Introduction
Anti-mullerian hormone (AMH), also termed mullerian
inhibiting substance, is a dimeric glycoprotein member of the
transforming growth factor-β superfamily (1,2). AMH
was first detected using a radioimmunoassay in mature bovine
follicular fluid (3). In females,
the expression of AMH is confined to the granulosa cells (GCs) of
follicles in the ovary. Its expression has been detected in the GCs
of follicles in several species, including mice (4), rats (5,6),
sheep (7) and humans (8). The expression of AMH begins in the
primary follicles and peaks in the preantral and small antral
follicles. However, the levels of AMH gradually decline during the
final stages of folliculogenesis, are not expressed in primordial
follicles and disappear in atretic follicles (8,9).
A previous study observed that AMH can inhibit the
initial recruitment of primordial follicles (10) and tumour cell proliferation
(11). Other studies performed in
animals have revealed that AMH can attenuate the sensitivity of
follicles to follicle-stimulating hormone (FSH), reduce aromatase
activity and the quantity of LH receptors in FSH-stimulated
granulosa cells (12), and inhibit
testosterone synthesis by thecal cells (13) by the binding of AMH to specific
type II receptors (AMHRII) expressed in granulosa and theca cells.
Previous detailed investigations have validated the use of serum
AMH levels as a quantitative marker for ovarian reserve and ovarian
dysfunction in in vitro fertilization (IVF) (14).
Gonadotrophin-releasing hormone (GnRH) agonist
(GnRHa), a decapeptide, is similar in structure to native GnRH and
binds to the GnRH receptors. Compared with native GnRH, GnRHa has a
higher affinity for GnRH receptors of the pituitary, higher
resistance to enzymatic breakdown and a prolonged half-life,
leading to its slow dissociation from GnRH receptors (15) and the concomitant desensitization
of pituitary GnRH receptors. This hypogonadotropic state is
referred to as downregulation (15). In the 1980s, GnRHa was
experimentally introduced in the mid-luteal phase of the preceding
cycle during controlled ovarian hyperstimulation (COH) (16). By inhibiting the pituitary-ovary
axis, GnRHa prevented premature luteinizing hormone (LH) surges
during COH.
The way in which the levels of AMH in Uthe serum
change during the process of downregulation remains controversial.
A previous study found that AMH levels in the sera of patients with
stage II-IV endometriosis are normal 4 and 8 weeks following
prolonged downregulation with goserelin administration (17). However, another report found that
circulating AMH declines following the first 3 months of prolonged
GnRHa treatment in girls with central precocious puberty and early
puberty (18). In accordance with
a GnRHa-mediated decrease in serum levels of AMH, a reduction in
the levels of AMH were observed following GnRHa treatment in
premenopausal women with breast cancer (19). It has been suggested that GnRHa
increases the mRNA expression of AMH in cultured human granulosa
cells (hGCs) and in the human HGL5 granulosa cell line (20). By contrast, evidence implying a
direct effect of GnRHa on suppression of AMH expression in the
ovaries has been obtained from observations that AMH concentrations
in follicular fluid from females treated with GnRHa are lower,
compared with untreated controls (21).
Previous studies on the effects of GnRHa on the
expression of AMH have all involved examination of the serum levels
of AMH in vivo and GCs in vitro. The effects of GnRHa
in vivo on the expression of AMH in follicles at varying
follicular stages during downregulation remain to be elucidated.
Understanding the changes of AMH in GCs can enable verification of
serum AMH and provide evidence supporting the further utilization
of serum AMH. The purpose of the present study was to investigate
the effects of different doses of GnRHa administration on the
expression of AMH at varying follicular stages during the process
of downregulation in vivo.
Materials and methods
Animals
All experimental procedures in the present stduy
were performed at the Experimental Animal Centre of Shantou
University Medical College (SUMC; Shantou, China), according to the
international ethical guidelines and with the approval of the SUMC
Ethics Committee. (SUMC2013-129). Adult female KM mice (5-6 weeks
old) were obtained from the Animal Center of SUMC and were housed
under a 12/12 h light/dark cycle at 22-26°C and 50-60% humidity.
