Introduction
Systemic lupus erythematosus (SLE) is a complex
autoimmune disease, the origin of which remains to be elucidated.
It is considered to affect virtually every organ in the human body
(1). SLE is primarily caused by
autoantibodies and immune complex (IC) deposition. Enhanced
apoptosis in conjunction with the defective clearance of apoptotic
cells results in high levels of autoantibodies (2). Lupus nephritis (LN) is a major cause
of morbidity and mortality in patients with SLE (3). The general consensus is that 60% of
patients with lupus may develop clinically relevant nephritis at a
certain time-point during the course of their illness (4). The prompt recognition and treatment
of renal disease is important, as an early response to therapy is
correlated with an improved outcome (5). Similar to other inflammatory and
autoimmune conditions, the pathogenic processes which contribute to
SLE involve complex interactions between immunocytes and
nonimmunocytes, including endothelial and epithelial cells, through
a number of receptor-ligand systems (1).
Signal transduction through CD28 is important in
regulating the initial response of a T cell to an antigen (6). To date, two distinct CD28 ligands,
B7-1 (CD80) and B7-2 (CD86), have been identified (7). These ligands also bind to cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4), a receptor closely
associated with CD28, which is expressed on activated T cells
(7). B7-1 is expressed on the
surface of antigen-presenting cells (APCs), and its combination to
the receptor CD28 is able to generate co-stimulatory signals, which
are essential in the primary immune response (8). Without the co-stimulatory signal, T
cells enter into a state of anergy, tolerance and even apoptosis
(9). By contrast, the
hyper-reaction of the B7/CD28 signal is closely associated with the
occurrence of autoimmune diseases (10).
RNA interference (RNAi) is a process that is
mediated by double stranded (ds)RNA dependent on small interfering
RNA (siRNA) of 21–23 nucleotides, which is able to silence the gene
expression of specific genes. It is also termed
post-transcriptional gene silencing, as it is able to suppress
target gene expression quickly, specifically and efficiently
(11). It is well-established that
specific antibody binding to an antigen is able to inhibit or
promote various biological effects (12). Neutralization of B7 with anti-B7
antibody inhibits or downregulates its binding to its receptor CD28
(13) and therefore, delays the
activation and immune response of B and T cells.
The present study was designed to determine the
putative effect of preventative therapy with B7-1 short hairpin RNA
(shRNA) or neutralizing anti-B7 antibody on a murine model of LN
and to examine the potential mechanisms through which this therapy
may be acting to improve clinical outcomes. C57BL6 J mice were
treated with either B7-1 shRNA or neutralizing anti-B7 antibody to
examine the implications of these results in the understanding of
LN and the possible roles for B7/CD28.
Materials and methods
Reagents and antibodies
Pristane was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Phycoerythrin (PE)-conjugated monoclonal
anti-mouse CD11b (cat. no. 12-0112), CD11c (cat. no. 12-0114),
Myeloid differentiation antigen, Ly-6G (Gr1; cat. no. 12-5931) and
CD21 (cat. no. 12-0212) antibody, allophycocyanin (APC)-conjugated
monoclonal anti-mouse major histocompatibility complex (MHC)-II IgG
(cat. no. 17-5321), fluorescein isothiocyanate (FITC)-conjugated
monoclonal anti-mouse B7-1 and CD86 IgG (cat. nos. 11-0801 and
11-0862) and mouse interleukin (IL)-4 and interferon (IFN)-γ ELISA
ready-set-go kit were purchased from eBioscience (San Diego, CA,
USA), FITC-conjugated goat anti mouse immunoglobulin G (IgG)
antibody were obtained from Jackson ImmunoResearch Labs (West
Grove, Pennsylvania, USA).
Cell culture
293T, L929 and K-562 cell lines (American Type
Culture Collection, Manassas, VA, USA) were grown in RPMI-1640
(Life Technologies, Thermo Fisher Scientific, Grand Island, NY,
USA) culture medium containing 10% fetal bovine serum (FBS;
HyClone, Logan, UT, USA), 100 μg/ml streptomycin and 100
U/ml penicillin (Sigma-Aldrich). The cells were maintained at 37°C
with 5% CO2 and were progressively passaged every two
days.
Animals
All procedures were approved by the Guideline for
the Care and Use of Laboratory Animals on the Chinese Medical
Academy and the Soochow University Animal Care Committee (Suzhou,
China). Specific pathogen-free C57BL/6 J female mice (7–8 weeks
old; weighing 20–25 g) were obtained from Shanghai Laboratory
Animal Centre (Shanghai, China) and were maintained in our animal
facility under specific pathogen-free conditions. A 12-h day/night
cycle was maintained during the entire course of the study at
18–22° and 50–60% humidity. Animals were housed in groups of five,
fed a standard diet and had ad libitum access to water.
Mouse anti-human B7-1 monoclonal antibody
(mAb) production
The hybridoma cell line from our laboratory
(14), which had been observed to
secrete anti-human B7-1 antibody with the highest titers was mass
cultured for hybridoma injection at the 5th-6th passage. A total of
20 BALB/c mice (female, 6–8 weeks old) were intraperitoneally
injected with sterile paraffin oil (0.5 ml per mouse) seven days
prior to hybridoma injection. Each mouse was injected with
1–2×106 hybridoma cells. After 7–10 days, ascites were
collected and centrifuged at 10,000 × g for 30 min to obtain the
supernatant. The supernatant of the ascites was further purified
with a protein G sepharose 4B column using a fast protein liquid
chromatography system (GE Pharmacia, Ramsey, MN, USA) according to
the manufacturer's instructions. The purity and concentration of
the purified mAb was analyzed using 10% SDS-PAGE (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and the Bradford protein
assay (15), respectively.
