Introduction
Epidemiological evidence has indicated a highly
significant association between the Helicobacter pylori
infection and the development of duodenal ulcers and distal gastric
adenocarcinoma. In 1994, H. pylori was categorized as a
class I carcinogen/definite human carcinogen by the World Health
Organization (1). Current
antibiotic-based therapeutic methods are not practical for global
control (2), therefore, vaccines
against the H. pylori infection are those that were
developed in the past (3). H.
pylori protein vaccines require an effective adjuvant (4) as H. pylori proteins exhibit a
low immunogenicity, therefore, vaccination with an H. pylori
antigen alone cannot induce a high enough immune response to
deplete the Helicobacter infection and protect the gastric
mucosa (5). Cholera toxin (CT) and
heat-labile Escherichia coli enterotoxin (LT) are generally
regarded as the most powerful mucosal adjuvants (6,7);
however, their use in humans is hampered by their particularly high
toxicities. CT and LT have been restructured to reduce their
toxicities (8), however this
resulted in a reduction of their adjuvant effects.
Chitosan, a polymer of D-glucosamine and a natural
product derived from chitin, is accessible, and demonstrates good
bioadhesion, biodegradability and biocompatibility without
immunogenicity, toxicity or side-effects (9); thus, chitosan has been used in
mucosal vaccines as an adjuvant (10).
Numerous studies have indicated that chitosan
effectively elicits a local (particularly mucosal local) immune
response, enhances the ability of antigenic delivery systems and
performs adjuvant activity in vaccines (11). It has been reported that
Neisseria meningitidis and Bordetella pertussis
vaccines with chitosan as the adjuvant successfully induced a
protective immune response (12).
Our previous study demonstrated that oral
administration of H. pylori whole-cell sonicate plus
chitosan as the adjuvant protected mice against H. pylori
infection (13). Furthermore, it
has been shown that, as an adjuvant in vaccines for H.
pylori protection, chitosan is more effective than CT in immune
protection against H. pylori infection (14). However, to the best of our
knowledge, there have been no reports regarding chitosan as an
adjuvant for the H. pylori therapeutic vaccine and the
immunoprotection mechanism remains unclear. Therefore, in the
present study, mice were infected with H. pylori and then
vaccinated using an H. pylori protein vaccine with chitosan
as the adjuvant. This was to delineate the therapeutic effect of
the H. pylori vaccine and the potential mechanism against
H. pylori infection in comparison to a H. pylori
vaccine with CT as the adjuvant.
Materials and methods
Reagents and bacterial strains
Chitosan and 88.5% deacetylated chitosan powder were
purchased from Shanghai Qisheng Biological Preparation Co., Ltd.
(Shanghai, China). Rabbit anti-rat IgG1 (cat. no. PA1-86329; Zymed
Life Technologies, Carlsbad, CA, USA), IgG2a (cat. no. 61-0220;
Zymed Life Technologies) and IgA (cat. no. Sab3700520;
Sigma-Aldrich, St. Louis, MO, USA), and goat anti-mouse IgG (cat.
no. A27025; Zymed Life Technologies) peroxidase conjugate were
purchased from Zymed Life Technologies (Carlsbad, CA, USA). CT was
purchased from Sigma-Aldrich. Enzyme-linked immunosorbent assay
(ELISA) kits for interleukin (IL)-2, interferons (IFNs), IL-12,
IL-4, and IL-10 were purchased from eBioscience, Inc. (San Diego,
CA, USA). Polymerase chain reaction (PCR) primers were purchased
from Shanghai Sheng Gong Biological Engineering Technology Service
Co., Ltd. (Shanghai, China) Goat anti-mouse TLR4 polyclonal
antibody (cat. no. sc-12511) was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Rabbit anti-rat Foxp3
polyclonal antibody (cat. no. bs-10211R) was purchased from Beijing
Bo Orson Biological Technology Co., Ltd., (Beijing, China) and the
H. pylori Sydney strain 1 (SS1) was provided by the H.
pylori Strain Pool (Chinese Centre for Disease Control, China).
An 450 enzyme microplate reader was purchased from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA). A PCR thermal cycler was
purchased from PerkinElmer, Inc. (Waltham, MA, USA). A JS680C gel
imaging analysis system was purchased from Shanghai Peiqing Science
and Technology Co., Ltd (Shanghai, China) and the ECP3000
electrophoresis apparatus was purchased from Beijing Liuyi
Instrument Factory (Beijing, China). A BH-2 stereo-binocular
microscope was purchased from Suzhou REIT Image Technology Co.,
Ltd. (China).
