Introduction
Lumican is one of the major extracellular proteins
in the interstitial extracellular matrix (ECM) of the skin, corneal
stroma, sclera, aorta, muscle, lung, kidney, bone, cartilage and
intervertebral discs (1). It is a
member of the family of small, leucine-rich proteoglycans (SLRP),
with a core protein of 30–50 kDa comprising a signal peptide, a
negatively charged N-terminal domain, a highly conserved
leucine-rich internal domain and a carboxyl-terminal domain
(2). The protein core and the
glycan chains of lumican can interact with various cellular
effectors (3), including
cytokines, growth factors and cell surface receptors, to modulate
cell adhesion, proliferation and migration (4). The primary function of lumican is to
produce rigidity of collagen fibers (5). In addition, lumican is important in
cellular migration and cell differentiation, and has a paracrine
function (6); therefore, lumican
is classified as a matrikine. Lumican is also involved in cancer
cell proliferation and metastasis (7). It has been reported that
lumican-deficient (Lum−/−) mice are defective in immunological
responses through the Fas-Fas ligand pathway (8). Lumican has also been demonstrated to
regulate the host response to pathogen-associated molecular
patterns (9). Therefore, the
Lum−/− mice are hypo-responsive to bacterial lipopolysaccharide
(LPS) endotoxins and Lum−/− macrophages in cell culture produce
lower levels of pro-inflammatory cytokines in response to LPS
(10). Lumican facilitates the
innate immune response by binding LPS and transferring the LPS
signal to toll-like receptor (TLR)4. However, the detailed
immunological role of lumican remains to be elucidated.
Gram-negative bacteria-induced sepsis remains the
leading cause of acute renal failure (11). One of the mechanisms of
sepsis-induced acute renal failure involves the release of
bacterial endotoxin into the circulation, which activates
interconnected inflammatory cascades in the kidney, ultimately
leading to renal injury (12,13).
The production of inflammatory mediators is important in the
pathophysiology of inflammation in renal injury. The present study
determined the role of lumican in LPS-induced systemic inflammation
and renal injury using a mouse model of endotoxemia. It was
demonstrated that lumican exacerbated LPS-induced inflammation and
renal injury in murine models of sepsis. The potential mechanism
may be partly through the adjustment of the TLR4-nuclear factor
(NF)κB pathway.
Materials and methods
Experimental animals
C57 mice were cross-bred with EF1-Lum transgenic
mice overexpressing lumican under the control of the EF1 promoter.
Following genotyping, the transgenic EF1-Lum mice from one line
were used in the present study and were compared with age-matched
and strain-matched C57 mice. A total of 20 male C57BL/6 mice and 20
lumican transgenic mice at 8–10 weeks of age, weighing 20–25 g,
were obtained from the Experimental Animal Center of China Medical
University (Shenyang, China). The mice were housed in rooms
maintained at 26°C and a 12-h light/dark cycle for at least one
week to acclimatize to the surroundings, with free access to water
and standard mouse chow. The study was approved by the ethics
committee of the China Medical University (Shenyang, China) and
conformed with the guide for the care and use of laboratory animals
published by the National Institutes of Health (Bethesda, MA,
USA).
Experimental protocols
The mice were randomly divided into four groups:
Wild-type control (WT CTR), wild-type LPS (WT LPS), lumican
transgenic control (TG CTR) and transgenic LPS (TG LPS). A mouse
model of endotoxemia, with intraperitoneal injection of a dose of
LPS (Escherichia coli serotype 055: B5; Sigma-Aldrich, St.
Louis, MO, USA) was used. Various doses of LPS were used (10, 15
and 20 mg/kg body weight) and the lowest dose was selected since it
was sufficient to induce septic shock in wild-type mice. Each mouse
in the LPS group was administered 10 mg/kg body weight LPS. In the
control group, an equal volume of sterile saline (Beijing Tiantan
Biological Products Co., Ltd, Beijing, China) was administered.