Lentivirus-mediated shRNA knockdown of
B7-1 gene expression
The recombinant lentivirus was constructed according
to the manufacturer's instructions with certain modifications.
Briefly, four pairs of shRNA fragments were hybridized with
synthesized sense and anti-sense oligonucleotides (Table I). This hybridized B7-1 shRNA
fragment was cloned into the pGLV-green fluorescent protein (GFP)
plasmid. 293T cells were co-transfected with 4 μg pGLV-GFP,
4 μg pLV/Helper-SL3, 4 μg pLV/Helper-SL4 and 4
μg pLV/Helper-SL5 along with 40 μl Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA). This recombinant
lentivirus was designated B7-1 lenti. The titers of B7-1 lenti were
determined with a GFP assay. Negative control lenti contained a
scrambled shRNA fragment, an irrelevant shRNA with random
nucleotides with a GC ratio similar to that of the B7-1 shRNA. When
the L929 cell line reached 60–70% confluence, lentiviral vectors
were added to the culture (serum-free medium) at a multiplicity of
infection of 20, 40 and 60 in the presence of 5 ng/ml polybrene.
Half of the medium was replaced every other day and the cultures
were examined by fluorescent microscopy (IX71; Olympus, Tokyo,
Japan) two days following transfection.
 | Table ISequences of the four pairs of shRNA
fragments used in the present study |
Table I
Sequences of the four pairs of shRNA
fragments used in the present study
| shRNA | Sequence |
|---|
| shRNA-256 |
| Forward |
5′-GATCCGCAATTGTCAGTTGATGCAGGTTCAAGAGACCTGCATCAACTGACAATTGCTTTTTTG-3′ |
| Reverse |
5′-AATTCAAAAAAGCAATTGTCAGTTGATGCAGGTCTCTTGAACCTGCATCAACTGACAATTGCG-3′ |
| shRNA-409 |
| Forward |
5′-GATCCGCCGTTACAACTCTCCTCATGTTCAAGAGACATGAGGAGAGTTGTAACGGCTTTTTTG-3′ |
| Reverse |
5′-AATTCAAAAAAGCCGTTACAACTCTCCTCATGTCTCTTGAACATGAGGAGAGTTGTAACGGCG-3′ |
| shRNA-620 |
| Forward |
5′-GATCCGGAAAGAGGAACGTATGAAGTTTCAAGAGAACTTCATACGTTCCTCTTTCCTTTTTTG-3′ |
| Reverse |
5′-AATTCAAAAAAGGAAAGAGGAACGTATGAAGTTCTCTTGAAACTTCATACGTTCCTCTTTCCG-3′ |
| shRNA-807 |
| Forward |
5′-GATCCGGCATCAATACGACAATTTCCTTCAAGAGAGGAAATTGTCGTATTGATGCCTTTTTTG-3′ |
| Reverse |
5′-AATTCAAAAAAGGCATCAATACGACAATTTCCTCTCTTGAAGGAAATTGTCGTATTGATGCCG-3′ |
Establishment of pristane-induced lupus
model
C57BL/6 J mice were inoculated once with 0.5 ml
pristane (2,6,10,14-tetramethyl-pentadecane; Sigma-Aldrich). The
mice were divided into four groups: B7-1 antibody, IgG isotype,
B7-1 shRNA and wild-type group. The B7-1 antibody mice were treated
with 200 μg B7-1 antibody through an intravenous injection
in the tail on day 1, 3, 5, 8 and 15 every month for three months
after the pristane injection. The IgG isotype mice were treated
with IgG control in parallel to the B7-1 antibody mice. The B7-1
shRNA mice were treated with 0.4×108 TU LV-B7-1 shRNA on
days 1 and 60 through an intravenous injection in the tail
following pristane injection. The mice were bled every month
following pristane inoculation and the sera were frozen for
analysis of the autoantibodies. All mice were monitored for
proteinuria once a month and were sacrificed via cervical
dislocation at 8 or 10 months to harvest their kidneys. Kidney
disease was assessed in mice treated with B7-1 antibody, B7-1 shRNA
and in wild-type littermates. Proteinuria was measured on a 0–4
scale using a colorimetric assay strip for albumin (Albustix;
Bayer, Elkhart, IN, USA), with scoring performed as follows: 0,
(absent); 1, 30 mg/dl (mild); 2, 100 mg/dl (moderate); 3, 300 mg/dl
(moderate to severe); and 4, 2000 mg/dl (severe). The experiment
included 60 mice, which were allocated into six groups (10 mice per
group).
Phenotypic spleen population analysis by
flow cytometry
For the collection of spleen cells, tissue of the
spleen was gently scraped off with scissors. Cell suspensions were
then passed through a nylon filter with 100-μm pore size
(Merck Millipore, Darmstadt, Germany). The spleen cells were
pelleted by centrifugation at 1,000 × g for 5 min and then
re-suspended in ammonium chloride-potassium lysis buffer (Amersham
Pharmacia Biotech, Piscataway, NJ, USA) to remove erythrocytes.