Animals
Female BALB/c mice (age, 6–8 weeks; mean weight,
22.5 g) were purchased from the Animal Center of the Chinese
Academy of Sciences (Shanghai, China). The mice were housed in a
specific pathogen-free environment with free access to food and
water. All animal experiments were conducted in accordance with
principles stated in the Guide for the Care and Use of Laboratory
Animals. The experimental protocols were approved by the Ethics
Committee of The First Affiliated Hospital of Nanchang
University.
H. pylori culture
The H. pylori SS1 was used throughout the
investigation. H. pylori was grown in a Campylobacter
agar base, containing 10% sheep blood, under microaerobic
conditions (5% O2, 10% CO2 and 85%
N2) at 37°C for 2–3 days.
Preparation of H. pylori antigen
After culturing for 2–3 days, the H. pylori
SS1 was eluted with phosphate-buffered saline (PBS) and centrifuged
at 10,000 × g and 4°C for 10 min. The pellet was washed and
sonicated. Following centrifugation at 8,000 × g and 4°C for 30
min, the supernatant was collected and stored at −80°C until use.
The protein concentration was determined using a bicinchoninic acid
(BCA) assay.
Preparation of chitosan particles and
solution
Deacetylated (88.5%) chitosan powder was suspended
in saline to a final concentration of 10 mg/ml and sonicated twice
(output, 80 Hz). The small particles in the supernatant were
removed. The chitosan particles were collected by further
centrifugation at 140 × g for 10 min. Chitosan stock solution [3%
(w/w)] was prepared from 88.5% deacetylated chitosan powder in 0.8%
(v/v) acetic acid and 0.9% (w/v) saline.
H. pylori infection
Each mouse was orally administered with
1×109 colony-forming units (CFUs) of H. pylori
per liter five times every other day. Twelve weeks after the last
inoculation, four mice were euthanized, and the stomachs were
removed to ascertain whether the H. pylori infection model
had been established.
H. pylori vaccination
The infected BALB/c mice were orally immunized in
the following groups at days 0, 7, 14 and 21: i) Control (PBS
alone), 12 mice; ii) H. pylori antigen alone, 11 mice; iii)
H. pylori antigen plus 0.5% chitosan solution, 12 mice; iv)
H. pylori antigen plus CT (5 µg/mouse), 11 mice (one
mouse died); and v) H. pylori antigen plus chitosan
particles (500 µg/mouse), 12 mice. Four weeks after the
final vaccination, saliva and blood samples were collected. The
stomachs were isolated and cut longitudinally into two sections.
One was used for examination of H. pylori, and the other was
used for histology and immunological assays.
Assessment of bacterial load in the
stomach
The bacterial load in the stomach was determined by
quantitative culture of H. pylori and Giemsa staining. H.
pylori-negative was defined when the culture of H.
pylori and the Giemsa staining were negative. H.
pylori-positive was defined when either the culture of H.
pylori or the Giemsa staining was positive. For assessment of
H. pylori colonization, the weighed stomachs were
homogenized in Brucella broth and spread over a serum plate. The
plates were incubated for 3–7 days, and the H. pylori
colonies were counted and calculated as CFUs per gram of stomach
tissue. For Giemsa staining, the colonization was assessed by
semi-quantitative analysis of H. pylori in the gastric
mucosa (0, nil; 1, 1–2 cells/crypt; 2, 3–10 cells/crypt; 3, 11–20
cells/crypt; and 4, >21 cells/crypt).
Determination of H. pylori-specific
antibody levels in the gastric mucosa and saliva
The H. pylori-specific antibodies, IgG, IgG1,
and IgG2a in sera, and IgA in the gastric mucosa and saliva were
detected by indirect ELISA. After weighing, the gastric mucosa was
homogenized in PBS and the homogenates were centrifuged at 3,000 ×
g at 4°C for 20 min. The supernatant was harvested and diluted at
1:2. The sera and saliva were diluted at 1:100 and 1:5,
respectively. Peroxidase-conjugated rabbit anti-rat IgG1, IgG2a or
IgA was diluted at 1:1,000 and peroxidase-conjugated goat
anti-mouse IgG secondary antibody was diluted at 1:2,000. The
antibody levels of each immunized group from the sera and saliva
were represented as relative levels to the mock-immunized control
group. The IgA levels in the gastric mucosa were represented as
relative levels (per gram wet weight of the gastric mucosa) to the
mock-immunized group.
