular mechanism, and therapeutic intervention. J Am Soc
Nephrol. 15:1–12. 2004. View Article : Google Scholar
|
|
17
|
Wilkins-Port CE and Higgins PJ: Regulation
of extracellular matrix remodeling following transforming growth
factor-beta1/epidermal growth factor-stimulated
epithelial-mesenchymal transition in human premalignant
keratinocytes. Cells Tissues Organs. 185:116–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hills CE and Squires PE: The role of TGF-β
and epithelial-to mesenchymal transition in diabetic nephropathy.
Cytokine Growth Factor Rev. 22:131–139. 2011.PubMed/NCBI
|
|
19
|
Ina K, Kitamura H, Tatsukawa S and
Fujikura Y: Significance of α-SMA in myofibroblasts emerging in
renal tubulointerstitial fibrosis. Histol Histopathol. 26:855–866.
2011.PubMed/NCBI
|
|
20
|
Tylicki L, Lizakowski S and Rutkowski B:
Renin-angiotensin-aldosterone system blockade for nephroprotection:
current evidence and future directions. J Nephrol. 25:900–910.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wolf G: Renal injury due to
renin-angiotensin-aldosterone system activation of the transforming
growth factor-beta pathway. Kidney Int. 70:1914–1919.
2006.PubMed/NCBI
|
|
22
|
Esteban V, Lorenzo O, Rupérez M, et al:
Angiotensin II, via AT1 and AT2 receptors and NF-kappaB pathway,
regulates the inflammatory response in unilateral ureteral
obstruction. J Am Soc Nephrol. 15:1514–1529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allison AC: Mechanisms of action of
mycophenolate mofetil. Lupus. 14(Suppl 1): s2–s8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roos N, Poulalhon N, Farge D, Madelaine I,
Mauviel A and Verrecchia F: In vitro evidence for a direct
antifibrotic role of the immunosuppressive drug mycophenolate
mofetil. J Pharmacol Exp Ther. 321:583–589. 2007. View Article : Google Scholar : PubMed/NCBI
|