Following centrifugation for 10 min at 1,200 × g, the spleen cells
were washed twice with RPMI-1640 medium with 5% FBS. The cells were
co-stained with PE-conjugated monoclonal antimouse CD11b IgG and
FITC-conjugated anti-B7-1 antibody, PE-conjugated anti-CD11c
antibody and FITC-conjugated anti-B7-1 antibody, or PE-conjugated
anti-CD21 antibody and FITC-conjugated anti-B7-1 antibody,
respectively. In other experiments, the spleen cells were stained
with PE-conjugated anti-CD11b antibody, PE-conjugated anti-CD11c
antibody, PE-conjugated anti-CD21 antibody, PE-conjugated anti-Gr1
antibody, respectively, or co-stained with PE-conjugated anti-CD21
antibody and FITC-conjugated anti-CD86 antibody, or PE-conjugated
anti-CD21 antibody and FITC-conjugated anti-APC-MHC-II antibody,
respectively. Stained cells were analyzed using a BD FACSCalibur
flow cytometer (BD Biosciences, Mountain View, CA, USA) and
CellQuest software version 1.0 (BD Biosciences).
Renal histology
Paraffin sections (4-μm thick) of kidneys
fixed in 4% paraformaldehyde were stained with hematoxylin and
eosin at room temperature for 10 min and 5 min, respectively
(Sigma-Aldrich). The stained sections were scored for the following
features on a 0–3 scale in a blinded manner: i) Glomerular activity
score (GAS), which included glomerular proliferation, karyorrhexis,
fibrinoid necrosis, cellular crescents, inflammatory cells and
hyaline deposits; ii) tubulointerstitial activity score (TIAS),
which included interstitial inflammation, tubular cell pyknosis,
nuclear activation, cell necrosis and cell flattening, and
epithelial cells or macrophages in tubular lumens; iii) chronic
lesions score (CLS), which included glomerular scars,
glomerulosclerosis, fibrous crescents, tubular atrophy, and
interstitial fibrosis and iv) vascular lesion score, which included
arterial/arteriolar lesions. The raw scores assigned by various
readers were averaged to obtain a mean score for each of the
individual features. The mean scores for individual features were
calculated to obtain the four main scores (GAS, TIAS, CLS and
vascular lesion score) and then all four scores were summed to
determine the composite kidney biopsy score.
Renal immunostaining
Frozen kidney sections were fixed with methanol and
acetone (1:1) for 5 min. The slides were then washed and incubated
with FITC-conjugated goat anti-mouse IgG (Sigma-Aldrich). The
slides were assessed by three individuals in a blinded manner.
Detection of autoantibodies against
anti-nuclear antibody (A NA) and double stranded (dsDNA)
ANA and dsDNA levels were detected by indirect
immunofluorescence according to the manufacturer's instructions
(Euroimmun, Lübeck, Germany). Undiluted samples were layered on ANA
slides and incubated for 30 min. Following washing,
fluorescein-labeled anti-mouse globulin was added to each slide and
incubated for a further 30 min. The slides were then embedded with
Floursave mounting reagent (Merck Millipore) and examined under a
fluorescence microscope (Olympus). Fluorescence intensity was
scored as follows: 4+, (very bright green), 3+, (bright green), 2+,
(green) and 1+, (faint green).
Detection of cytokines IL-4 and IFN-γ in
sera
Mice sera were collected eight months following
pristane injection. A standard sandwich ELISA was used to measure
cytokines IL-4 and IFN-γ in the sera. IL-4 and IFN-γ levels were
measured using the mouse cytokine ready-set-go kit according to the
manufacturer's instructions.
Scanning electron microscopy (SEM)
The renal specimens were collected and fixed in 2.5%
glutaraldehyde buffer (pH 7.4) for 2 h The renal specimens were
washed twice with phosphate buffered saline (Bio-Rad Laboratories,
Inc.) for 15 min and then dehydrated using serial dilutions of
alcohol (50, 70, 80, 90, 95, 99 and 100%). Critical point drying of
specimens was performed with liquid CO2 and specimens
were sputter-coated with gold and examined using SEM (S-450;
Hitachi Ltd., Tokyo, Japan).
Statistical analysis
All results are expressed as the mean ± standard
deviation. Levels of antibodies and cytokines, lymphocyte
percentages and quantities, as well as renal scores were compared
using Student's t-test or the Mann-Whitney U test. Frequencies of
antibodies and proteinuria were compared using the two-sided
Fisher's exact test. Statistical calculations were performed with
Prism 5 software (GraphPad Software, La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
B7-1 deficiency reduces proteinuria
production
To determine whether the B7-1/CD28 signaling pathway
is involved in the development of pristane-induced lupus, the
B7-1/CD28 pathway was blocked in mice by treatment with
neutralizing anti-mouse B7-1 antibody or B7-1 shRNA. All mice were
bled prior to and at four and eight months post-inoculation of
pristane and monitored for proteinuria. Proteinuria developed late
and was alleviated in the pristane-inoculated B7-1 antibody or B7-1
shRNA mice as compared with that in the control littermates
(Table II). At eight months
post-inoculation, 50% of the mice in the control group, but none in
the B7-1 antibody or B7-1 shRNA group, exhibited 100 mg/dl
(moderate to severe) proteinuria (P<0.05). The frequency of
moderate to severe proteinuria remained high in control mice at 10
months (P<0.005). None of the B7-1 antibody or B7-1 shRNA mice,
but 63% of the control mice developed severe (300 mg/dl)
proteinuria at 10 months post-inoculation (P<0.05).