Determination of cytokines in the gastric
mucosa by ELISA
After weighing, the gastric mucosa was homogenized
in PBS and the homogenates were centrifuged at 3,000 × g at 4°C for
20 min. ELISA kits were used to quantify IL-2, IFN, IL-12, IL-4 and
IL-10 in the supernatants (diluted at 1:2) following
centrifugation. The results were represented as pg/mg wet weight of
the gastric mucosa.
Determination of TLR4 and Foxp3 mRNA
contents in the gastric mucosa by reverse transcription
(RT)-PCR
Total RNA was isolated from the mouse gastric mucosa
to determine TLR4 and Foxp3 mRNA levels within the gastric mucosa
using RT-PCR. The cDNA from each sample served as a template for
subsequent PCR assays to assess the TLR4 and Foxp3 mRNA levels,
which were normalized to the expression of β-actin. Each
50-µl PCR consisted of 25 pmol of each primer, 10 mM Tris
(pH 8.3), 1.5 mM MgCl2, 200 µM dNTPs, and 0.5
µl of Taq enzyme. β-actin and TLR4 were amplified at 95°C
for 5 min (1 cycle); 95°C for 30 sec, 56°C for 30 sec and 72°C for
1 min (30 cycles); and 72°C for 5 min (1 cycle). Foxp3 was
amplified at 95°C for 2.5 min (1 cycle); 95°C for 30 sec, 57°C for
30 sec and 72°C for 1 min (32 cycles); 72°C for 5 min (1 cycle).
The PCR products were visualized following electrophoresis on 2%
agarose gels and the band intensities were quantified by
densitometry.
Determination of TLR4 and Foxp3 protein
expression in the gastric mucosa by immunohistochemistry
Snap-frozen biopsies were cut into 4-µm
sections to determine the levels of TLR4 and Foxp3 protein
expression in the gastric mucosa by immunohistochemistry. In the
TLR4-stained sections, positive cells were assigned to the cell
membrane or to yellow/brown-dyed plasma, and negative cells were
assigned to the cell membrane or to non-dyed plasma. In
Foxp3-stained sections, positive cells were assigned to the cell
nucleus or to yellow/brown-dyed plasma and negative cells were
assigned to the cell membrane or to non-dyed plasma. The
immunoreaction was graded according to the depth of the color and
the proportion of positive cells. The degree of dyeing was divided
into the following score grades and expressed as a percentage: 0,
Negative; 1, (yellow) weakly positive; 2 (light brown) positive;
and 3 (brown) strongly positive.
Statistical analysis
Differences in the eradication rate were analyzed by
Fisher's exact test. Differences in H. pylori-specific
antibody levels in the gastric mucosa among the experimental groups
were evaluated for statistical significance by analysis of variance
or Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Animal model of H. pylori infection
The animal model of H. pylori infection was
established for the different infection groups. Four mice were
euthanized 12 weeks after oral infection with H. pylori.
Positive H. pylori-infection was observed by H.
pylori culture and Giemsa staining of sections of the infected
stomachs (Fig. 1A). Gastric
inflammation was observed by hematoxylin and eosin (H&E)
staining (Fig. 1B), thus
indicating the H. pylori infection.
H. pylori infection among different
groups
To determine H. pylori infection in the
gastric mucosa following the therapeutic vaccination H.
pylori clearance was measured, and the H. pylori
colonization scores and density of the quantitative H.
pylori culture were obtained. Pathological tests of the gastric
mucosa were also conducted (Fig.
2).
After the therapeutic vaccination, the gastric
mucosae of H. pylori-infected mice were analyzed for H.
pylori infection. The H. pylori clearance in the groups
with chitosan as the adjuvant was identified to be significantly
greater than that of the mock-immunized control group (P<0.005)
and the H. pylori antigen-immunized group without any
adjuvant (P<0.05); in addition, the H. pylori clearance
in the group with CT as the adjuvant was significantly greater than
that in the control group (Fig.