Blood samples (0.5 ml) were collected from the inferior vena cava
of the mice while the mice were under anesthesia with isoflurane
(2%; Abbott Laboratories Co., Shanghai, China), at 24 h following
LPS injection. The blood samples were immediately centrifuged at
1,000 × g for 20 min and the serum was stored at −80°C. Following
the collection of blood, the mice were sacrificed by cervical
dislocation and the kidneys were excised. Each of these
experimental groups included ten mice.
Measurement of serum creatinine (SCr),
blood urea nitrogen (BUN) and cytokines
Blood was collected for the measurement of serum
creatinine and BUN, according to the manufacturer's instructions
(Nanjing Jiancheng, Nanjing, China). Selected cytokines were
measured by standard sandwich ELISA. Mouse ELISA kits for tumor
necrosis factor (TNF)α, interleukin (IL)-6, IL-4 and IL-10 were
obtained from R&D Systems (Minneapolis, MN, USA). Recombinant
cytokines were used as standard controls. The experimental samples,
negative controls and diluted standard markers were added into each
well. The absorbance was measured using a Biotek ELx808 absorbance
reader (BioTek Instruments, Inc., Winooski, VT, USA) 540 nm and the
total protein concentration was measured using a Bradford assay kit
(Bio-Rad Laboratories, Hercules, CA, USA).
Determination of the expression levels of
TLR4 and NFκB by western blot analysis
Renal tissue samples were lysed with lysis buffer
(Sigma-Aldrich) and the protein concentrations were determined
using a protein assay kit (Bio-Rad Laboratories). An equal quantity
of protein (50 µg) from tissue homogenates was separated
using 10% SDS-PAGE and was subsequently transferred onto
nitrocellulose membranes (Bio-Rad Laboratories). Following blocking
of the membrane with 5% non-fat milk in Tris-buffered saline
(Sigma-Aldrich) at room temperature for 1 h, the membrane was
incubated with mouse monoclonal primary antibodies against TLR4
(1:1,000; cat. no. 2246) and phospho-NFκB (1:1,000; cat. no. 3036)
both purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA) at 4°C for 12 h. The membranes were incubated with horseradish
peroxidase-conjugated secondary antibody (Cell Signaling
Technology, Inc.) for 1 h and signals were observed using an
enhanced chemiluminescence kit (Amersham Pharmacia, GE Healthcare,
Amersham, UK). The membranes were subsequently re-probed with a
mouse monoclonal antibody against actin (1:1,000; cat. no. 12262)
and a rabbit polyclonal antibody against NFκB (1:1,000; cat. no.
8242) both purchased from Cell Signaling Technology, Inc. at room
temperature for 2 h as indicators for equal loading of the samples.
Western blotting data were quantified by densitometric analysis
using ImageJ version 1.38× (NIH Image software, Bethesda, MA, USA).
Values are expressed as relative differences following
normalization against the expression levels of actin and NFκB.
Determination of renal cell apoptosis by
terminal deoxynucleotidyl transferase-mediated dUTP nickend
labeling (TUNEL)
Apoptotic cells were detected using a TUNEL assay
kit (Promega Corporation, Madison, WI, USA). Fresh kidney sections
were fixed in 10% buffered formalin and embedded in paraffin, and
4-µm slices were stained using the in situ Cell Death
Detection kit (Promega Corporation) according to the manufacturer's
instructions. Three tissue sections from each sample were randomly
selected and 10 microscopic fields per section were assessed by two
independent observers. In each field, the nuclei were quantified
and the percentage of TUNEL-positive nuclei was determined.