 | Table IIComparison of the degree of
proteinuria at 10 months post-inoculation (n=10). |
Table II
Comparison of the degree of
proteinuria at 10 months post-inoculation (n=10).
| Group | − | ± | + | ++ | +++ | ++++ |
|---|
| Negative
control | 7 | 2 | 1 | 0 | 0 | 0 |
| Model control | 0 | 0 | 0 | 1 | 5 | 3 |
| Lentivrius
control | 0 | 0 | 0 | 3 | 4 | 2 |
| B7-1
lentivriusa | 0 | 2 | 1 | 5 | 2 | 0 |
| IgG isotype
control | 0 | 0 | 0 | 3 | 3 | 3 |
| B7-1
Absa,b | 0 | 3 | 5 | 2 | 0 | 0 |
B7-1 deficiency reduces pristane-induced
autoantibody production
Pristane-inoculated BALB/c mice have been observed
to develop autoantibodies to several cellular antigens (Ags)
(16,17). These antibodies were detected with
an immunoprecipitation assay using a cell extract from the K-562
erythroleukemia cell line as a source of autoantigens. Generally,
reactivity to cellular Ags was lower in the sera from
pristane-inoculated B7-1 antibody mice and B7-1 shRNA mice than
that in the sera from pristane-inoculated control mice (Table III). Serum IgG anti-dsDNA
antibody levels, as measured by ELISA, were also lower in the B7-1
antibody mice and B7-1 shRNA mice those in the control mice at six
months post-inoculation (P<0.05; Table IV).
 | Table IIITiter of anti-nuclear antibody in the
sera at six months post-pristane inoculation (n=10). |
Table III
Titer of anti-nuclear antibody in the
sera at six months post-pristane inoculation (n=10).
| Group | − | + | ++ | +++ |
|---|
| Negative
control | 9 | 1 | 0 | 0 |
| Model control | 0 | 1 | 1 | 7 |
| Lentivrius
control | 0 | 2 | 2 | 5 |
| B7-1
lentivriusa | 1 | 4 | 4 | 1 |
| IgG isotype
control | 0 | 1 | 3 | 5 |
| B7-1
Absa,b | 3 | 6 | 1 | 0 |
 | Table IVTiter of anti-double-stranded DNA
antibody in the sera at six months post-pristane inoculation
(n=10). |
Table IV
Titer of anti-double-stranded DNA
antibody in the sera at six months post-pristane inoculation
(n=10).
| Group | − | + | ++ | +++ |
|---|
| Negative
control | 9 | 1 | 0 | 0 |
| Model control | 1 | 1 | 2 | 5 |
| Lentivrius
control | 2 | 1 | 2 | 4 |
| B7-1
lentivriusa | 2 | 3 | 3 | 2 |
| IgG isotype
control | 1 | 2 | 2 | 4 |
| B7-1 Absa | 3 | 3 | 2 | 2 |
B7-1 deficiency alleviates IC
deposits
As shown in Fig. 1,
immunofluorescence revealed that IC deposits in the renal glomeruli
of the mice administered with B7-1 antibody or B7-1 shRNA were
fewer than in those of the control mice.
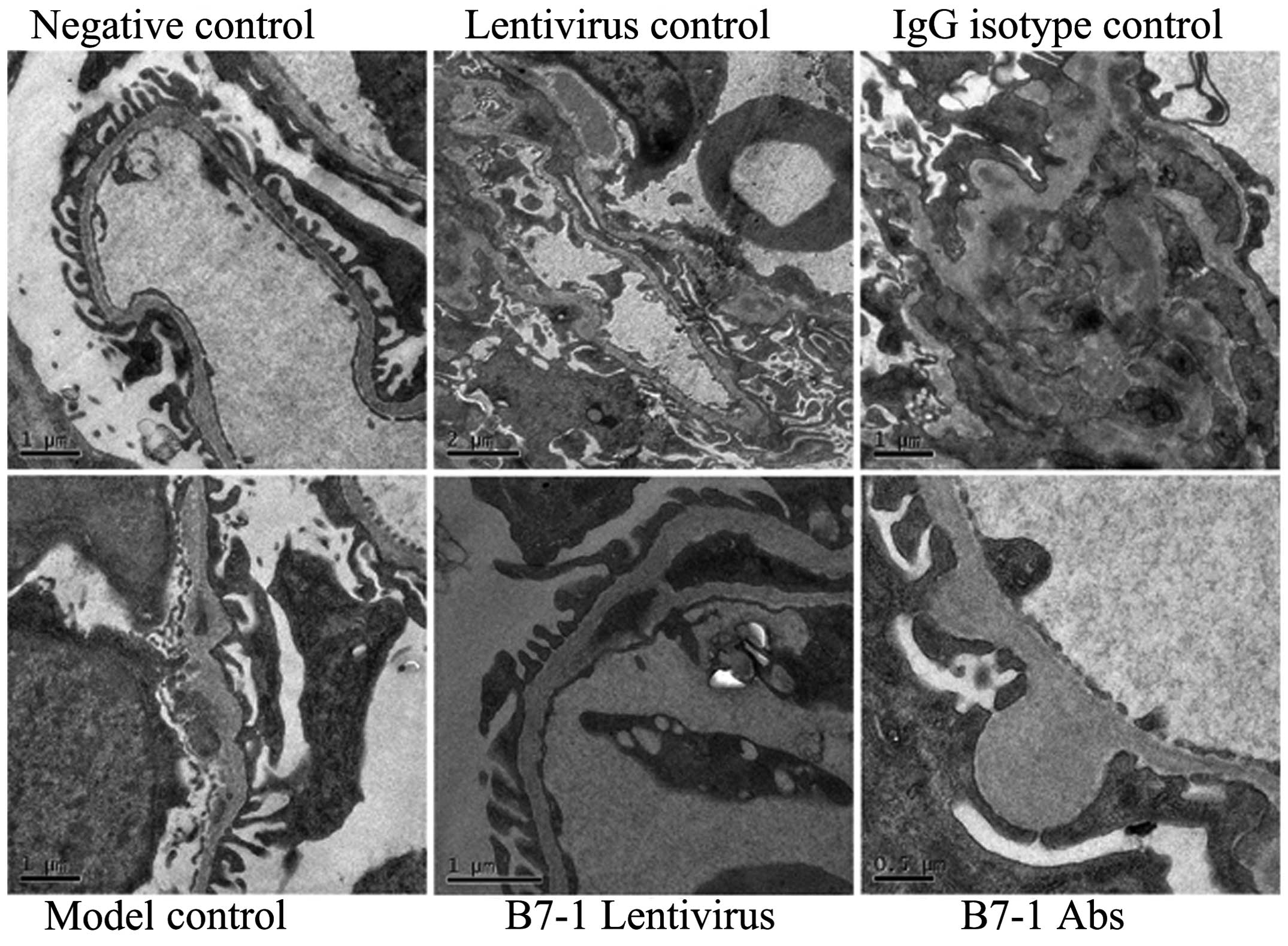
Analysis of renal ultrastructure
SEM showed electron-dense deposits in mouse
glomerular endothelial cells in all model groups, and