2A; P<0.05). The H. pylori colonization density in
the control group was significantly greater than that in any of the
other groups (Fig. 2B and Table I; P<0.05), and the H.
pylori colonization density in the groups with chitosan as the
adjuvant was significantly less than that of the H. pylori
antigen group without any adjuvants (Fig. 2E and Table I; P<0.05). No significant
difference was identified between the groups with chitosan as the
adjuvant and the group with CT as the adjuvant (Fig. 2A, B and E; P>0.05).
 | Table IHelicobacter pylori (Hp)
colonization density of a quantitative culture in the gastric
mucosa. |
Table I
Helicobacter pylori (Hp)
colonization density of a quantitative culture in the gastric
mucosa.
| Group | n | Median Hp
colonization density (colony forming units/g of gastric
mucosa) |
|---|
| Control | 12 |
1.4×108 |
| Hp antigen | 11 |
1.8×105a |
| Hp antigen +
chitosan solution | 12 | 0ab |
| Hp antigen +
chitosan particle | 12 | 0ab |
| Hp antigen +
cholera toxin | 11 | 0a |
Grades of gastritis
H. pylori clearance, H. pylori
colonization scores, H. pylori colonization density of the
quantitative culture, and grades of acute and chronic gastritis in
the gastric mucosa were measured to determine H. pylori
infection and gastritis in the gastric mucosa following therapeutic
vaccination.
The gastric mucosae of H. pylori-infected
mice were tested for gastritis after therapeutic vaccination. The
grade of acute gastritis in the groups with an adjuvant was
identified to be significantly lower than that of the control group
and the H. pylori antigen group without any adjuvant
(Fig. 3A; P<0.05). For acute
gastritis after H. pylori infection, no difference was
observed in the therapeutic effect between the groups with chitosan
as the adjuvant and the group with CT as the adjuvant (Fig. 3A; P>0.05). However, the vaccine
with chitosan as the adjuvant relieved chronic gastritis to a
greater extent than the vaccine with CT as the adjuvant (Fig. 3B; P<0.05). HE staining of the
gastric mucosa of the control group revealed an increased chronic
inflammation response (Fig. 3C).
The group with chitosan treatment revealed milder chronic
inflammation response compared with the group with CT as adjuvant
(Fig. 3D and E).
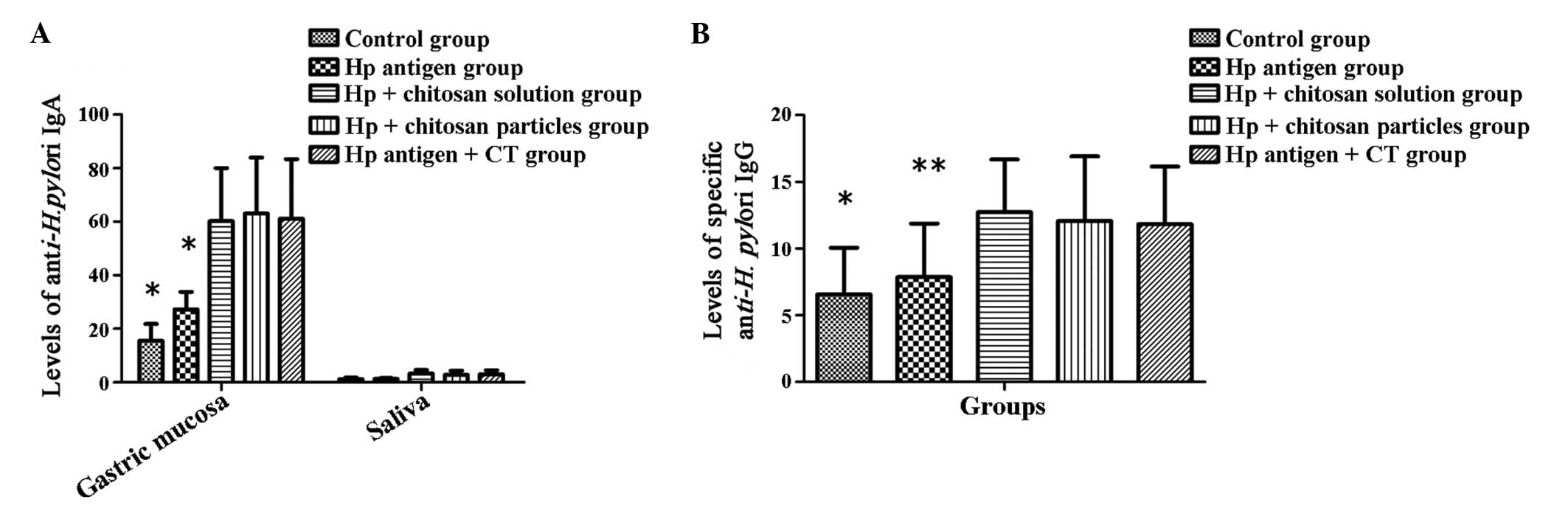
Levels of H. pylori-specific antibodies
in the gastric mucosa serum, and saliva
To determine the local immune response induced by
the therapeutic vaccination, the levels of H.
pylori-specific IgA and IgG antibodies in each group were
measured by ELISA.