Observation of pathological changes by
light microscopy and electron microscopy
24 h following LPS injection, the animals were
sacrificed by cervical dislocation, followed by immediate organ
collection for histological analysis. Fresh kidney tissue sections
were fixed in 10% buffered formalin (Sigma-Aldrich) and embedded in
paraffin, and 4-µm sections were stained with hematoxylin
and eosin (Sigma-Aldrich). Samples were assessed using an Olympus
CX22 light microscope (Olympus, Tokyo, Japan). For the severity
(score: 0–3) of renal cortical vacuolization, peritubular/proximal
tubule leukocyte infiltration, the percentage of proximal tubule
simplification and proximal tubule hypereosinophilia was assessed
by an experienced pathologist, in a blinded manner. The kidneys
were perfusion-fixed with 1.25% glutaraldehyde (Sigma-Aldrich) in
0.1 M phosphate buffer (pH 7.4) and were subsequently cut in
sagittal and horizontal cross sections for image analysis. Sections
(1 µm) were dried overnight at 45°C on gelatin-coated slides
(Sigma-Aldrich), stained at 60°C for 2 h in giemsa (Sigma-Aldrich),
cooled to room temperature, dehydrated, cleaned in xylene and
mounted in permount (Sigma-Aldrich). A JEOL 1011 transmission
electron microscope with a Hamamatsu Orca-HR Digital Camera (JEOL,
Inc., Peabody, MA, USA) and the Advanced Microscopy Techniques
Corp. AMT16000B image capture system (Advanced Microscopy
Techniques Corp., Danvers, MA, USA) were used. A pathologist
analyzed the samples and determined the levels of injury in a
blinded manner.
Statistical analysis
All values are expressed as the mean ± standard
error of the mean. Differences were compared by analysis of
variance, followed by Bonferroni correction for post-hoc t-test
where appropriate. P<0.05 was considered to indicate a
statistically significant difference. All statistical tests were
performed using SPSS software 13.0 (SPSS, Inc., Chicago, IL,
USA).
Results
BUN and creatinine changes
The present study assessed the serum BUN and
creatinine levels (Table I). LPS
injection caused a significant increase in serum BUN and SCr levels
compared with those in WT and TG mice in the control group
(P<0.05). However, the increase was attenuated in the WT group
(P<0.05).
 | Table IContents of serum BUN and SCr in each
group. |
Table I
Contents of serum BUN and SCr in each
group.
| Group | BUN (mmol/l) | SCr
(µmol/l) |
|---|
| WT CTR | 17.3±1.9 | 49.8±2.3 |
| WT LPS | 20.2±1.7a | 55.9±2.8a |
| TG CTR | 18.7±2.3 | 50.1±2.7 |
| TG LPS | 23.7±1.8a,b | 58.2±2.9a,b |
Release of inflammatory cytokines into
renal tissues
LPS treatment increased the expression levels of
TNF-α, IL-6, IL-4 and IL-10 compared with those in the control
group (P<0.05). However, the expression levels of TNF-α, IL-6,
IL-4 and IL-10 in the TG LPS group were significantly higher
compared with those in the WT LPS group (P<0.05; Fig. 1).
 | Figure 1Release of inflammatory cytokines into
renal tissue. Following treatment with lipopolysaccharides for 3,
6, 12 and 24 h, renal tissues were collected and selected
cytokines, including (A) TNFα, (B) IL-6, (C) IL-4 and (D) IL-10,
were measured by standard ELISA. *P<0.05, vs WT CTR;
#P<0.05, vs WT LPS. WT, wild-type; TG, transgenic
mice; IL, interleukin; TNF, tumor necrosis factor; prot,
protein. |
Expression levels of TLR4 and NFκB
following treatment with LPS
As shown in Fig. 2,
treatment with LPS caused an increase in the expression levels of
phospho-NFκB and TLR4 compared with those in the control group in
each strain of mice; however, the expression levels of phospho-NFκB
and TLR4 were reduced in the WT LPS group compared with those in
the TG LPS group.