electron-dense deposits also formed in certain basement membranes
(Fig. 2). Fused podocytes were
observed in numerous glomeruli and false chorion were formed on the
surface of podocytes, combined with basement membrane thickening.
However, electron-dense deposits in the mouse glomerular
endothelial cells in B7-1 lentivirus and B7-1 antibody treatment
groups were fewer than those in the model control group (Fig. 2).
B7-1 deficiency alleviates renal
lesions
The mice were sacrificed at 10 months to harvest the
kidneys and their renal histology was assessed (Fig. 3). Mild and focal
mesangio-proliferative glomerulonephritis was observed in the
majority of pristane-inoculated C57/BJ mice (Fig. 3) by light microscopy (CX41-12C02;
Olympus); however, none of the B7-1 antibody or B7-1 shRNA mice had
diffuse proliferative or chronic lesions. In the control group,
however, >60% of the mice developed diffuse proliferative
glomerulonephritis with fibrous crescents, glomerulosclerosis,
tubular atrophy and interstitial fibrosis (Fig. 3). Another pristane-inoculated
control mouse exhibited mild to moderate mesangio-proliferative
lesions.
Cytokine IL-4 and IFN-γ responses in
B7-1-deficient mice
Abnormalities in cytokine production contributes to
the development of lupus (18). To
determine whether the effects of B7-1/CD28 on the development of
lupus are associated with abnormalities in cytokine production, the
cytokine responses in the blood of pristane-inoculated B7-1
antibody, B7-1 shRNA and control mice were measured. As expected,
IL-4 and IFN-γ levels were significantly decreased in the B7-1
antibody and B7-1 shRNA mice, as compared with those in the control
mice. Such a cytokine profile, including increased type 1
cytokines, may contribute to the alleviation of autoimmune disease
in B7-1 antibody and B7-1 shRNA mice, respectively (Fig. 4).
Infiltration cell functions and
quantities are reduced in B7-1 deficient mice
To assess whether the function of immune cells is
also compromised in the B7-1 signaling pathway, spleen cells from
control- or pristane-inoculated B7-1 antibody and B7-1 shRNA mice
were analyzed for the activation and memory markers CD11b, CD11c,
CD21, Gr1, CD86 and APC-MHC-II using flow cytometry (Fig. 5). Significant decreases in the
induction of CD11b, CD11c, Gr1 and CD21 as well as CD86 and APC-MHC
II on B cells were observed at six months post-inoculation in the
B7-1 antibody and B7-1 shRNA treatment groups (P<0.05; Fig. 5), but no significant difference was
observed at earlier time-points, including 12 h and 10–12 days
post-inoculation (data not shown).