A significant difference (P<0.001) was observed
between the different groups in the level of anti-H. pylori
IgA in the gastric mucosa and saliva, and specific anti-H.
pylori IgG in the sera. The levels of H. pylori-specific
antibodies in the groups that were treated with a vaccine plus an
adjuvant were significantly greater when compared with those of the
control group and the H. pylori antigen groups (Fig. 4A and B; P<0.01-0.001). In
addition, no significant difference was identified between the
groups with chitosan as the adjuvant and the group with CT as the
adjuvant (Fig. 4A and B;
P>0.05).
Effect of TH1 and TH2
To determine the cellular immune response (TH1 and
TH2) induced by the therapeutic vaccination, ELISA was conducted to
establish the levels of cytokines in the gastric mucosa and the
ratio of IgG2a to IgG1 in the sera of each group.
The levels of cytokines, IL-12, IFN-γ, and IL-2 in
the groups administered with a vaccine plus an adjuvant were
observed to be significantly greater than those of the control
group and the H. pylori antigen group (P<0.001–0.05). The
level of IL-10 in the groups administered with a vaccine plus an
adjuvant was significantly greater than that of the control and the
H. pylori antigen groups (P<0.001–0.01), and the level of
IL-10 in the group administered with a vaccine plus chitosan as the
adjuvant was identified as significantly greater than that of the
group with CT as the adjuvant (P<0.01). The level of IL-4 in the
group administered with a vaccine plus chitosan as the adjuvant was
significantly greater than that of the control group, the H.
pylori antigen group and the group with CT as the adjuvant
(P<0.001–0.05). The level of IL-10 in the group administered
with a vaccine plus CT as the adjuvant was found to be
significantly greater than that of the control group (Fig. 5; P<0.05).
The ratio of IgG2a to IgG1 in the sera of the groups
administered with a vaccine plus an adjuvant was significantly less
than that of the control and the H. pylori antigen groups
(Fig. 6; P<0.01–0.05).
TLR4 mRNA and protein expression levels
in the gastric mucosa
To determine the role of TLR4 in vaccination therapy
following H. pylori infection, TLR4 mRNA and protein
expression levels in the gastric mucosa were measured by RT-PCR and
immunohistochemical staining. The primer to amplify the 348-bp
β-actin cDNA were as follows: Forward, GAC GAT ATC GCT GCG CTG and
reverse, GTA CGA CCA GAG GCA TAC AGG. The primer to amplify the
438-bp TLR4 cDNA were as follows: Forward, CAG CTT CAA TGG TGC CAT
CA and reverse, CTG CAA TCA AGA GTG CTG AG.
The expression of TLR4 mRNA and the positive cell
scores of TLR4 in the gastric mucosa of the vaccine groups were
found to be significantly greater than those of the control and the
H. pylori antigen groups (Fig.
7A–E; P<0.001).
 | Figure 7TLR4 mRNA and protein expression
levels in the gastric mucosa. (A) *P<0.001 compared
with the control group and the Hp antigen group. (B) Lane M, 100-bp
DNA ladder; lanes 1–4, β-actin; lanes 5–8, TLR4; lanes 1 and 5,
control group; lanes 2 and 6, Hp antigen group; and lanes 3 and 7,
Hp antigen + chitosan solution group; lanes 4 and 8, Hp antigen +
chitosan particle group. (C–E) TLR4 immunohistochemical staining of
the gastric mucosa (magnification, x400). (C) The control group
showed few positive cells. (D) The group with chitosan as the
adjuvant showed abundant positive cells. (E) The group with CT as
the adjuvant showed abundant positive cells. No significant
difference was observed between the group with chitosan as the
adjuvant and the group with CT as the adjuvant (P>0.05). Hp,
H. pylori; CT, cholera toxin. |
Expression levels of Foxp3 mRNA and
protein in the gastric mucosa
To determine the role of Foxp3 in vaccination
therapy following H. pylori infection, the expression levels
of Foxp3 mRNA and protein were measured in the gastric mucosa by
RT-PCR and immunohistochemical staining. In RT-PCR, the
amplification primers were: Forward, 5′-GAC GAT ATC GCT GCG CTG-3′;
and reverse, 5′-GTA CGA CCA GAG GCA TAC AGG-3′ for β-actin (cDNA
length, 348 bp); and forward, 5′-GGC CCT TCT CCA GGA CAG A-3′ and
reverse, 5′-GCT GAT CAT GGC TGG GTT GT-3′ for Foxp3 (cDNA length,
218 bp).