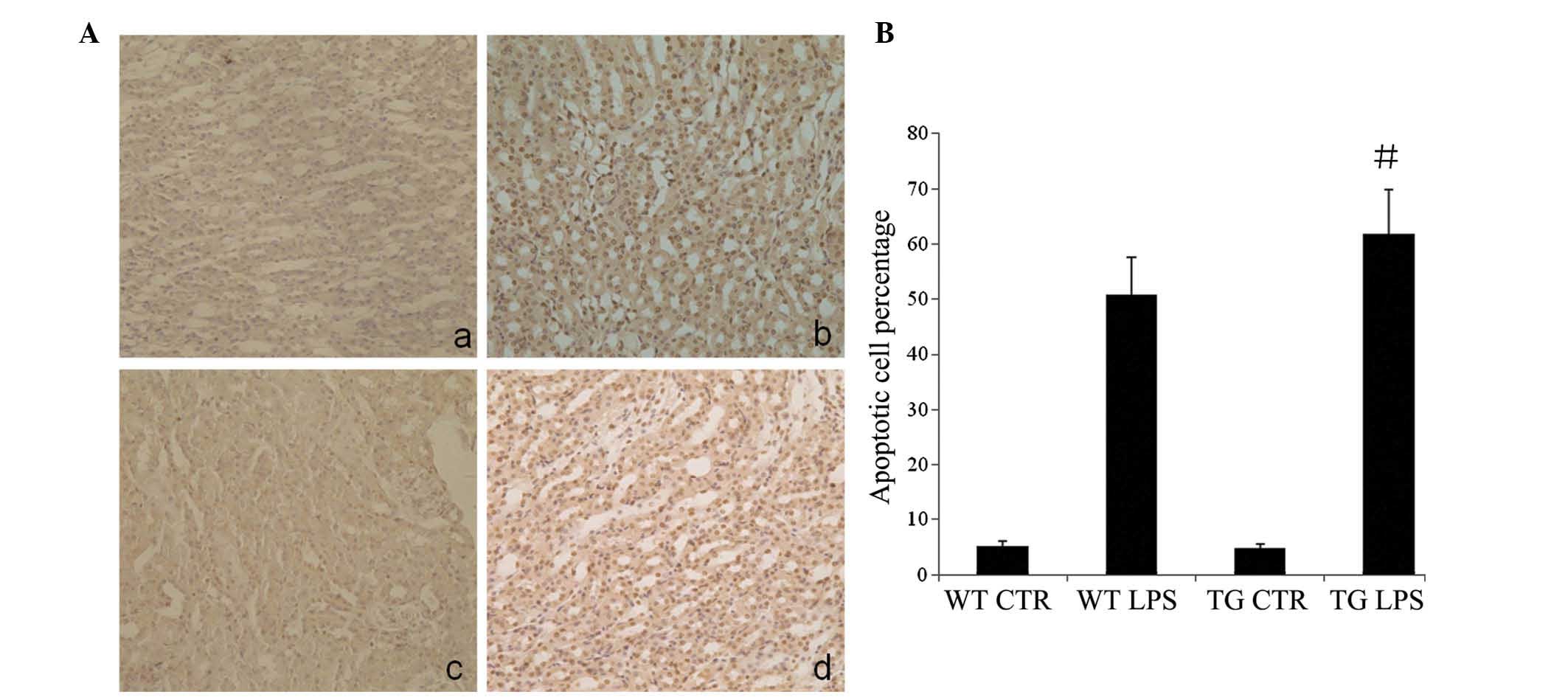
Apoptosis in renal tissues following
treatment with LPS
LPS increased the TUNEL-positive staining in the WT
and TG groups compared with that in the control group (P<0.05),
particularly in the renal tubular epithelial cells. However, the
increase was significantly lower compared with that in the TG LPS
group (P<0.05; Fig. 3).
Pathological changes of kidney
tissues
Kidney tissue sections were stained with hematoxylin
and eosin, and the samples were assessed for the severity (score:
0–3) of renal cortical vacuolization, peritubular/proximal tubule
leukocyte infiltration, percentage of proximal tubule
simplification and proximal tubule hypereosinophilia by an
experienced pathologist, in a blinded manner. As shown in Fig. 4, the control group exhibited no
clear abnormalities under the light microscope, with the exception
of occasional edema of renal tubular epithelial cells. In the LPS
group, swelling and necrosis of proximal or distal tubules,
inflammatory cell infiltration and edema of connective tissue in
the renal interstitium was observed (Fig. 4A and B). Administration of LPS also
caused glomerular ultrastructural changes in the mice. Transmission
electron microscopy of glomeruli from mice which had received LPS
demonstrated significant ultrastructural changes compared with the
controls (Fig. 4C). None of these
pathologic changes were observed in the kidneys of the control
mice, whereas in the LPS group, podocytes appeared swollen, foot
process effacement was present, the urinary space was diminished
and certain samples contained extensive vacuoles of unknown
significance.