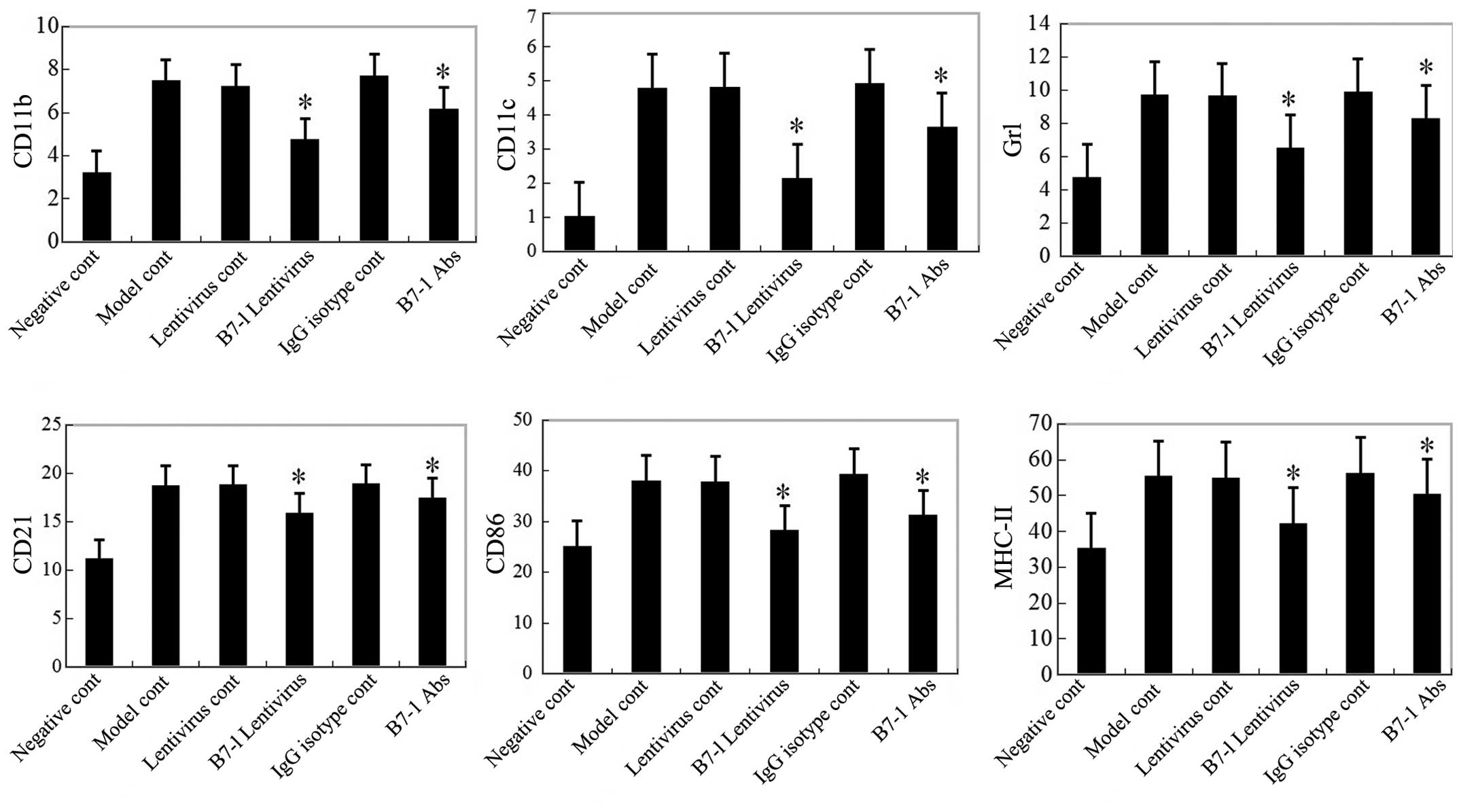 | Figure 5Infiltrating cell functions and
numbers are reduced in B7-1-deficient mice six months following
pristane inoculation. Expression of CD11b, CD11c, Gr1 and CD21 on
cells in spleens from each group were detected using FACS. CD86 and
MHC-II expression in CD21+ B cells from spleens of each
group were also detected using FACS. *P<0.05 compared with the
model control group, the expression of CD11b, CD11c, Gr1 and CD21
on cells in the spleen and the expression of CD86 and MHC-II on
CD21+ B cells were all markedly inhibited in the B7-1
lentivirus-transfected group (473±0.48, 2.13±0.71, 6.50±1.58,
15.87±0.87, 28.21±1.32 and 42.26±3.17%) and B7-1 antibody treatment
group (6.15±1.26, 3.65±0.88, 8.32±1.06, 1745±0.97, 31.21±2.35 and
50.26±3.25%). FACS, fluorescence-activated cell sorting; MHC, major
histocompatibility complex; IgG, immunoglobulin G; Abs, antibodies;
MHC, major histocompatibility complex; cont, control. |
Discussion
B7-1, which is expressed on the APC surface, is an
important co-stimulatory molecule (19). Its receptor, CD28/CTLA-4 is
expressed on T cells. The B7-1/CD28 signaling pathway is important
in regulating the immune responses for the promotion of T-cell
proliferation, T-helper (Th)1 and Th2 differentiation, antibody
production and Ig type conversion, amongst others. However,
excessive activation of the B7-1/CD28 signaling pathway may induce
autoimmune diseases (20).
Blocking this pathway may inhibit T- or B-cell responses, or even
induce immune tolerance (21). The
methods of inhibiting the B7/CD28 signaling pathway include the
application of antisense oligonucleotides (19,22),
gene knockout and monoclonal antibody blocking (23,24).