The expression of Foxp3 mRNA and the positive cell
score of Foxp3 in the gastric mucosa of the vaccine groups were
identified to be significantly less than those of the control and
the H. pylori antigen groups (Fig. 8A–F; P<0.001, vaccine groups, vs.
control and H. pylori antigen groups).
Discussion
To investigate the H. pylori therapeutic
vaccine with chitosan as the adjuvant, mice were infected with
H. pylori and vaccinated with an H. pylori protein
vaccine with chitosan as the adjuvant to delineate the therapeutic
effect of the H. pylori vaccine and establish the potential
mechanism against H. pylori infection in comparison with an
H. pylori vaccine with CT as the adjuvant. It was found that
the effect of the H. pylori therapeutic vaccine with
chitosan as the adjuvant was equivalent to the H. pylori
therapeutic vaccine with the traditional mucosal adjuvant, CT.
In the present study, the eradication rate of the
H. pylori vaccine with chitosan as the adjuvant was found to
be 58.33%, which was approximately that of the H. pylori
vaccine with CT as the adjuvant (45.45%), and significantly greater
(P<0.005–0.05) than that of the H. pylori antigen alone
and control groups. These results indicate that chitosan may act as
a substitute for CT as a mucosal adjuvant for a H. pylori
therapeutic vaccine. Furthermore, in mice where the H.
pylori infection had not been eradicated, the density of H.
pylori colonization in the gastric mucosa of the H.
pylori-vaccinated groups with chitosan as the adjuvant was
significantly (P<0.05) less than that of the group with CT as
the adjuvant, demonstrating that the adjuvant activity of chitosan
was stronger than that of CT. Therefore, the present study
demonstrated that the degree of chronic gastritis in the groups
with chitosan as the adjuvant was significantly less than that of
the groups without chitosan as the adjuvant; thus, indicating that
the H. pylori vaccine with chitosan as the adjuvant reduced
H. pylori-induced chronic gastritis.
The effect of humoral immunity in H. pylori
immunotherapy was also investigated in the present study. It was
found that the H. pylori vaccine with chitosan or CT as the
adjuvant elicited anti-H. pylori IgG in mice, which may be
utilized in immunotherapy. Furthermore, chitosan as the adjuvant in
the H. pylori vaccine induced a systemic humoral immune
response. Similarly, in a study of chitosan co-administered with an
influenza virus subunit vaccine, local and serum antibody levels
were observed to be markedly enhanced (9). In the present study, it was found
that the level of anti-H. pylori IgA in saliva and the
gastric mucosa in the groups with chitosan as the adjuvant was
significantly greater than that of the control group and the groups
without any adjuvant (although it was not significantly different
from that of the group with CT as the adjuvant). These results
indicate that the local secretory IgA antibody may correlate well
with the protection function of the H. pylori vaccine.
Similarly, IgA has previously been presented as critical in immune
protection of an H. pylori vaccine with CT as the adjuvant
(10) and immune protection of an
H. felis vaccine with LT as the adjuvant (11).
The levels of Th1 cytokines (IFN-γ and IL-12) and
Th2 cytokines (IL-10 and IL-4) in the groups administered with a
vaccine plus an adjuvant were significantly greater than those of
the control group and the groups without an adjuvant. Furthermore,
it was found that the H. pylori vaccine with chitosan or CT
as the adjuvant significantly increased the levels of Th1 and Th2
cytokines in the gastric mucosa of mice, as well as in the sera.