Discussion
Proteoglycans are widely distributed in the stromal
tissue of mammals and are considered to be closely associated with
the ECM and growth factors (14).
Proteoglycans belong to the SLRP family and are characterized by
the presence of multiple adjacent leucine-rich regions, comprising
20–29 amino acid residues, which may be repeated up to 30 times
(2). Members of SLRP family
include keratocan, mimecan, decorin, biglycan fibromodulin,
epiphycan, osteoadherin and lumican (15). SLRPs, including lumican, have
important functions in cell migration, cell proliferation, tissue
repair and tumor growth, in addition to their ECM functions in
tissue hydration and collagen fibrillogenesis (2). Lumican was first isolated from chick
cornea and has also been reported to be localized in the skin
dermis, lung, bone, cartilage and heart of adult mice (3). In adult cornea, lumican is present as
keratan sulfate proteoglycans (16); however, in non-corneal tissues,
lumican exists as low- or non-sulfated glycoprotein (17). Gene-targeting studies indicate that
lumican is important in determining the structural phenotype of the
mature collagen fibril in various tissue types. In addition, it is
hypothesized that lumican sequesters in the pericellular matrix and
interacts with cell surface proteins for specific cellular
functions. Lumican-deficient mice suffer from increased skin
fragility and corneal opacities as a result of abnormal fibril
assembly and altered interfibrillar spacing, indicating lateral
fusion of collagen fibrils (18).
By contrast, lumican mediates Fas-Fas ligand (FasL)-induced
apoptosis by inducing Fas in mouse embryonic fibroblasts (19). Lumican, by binding and signaling
via FasL, increases the synthesis and secretion of pro-inflammatory
cytokines and the recruitment of macrophages and neutrophils
(20). The present study
demonstrated that bacterial LPS is sensed by the TLR4 signaling
pathway and is regulated by lumican. LPS endotoxins from the cell
wall of Gram-negative bacteria are recognized by the TLR4 signaling
pathway (21). LPS sensing begins
with its binding to the LPS-binding protein in the blood. The LPS
recognition complex also requires soluble MD-2 protein, heat shock
proteins and additional factors, which remain to be identified
(22–24). The TLR4 signaling pathway involves
the phosphorylation of inhibitor of NF-κB kinase, nuclear
translocation and the activation of NF-κB. The NF-κB transcription
factor upregulates pro-inflammatory cytokines, including TNFα and
IL-6, and increases microbicidal activities (25,26).
TNFα is a cytokine prototype and is often used to assess the host
innate immune response. These mediators assist in clearing
infections. However, unrestricted systemic overproduction of
pro-inflammatory cytokines and proteins can lead to severe sepsis,
multiple organ failure and mortality. Host response to infection
and bacterial endotoxins is being investigated at multiple levels
to define the events leading to sepsis and septic shock.
Understanding the molecular events from pathogen recognition to
inflammatory mediators is becoming important in the treatment of
sepsis and for identifying patients at risk.
Bacterial infection causes shock, acute respiratory
failure, multiple organ dysfunction syndrome and disseminated
intravascular coagulation. Despite an improved understanding of the
epidemiology, pathophysiology and genetic pre-disposition to
sepsis, morbidity and mortality associated with severe sepsis and
septic shock remain high throughout the world (27,28).
Renal failure is one of the most common and life-threatening
diseases during septic shock.