RNAi technology is currently widely used in
molecular biology and cell biology. It is able to quickly and
specifically inhibit the expression of target genes, therefore it
has been used simply and effectively in gene knockout studies
(25). In the present study, a
murine B7-1 gene RNAi lentiviral vector was successfully
constructed. It was able to effectively silence the L929
membrane-type molecule B7-1 to 73.2%. In addition, the functional
mouse anti-human B7-1 monoclonal antibody, 4E5 was also prepared
for comparison with the B7-1 shRNA. In the present study, the
immunofluorescence and flow cytometry results revealed that in the
B7-1 shRNA lentivirus infection g r o u p, C D11b
+B7-1+, CD11c+B7-1+ and
CD21+B7-1+ cells in the spleen were
significantly decreased compared with those in the control group.
It was indicated that a recombinant lentivirus was able to
effectively interfere with the APC surface expression of the B7-1
molecule.
Previous studies have revealed that T lymphocytes,
in particular Th cells, are able to promote the proliferation and
activation of B lymphocytes through the secretion of cytokines,
resulting in a large number of pathogenic autoantibodies and ICs,
leading to organ damage (26,27).
Therefore, it is important in LN occurrence and development. In
recent years, there have been a number of studies on the
correlation between the balance of Thl/Th2 cells and SLE, or
Thl/Th2 abnormal cytokine secretion and SLE. However, this
correlation remains to be fully elucidated. Lazarus et al
(28) and Hasegawa et al
(29) observed that in humans and
in a mouse model of lupus, when Th1 cell secretion of IFN-γ was
increased, the development of the disease was more damaging, while
Th2-cell secretion had the inverse function. Amel-Kashipaz et
al (30) have indicated that
the Th2-cell proportion in the peripheral blood of patients with
SLE was significantly higher than that in the peripheral blood of
normal control individuals. The authors suggested that Th2
dominance was correlated with the pathogenesis of SLE. The Th2
proportion skew may be due to the inhibition to Th1 proliferation
and activation, Thl-type cytokine production or Thl cytokine gene
transcription by cytokines secreted by Th2 (30,31).
Szegedi et al (32)
identified that Thl and Th2-type cytokine levels in the serum of
patients with SLE were elevated and that Th1 and Th2 may function
together in SLE. Consistent with the study by Szegedi et al
(32), the present study revealed
that in the model group, IL-4 and INF-γ levels in mouse serum were
significantly higher than those in the normal control group. In the
lentiviral interference group and B7-1 antibody early intervention
group, IL-4 and INF-γ levels in mouse serum were significantly
lower than those in the model group. Thus, it was suggested that
mouse B7-1 shRNA lentivirus and a B7-1 antibody are able to inhibit
the production of inflammatory cytokines, including IL-4 and
INF-γ.
In the present study, it was identified that mouse
B7-1 shRNA lentiviral vector intervention at the gene level or B7-1
monoclonal antibody intervention at the protein level were able to
effectively inhibit the early activation of immune cells, reduce
ANA antibody and proteinuria production and decrease IC deposition,
thereby reducing renal pathological damage. However, the B7-1
monoclonal antibody was more effective than the B7-1 shRNA
lentivirus. A possible reason for this difference may be that B7-1
antibodies directly bind and neutralize the antigen of B7-1, which
is able to quickly react and affect inflammation and immune cell
activation. However, the lentiviral vector interference effect was
slower due to several indirect steps. Firstly, a delivery system is
required to efficiently and safely transport siRNA for the target
gene into the associated tissues or organs. Secondly, the effect of
siRNA is generally considered to be sequence-specific; however, the
effect is occasionally non-specific. Finally, the RNAi system may
activate the IFN system and induce non-specific mRNA, which is not
biologically efficient. With RNAi technology improving, such issues
are likely to be resolved in the near future (33).
In conclusion, specific RNAi and antibodies are able
to inhibit B7-1-mediated signaling pathways, thereby reducing the
activation of immune cells and reversing pathological damage in a
lupus nephritis model. At present, increasing numbers of treatments
for SLE are under investigation (34); however, inhibition of
co-stimulatory molecules is expected to present an efficacious,
specific and non-toxic treatment for the biological intervention
against SLE.
Acknowledgments
The present study was supported by the Foundation of
the National Basic Research Program of the Ministry of Science and
Technology (grant no. 2009ZX09103-705) and the National Natural
Science Foundation of China (grant no. 81373236).
References
|
1
|
Tenbrock K, Juang YT, Kyttaris VC and
Tsokos GC: Altered signal transduction in SLE T cells. Rheumatology
(Oxford). 46. pp. 1525–1530. 2007, View Article : Google Scholar
|
|
2
|
Ohl K and Tenbrock K: Inflammatory
cytokines in systemic lupus erythematosus. J Biomed Biotechnol.
2011:4325952011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kinoshita K, Kishimoto K, Shimazu H, et
al: Successful treatment with retinoids in patients with lupus
nephritis. Am J Kidney Dis. 55:344–347. 2010. View Article : Google Scholar
|
|
4
|
Saxena R, Mahajan T and Mohan C: Lupus
nephritis: current update. Arthritis Res Ther. 13:2402011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bertsias G, Gordon C and Boumpas DT:
Clinical trials in systemic lupus erythematosus (SLE): lessons from
the past as we proceed to the future - the EULAR recommendations
for the management of SLE and the use of end-points in clinical
trials. Lupus. 17:437–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thompson CB: Distinct roles for the
costimulatory ligands B7-1 and B7-2 in T helper cell
differentiation? Cell. 81:979–982. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
King CL, Xianli J, June CH, Abe R and Lee
KP: CD28-deficient mice generate an impaired Th2 response to
Schistosoma mansoni infection. Eur J Immunol. 26:2448–2455. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hart DN: Dendritic cells: unique leukocyte
populations which control the primary immune response. Blood.