The increased anti-H. pylori IgG2a and IgG1 levels may
induce the mixed immune response of Th1 and Th2. In addition, the
ratio of IgG2a to IgG1 in the serum of the groups with chitosan as
the adjuvant was found to be less than that of the group with CT as
the adjuvant. In addition, the levels of Th2 cytokines,
particularly IL-4, in the gastric mucosa of the groups with
chitosan as the adjuvant were significantly greater than those of
the group with CT as the adjuvant. These results reveal that the
H. pylori vaccine with chitosan as the adjuvant may recover
Th2 immunity that is suppressed by the H. pylori infection,
as well as regulate the balance of Th1 and Th2 inflammatory cells,
thus facilitating clearance of the H. pylori infection. A
previous study reported that IFN-γ, produced by Th1 cells, induced
an increase of the major histocompatibility complex II antigen to
recruit inflammatory cells to the gastric mucosa to damage the
mucosal tissue, which benefited the clearance of H. pylori
to a certain degree (12). The
H. pylori vaccine with Freund's adjuvant was identified to
be more effective against the H. pylori infection by
inducing the mixed reaction of Th1 and Th2 rather than only
inducing the reaction of Th2 (13). It was demonstrated in the present
study that the effective treatment of H. pylori infection by
the H. pylori vaccine was closely associated with Th1 and
Th2 cells. Therefore, chitosan as the adjuvant in the H.
pylori vaccine may be more effective than CT as the adjuvant
for the immunotherapy of H. pylori infection, as it is less
toxic.
In the present study, following therapeutic
vaccination, the expression of TLR4 mRNA in the gastric mucosa and
the number of TLR4-positive cells were found to be significantly
increased. The low response to H. pylori in the gastric
epithelial cells may be corrected and the H. pylori immune
tolerance might be damaged, which may benefit clearance of the
H. pylori infection. Furthermore in the present study, it
was observed that in the vaccination group, the expression of TLR4
mRNA in the gastric mucosae of the mice where the H. pylori
infection had been eradicated was significantly greater than that
of the mice where the H. pylori infection had not been
eradicated. This indicates that TLR4 may benefit the
antibody-mediated H. pylori clearance, which is critical in
H. pylori vaccine immunotherapy. This result is in
accordance with a previous study in which TLR4 was determined to be
essential for antibody-mediated clearance of bacteria (14).
The effect of regulatory T cells in H. pylori
immunotherapy was also investigated in the present study. The
changes of Foxp3 mRNA and Foxp3-positive cells in the gastric
mucosa following vaccination were analyzed and Foxp3 mRNA and the
number of Foxp3-positive cells in the gastric mucosa were observed
to be significantly reduced. Vaccination reduced the level of
CD4+CD25+Foxp3+Treg in the gastric
mucosa, relieved the immune response depression and damaged the
H. pylori immune tolerance, thus benefiting the H.
pylori infection clearance. In addition, it was found that the
expression of Foxp3 mRNA in the gastric mucosa of mice where the
H. pylori infection had been eradicated was significantly
less than that in the mice where the H. pylori infection had
not been eradicated. Previous studies with an H. pylori
infection mouse model demonstrated that a certain degree of
inflammation is required to reduce the bacterial load in the
stomach and that the absence of regulatory T cells is associated
with increased gastric inflammation and a decreased bacterial load
(15–17), indicating that the enhancement of
Treg, induced by H. pylori infection, may inhibit the immune
response. It was found in the present study that Treg, in
CD25-depleted mice, reduced H. pylori loads in the H.
pylori-infected gastric mucosa, increased the numbers of
mucosal T cells, B cells and macrophages, and increased the titers
of H. pylori-specific IgG1 and IgG2a antibodies. These
results indicated that the depression of Treg favored H.
pylori clearance. Therefore, after H. pylori vaccine
inoculation, the significant depression of
CD4+CD25+Foxp3+Treg in the gastric
mucosa may participate in the process of H. pylori infection
clearance.
In conclusion, the H. pylori vaccine with
chitosan as the adjuvant was found to effectively increase the
H. pylori elimination rate. Furthermore, the H.
pylori vaccine with chitosan or CT as the adjuvant induced
specific anti-H. pylori IgA in the gastric mucosa, and
non-specific secretory IgA and specific anti-H. pylori IgG
in the sera. In addition, the H. pylori vaccine with
chitosan or CT as the adjuvant promoted Th1 and Th2 cytokines, and
decreased the ratio of IgG2a to IgG1. The H. pylori vaccine
with chitosan as the adjuvant effectively increased the humoral
immune response, the Th1 and Th2 cell immune reaction, as well as
balancing the Th1 and Th2 response; all of which may contribute to
clearance of the H. pylori infection. Furthermore, following
H. pylori vaccination, the TLR4 expression in the mouse
gastric epithelial cells increased and the number of
CD4+CD25+Foxp3+Treg decreased,
which may influence the process of H. pylori infection
clearance. The effect of the H. pylori therapeutic vaccine
with chitosan as the adjuvant was equivalent to the H.
pylori therapeutic vaccine with the traditional mucosal
adjuvant, CT. Thus, these findings may promote the use of chitosan
as an adjuvant for the H. pylori therapeutic vaccine.