The present study of the ECM protein identified
lumican as a novel modulator of the host response to inflammation
and sepsis. Lumican modulates host sensing of bacterial LPS by TLR4
in a mouse model of LPS-induced systemic inflammation. The serum
levels of TNF-α, IL-6, IL-4 and IL-10 were examined. These
cytokines are normally markedly induced by LPS and are secreted
during the early phase of the inflammatory response, therefore
exerting an important role in organ dysfunction (29). TNFα is a primary mediator of
inflammation and its release leads to the activation of other
cytokines, including IL-6, which is associated with cellular damage
(30,31). On the other hand, IL-6 may be a
more consistent predictor of sepsis and appears to correlate better
with sepsis severity and mortality (32). It was demonstrated that Lum+/+ mice
are hyper-responsive to LPS-induced septic shock, with further
induction of pro-inflammatory cytokines, including TNFα and IL-6,
and anti-inflammatory cytokines, including IL-4 and IL-10, in the
serum. LPS treatment successfully induced multiple organ
dysfunction syndrome in mice, including acute renal failure
indicated by increased BUN and SCr levels, increased TUNEL-positive
staining, particularly in tubular epithelial cells, and
histological damage in the kidney, accompanied with activation of
the TLR4 pathway. Lum+/+ mice exhibited a more severe injury
compared with wild-type mice.
In conclusion, the present study demonstrated that
LPS caused excessive apoptosis of renal tubular cells via the TLR4
signal transduction pathway, decreased the number of renal tubular
cells and resulted in ARF. Lumican may be involved in LPS-induced
ARF in mice.
Acknowledgments
This study was supported, in part, by a grant from
the National Natural Science Foundation of China (no.
81100109).
References
|
1
|
Chen S and Birk DE: The regulatory roles
of small leucine-rich proteoglycans in extracellular matrix
assembly. FEBS J. 280:2120–2137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schaefer L: Small leucine-rich
proteoglycans in kidney disease. J Am Soc Nephrol. 22:1200–1207.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nikitovic D, Katonis P, Tsatsakis A,
Karamanos NK and Tzanakakis GN: Lumican, a small leucine-rich
proteoglycan. IUBMB Life. 60:818–823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naito Z: Role of the small leucine-rich
proteoglycan (SLRP) family in pathological lesions and cancer cell
growth. J Nippon Med Sch. 72:137–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brézillon S, Pietraszek K, Maquart FX and
Wegrowski Y: Lumican effects in the control of tumour progression
and their links with metalloproteinases and integrins. FEBS J.
280:2369–2381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malinowski M, Pietraszek K, Perreau C, et
al: Effect of lumican on the migration of human mesenchymal stem
cells and endothelial progenitor cells: involvement of matrix
metallopro-teinase-14. PLoS One. 7:e507092012. View Article : Google Scholar
|
|
7
|
Goldoni S and Iozzo RV: Tumor
microenvironment: Modulation by decorin and related molecules
harboring leucine-rich tandem motifs. Int J Cancer. 123:2473–2479.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vij N, Roberts L, Joyce S and Chakravarti
S: Lumican regulates corneal inflammatory responses by modulating
Fas-Fas ligand signaling. Invest Ophthalmol Vis Sci. 46:88–95.
2005. View Article : Google Scholar
|
|
9
|
Lohr K, Sardana H, Lee S, Wu F, Huso DL,
Hamad AR and Chakravarti S: Extracellular matrix protein lumican
regulates inflammation in a mouse model of colitis. Inflamm Bowel
Dis. 18:143–151. 2012. View Article : Google Scholar
|
|
10
|
Shao H, Scott SG, Nakata C, Hamad AR and
Chakravarti S: Extracellular matrix protein lumican promotes
clearance and resolution of Pseudomonas aeruginosa keratitis in a
mouse model. PLoS One. 8:e547652013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Howell GM, Gomez H, Collage RD, et al:
Augmenting autophagy to treat acute kidney injury during
endotoxemia in mice. PLoS One. 8:e695202013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui WY, Tian AY and Bai T: Protective
effects of propofol on endotoxemia-induced acute kidney injury in
rats. Clin Exp Pharmacol Physiol. 38:747–754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nair AR, Masson GS, Ebenezer PJ, Del Piero
F and Francis J: Role of TLR4 in lipopolysaccharide-induced acute
kidney injury: protection by blueberry. Free Radic Biol Med.
71:16–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Juneja SC and Veillette C: Defects in
tendon, ligament, and enthesis in response to genetic alterations
in key proteoglycans and glycoproteins: a review. Arthritis.