90:3245–3287. 1997.PubMed/NCBI
|
|
9
|
Nossal GJ: Negative selection of
lymphocytes. Cell. 76:229–239. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong GC, Chuang PH, Chang KC, et al:
Blocking effect of an immuno-suppressive agent, cynarin, on CD28 of
T-cell receptor. Pharm Res. 26:375–381. 2009. View Article : Google Scholar :
|
|
11
|
Fire A: RNA-triggered gene silencing.
Trends Genet. 15:358–363. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yelton DE and Scharff MD: Monoclonal
antibodies: a powerful new tool in biology and medicine. Annu Rev
Biochem. 50:657–680. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCoy KD and Le Gros G: The role of CTLA-4
in the regulation of T cell immune responses. Immunol Cell Biol.
77:1–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi Q, Gao ZY, Xie F, Wang LF, Gu YP, Yang
TJ, Huang L, Qian QH and Qiu YH: A novel monoclonal antibody
against human CD80 and its immune protection in a mouse lupus-like
disease. Int J Immunopathol Pharmacol. 24:583–593. 2011.PubMed/NCBI
|
|
15
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Satoh M and Reeves WH: Induction of
lupus-associated autoantibodies in BALB/c mice by intraperitoneal
injection of pristane. J Exp Med. 180:2341–2346. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Richards HB, Satoh M, Jennette JC, Croker
BP, Yoshida H and Reeves WH: Interferon-gamma is required for lupus
nephritis in mice treated with the hydrocarbon oil pristane. Kidney
Int. 60:2173–2180. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dias N and Stein CA: Antisense
oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther.
1:347–355. 2002.PubMed/NCBI
|
|
19
|
Zang X and Allison JP: The B7 family and
cancer therapy: Costimulation and coinhibition. Clin Cancer Res.
13:5271–5279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Howard LM, Kohm AP, Castaneda CL and
Miller SD: Therapeutic blockade of TCR signal transduction and
co-stimulation in autoimmune disease. Curr Drug Targets Inflamm
Allergy. 4:205–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kremer JM, Genant HK, Moreland LW, et al:
Effects of abatacept in patients with methotrexate-resistant active
rheumatoid arthritis: a randomized trial. Ann Intern Med.
144:865–876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyagishi M, Hayashi M and Taira K:
Comparison of the suppressive effects of antisense oligonucleotides
and siRNAs directed against the same targets in mammalian cells.
Antisense Nucleic Acid Drug Dev. 13:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakajima A, Azuma M, Kodera S, et al:
Preferential dependence of autoantibody production in murine lupus
on CD86 costimulatory molecule. Eur J Immunol. 25:3060–3069. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cunnane G, Chan OT, Cassafer G, et al:
Prevention of renal damage in murine lupus nephritis by CTLA-4Ig
and cyclophosphamide. Arthritis Rheum. 50:1539–1548. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kubowicz P, Żelaszczyk D and Pękala E:
RNAi in clinical studies. Curr Med Chem. 20:1801–1816. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Talaat RM, Mohamed SF, Bassyouni IH and
Raouf AA: Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus
erythematosus (SLE) patients: Correlation with disease activity.
Cytokine. 72:146–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dolff S, Bijl M, Huitema MG, Limburg PC,
Kallenberg CG and Abdulahad WH: Disturbed Th1, Th2, Th17 and T(reg)
balance in patients with systemic lupus erythematosus. Clin
Immunol. 141:197–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lazarus M, Hajeer AH, Turner D, et al:
Genetic variation in the interleukin 10 gene promoter and systemic
lupus erythematosus. J Rheumatol. 24:2314–2317. 1997.
|
|
29
|
Hasegawa K, Hayashi T and Maeda K:
Promotion of lupus in NZB x NZWF1 mice by plasmids encoding
interferon (IFN)-gamma but not by those encoding interleukin
(IL)-4. J Comp Pathol. 127:1–6. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amel-Kashipaz MR, Huggins ML, Lanyon P,
Robins A, Todd I and Powell RJ: Quantitative and qualitative
analysis of the balance between type 1 and type 2
cytokine-producing CD8 (−) and CD8(+) T cells in systemic lupus
erythematosus. J Autoimmun. 17:155–163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Solomou EE, Juang YT, Gourley MF, Kammer
GM and Tsokos GC: Molecular basis of deficient IL-2 production in T
cells from patients with systemic lupus erythematosus. J Immunol.
166:4216–4222. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Szegedi A, Simics E, Aleksza M, et al:
Ultraviolet-A1 phototherapy modulates Th1/Th2 and Tc1/Tc2 balance
in patients with systemic lupus erythematosus. Rheumatology
(Oxford). 44. pp. 925–931. 2005, View Article : Google Scholar
|
|
33
|
Svoboda P: Off-targeting and other
non-specific effects of RNAi experiments in mammalian cells. Curr
Opin Mol Ther. 9:248–257. 2007.PubMed/NCBI
|
|
34
|
Zhang JL, Sun DJ, Hou CM, et al: CD3 mAb
treatment ameliorated the severity of the cGVHD-induced lupus
nephritis in mice by up-regulation of Foxp3+ regulatory T cells in
the target tissue: kidney. Transpl Immunol. 24:17–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|