Acknowledgments
The present study was financially supported by the
National Natural Science Foundation of China (grant no.
30460052).
References
|
1
|
Sijun H and Yong X: Helicobacter pylori
vaccine: mucosal adjuvant & delivery systems. Indian J Med Res.
130:115–124. 2009.PubMed/NCBI
|
|
2
|
Barrette RW, Szczepanek SM, Rood D, et al:
Use of inactivated Escherichia coli enterotoxins to enhance
respiratory mucosal adjuvanticity during vaccination in swine. Clin
Vaccine Immunol. 18:1996–1998. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Synowiecki J and Al-Khateeb NA:
Production, properties, and some new applications of chitin and its
derivatives. Crit Rev Food Sci Nutr. 43:145–171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Lubben IM, Verhoef JC, Borchard G
and Junginger HE: Chitosan and its derivatives in mucosal drug and
vaccine delivery. Eur J Pharm Sci. 14:201–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Min M, Du N, Gu Y, Hode T, Naylor M,
Chen D, Nordquist RE and Chen WR: Chitin, chitosan, and glycated
chitosan regulate immune responses: the novel adjuvants for cancer
vaccine. Clin Dev Immunol. 2013:3870232013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang ML, Kang SG, Jiang HL, et al: In vivo
induction of mucosal immune responses by intranasal administration
of chitosan microspheres containing Bordetella bronchiseptica DNT.
Eur J Pharm Biopharm. 63:215–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie Y, Zhou NJ, Gong YF, et al: The immune
response induced by H. pylori vaccine with chitosan as an adjuvant
and its relation to immune protection. World J Gastroenterol.
13:1547–1553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong YF, Xie Y, Zhou NJ, et al: Cellular
immunity induced by H. pylori vaccine with chitosan as an adjuvant.
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 23:595–599. 2007.In Chinese.
PubMed/NCBI
|
|
9
|
Bacon A, Makin J, Sizer PJ, et al:
Carbohydrate biopolymers enhance antibody responses to mucosally
delivered vaccine antigens. Infect Immun. 68:5764–5770. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goto T, Nishizono A, Fujioka T, Ikewaki J,
Mifune K and Nasu M: Local secretory immunoglobulin A and
postimmunization gastritis correlate with protection against
Helicobacter pylori infection after oral vaccination of mice.
Infect Immun. 67:2531–2539. 1999.PubMed/NCBI
|
|
11
|
Lee CK, Weltzin R, Thomas WD Jr, et al:
Oral immunization with recombinant Helicobacter pylori urease
induces secretory IgA antibodies and protects mice from challenge
with Helicobacter felis. J Infect Dis. 172:161–172. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saldinger PF, Porta N, Launois P, et al:
Immunization of BALB/c mice with Helicobacter urease B induces a T
helper 2 response absent in Helicobacter infection.
Gastroenterology. 115:891–897. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenberg JC, Czinn SJ, Garhart CA, et al:
Protective efficacy of anti-Helicobacter pylori immunity following
systemic immunization of neonatal mice. Infect Immun. 71:1820–1827.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Litzinger MT, Fernando R, Curiel TJ,
Grosenbach DW, Schlom J and Palena C: IL-2 immunotoxin denileukin
diftitox reduces regulatory T cells and enhances vaccine-mediated
T-cell immunity. Blood. 110:3192–3201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nair S, Boczkowski D, Fassnacht M,
Pisetsky D and Gilboa E: Vaccination against the forkhead family
transcription factor Foxp3 enhances tumor immunity. Cancer Res.
67:371–380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson BD, Jing W and Orentas RJ:
CD25+ regulatory T cell inhibition enhances
vaccine-induced immunity to neuroblastoma. J Immunother.
30:203–214. 2007. View Article : Google Scholar : PubMed/NCBI
|