2013:1548122013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iozzo RV and Schaefer L: Proteoglycans in
health and disease: novel regulatory signaling mechanisms evoked by
the small leucine-rich proteoglycans. FEBS J. 277:3864–3875. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amjadi S, Mai K, McCluskey P and Wakefield
D: The role of lumican in ocular disease. ISRN Ophthalmol.
2013:6323022013. View Article : Google Scholar
|
|
17
|
Frey H, Schroeder N, Manon-Jensen T, Iozzo
RV and Schaefer L: Biological interplay between proteoglycans and
their innate immune receptors in inflammation. FEBS J.
280:2165–2179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chakravarti S: Functions of lumican and
fibromodulin: lessons from knockout mice. Glycoconj J. 19:287–293.
2002. View Article : Google Scholar
|
|
19
|
Vij N, Roberts L, Joyce S and Chakravarti
S: Lumican suppresses cell proliferation and aids Fas-Fas ligand
mediated apoptosis: implications in the cornea. Exp Eye Res.
78:957–971. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vij N, Roberts L, Joyce S and Chakravarti
S: Lumican regulates corneal inflammatory responses by modulating
Fas-Fas ligand signaling. Invest Ophthalmol Vis Sci. 46:88–95.
2005. View Article : Google Scholar
|
|
21
|
Ramachandran G: Gram-positive and
gram-negative bacterial toxins in sepsis: a brief review.
Virulence. 5:213–218. 2014. View Article : Google Scholar :
|
|
22
|
Maeshima N and Fernandez RC: Recognition
of lipid A variants by the TLR4-MD-2 receptor complex. Front Cell
Infect Microbiol. 3:32013.PubMed/NCBI
|
|
23
|
Tamura Y, Torigoe T, Kutomi G, Hirata K
and Sato N: New paradigm for intrinsic function of heat shock
proteins as endogenous ligands in inflammation and innate immunity.
Curr Mol Med. 12:1198–1206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lucas K and Maes M: Role of the Toll Like
receptor (TLR) radical cycle in chronic inflammation: possible
treatments targeting the TLR4 pathway. Mol Neurobiol. 48:190–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hildebrand D, Sahr A, Wölfle SJ, Heeg K
and Kubatzky KF: Regulation of Toll-like receptor 4-mediated immune
responses through Pasteurella multocida toxin-induced G protein
signalling. Cell Commun Signal. 10:222012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
da Silveira Cruz-Machado S, Carvalho-Sousa
CE, Tamura EK, et al: TLR4 and CD14 receptors expressed in rat
pineal gland trigger NFKB pathway. J Pineal Res. 49:183–192.
2010.PubMed/NCBI
|
|
27
|
Schorr CA, Zanotti S and Dellinger RP:
Severe sepsis and septic shock: management and performance
improvement. Virulence. 5:190–199. 2014. View Article : Google Scholar :
|
|
28
|
Balk RA: Systemic inflammatory response
syndrome (SIRS): where did it come from and is it still relevant
today? Virulence. 5:20–26. 2014. View Article : Google Scholar :
|
|
29
|
Li XJ, Zhang GX, Sun N, Sun Y, Yang LZ and
Du YJ: Protective effects of erythropoietin on endotoxin-related
organ injury in rats. J Huazhong Univ Sci Technolog Med Sci.
33:680–686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Striz I, Brabcova E, Kolesar L and
Sekerkova A: Cytokine networking of innate immunity cells: a
potential target of therapy. Clin Sci (Lond). 126:593–612. 2014.
View Article : Google Scholar
|
|
31
|
Khosravi R, Ka K, Huang T, Khalili S,
Nguyen BH, Nicolau B and Tran SD: Tumor necrosis factor-α and
interleukin-6: potential interorgan inflammatory mediators
contributing to destructive periodontal disease in obesity or
metabolic syndrome. Mediators Inflamm. 2013:7289872013. View Article : Google Scholar
|
|
32
|
Zhao Y and Li C: (Diagnostic value of a
combination of biomarkers in patients with sepsis and severe sepsis
in emergency department). Chin Crit Care Med. 26:153–158. 2014.In
Chinese.
|