All mice had free access to a standard pellet diet and water. The
estrous cycles of the adult female mice were determined in vaginal
smears, which were collected for 21 consecutive days. Only mice
that had more than two consecutive regular 4-day cycles were used.
These mice were randomly allocated into four experimental groups,
with 21 mice per group, as well as a control group containing three
mice.
Treatment
Treatment for downregulation involved a daily
intraperitoneal injection of GnRH agonist (Triptorelin; Ipsen
Pharma Biotech, France) between days 3 and 9 of the estrus cycle at
the following doses: 1.5 µg/100 g body weight (bw)/day, 3.0
µg/100 g bw/day, 4.5 µg/100 g bw/day and (4) 6.0 µg/100 gbw/day. Control mice
remained without any treatment.
Tissue collection
In each treatment group, three mice were sacrificed
each day following GnRH agonist administration, up to day 9. The
mice in the control group were sacrificed on day 0, corresponding
to day 3 of the estrus cycle. The ovaries were removed from each
animal and cleaned of surrounding fat.
Immunohistochemistry
The ovaries were fixed in 4% para-formaldehyde at
4°C overnight and embedded in paraffin. The ovaries were then
serially sectioned at a thickness of 4 µm. Every 10th
section was used. The tissue sections between days 0 and 7 were
obtained at the same time for each trial. Following dewaxing in
xylene, rehydration in a series of ethanols and antigen retrieval
in a microwave oven (15 min), endogenous peroxidase activity was
blocked with 3% H2O2 for 15 min. To block
non-specific binding sites, the sections were then incubated in 10%
normal donkey serum diluted in 0.01 M phosphate-buffered saline
(PBS) for 15 min. The sections were incubated at 4°C overnight with
polyclonal goat anti-MIS antibody (cat. no. sc-6886, Santa Cruz
Biotechnology, Santa Cruz, CA, USA), diluted 1:50 in 0.01 M PBS.
The sections were then incubated for 1 h with secondary horseradish
peroxidase (HRP)-conjugated donkey anti-goat IgG-HRP (cat. no
sc-2020; Santa Cruz Biotechnology), diluted 1:100 in 0.01 M PBS.
The detection of AMH was performed using chromogen,
3.3-diaminobenzidine (DAB; Gene Tech Shanghai Company Limited),
according to the manufacturer's instructions. The sections were
counterstained with hema-toxylin, and were then dehydrated and
mounted. For a negative control, the primary antibody was
excluded.
Quantification of
immunohistochemistry
The morphological classification of different stages
of follicles were, as defined previously (22,23).
The follicles were distinguished as follows: Primordial follicles
exhibited a single layer of flattened GCs; primary follicles
exhibited a single layer of cuboidal GCs; small preantral follicles
possessed between two and five layers of GCs; large preantral
follicles possessed more than five layers of GCs without an antrum;
small antral follicles contained fluid-filled spaces with fewer
than five GC layers, and follicles with five or more layers of GCs
and an antrum were considered large antral follicles. Only
follicles that contained a visible oocyte with a nucleus were
selected for immunohistochemical quantification.
Expression levels of AMH in follicles at
different developmental stages, at different administration times
and with different doses of GnRHa
For comparison of the expression levels of AMH in
the control and treatment groups between days 1 and 7 (seven
subgroups each day, n=3 per subgroup), 20 follicles of each stage
in each animal were examined. For the control group (day 0; n=3),
20 follicles of each stage in each animal were also examined. To
quantify the mean density of the expression of AMH from individual
follicles, analysis was performed using an image analysis system
linked to an Olympus camera (Olympus Corporation, Tokyo, Japan).
All images were captured under the same exposure times, and the
mean density of each follicle was measured using Image-Pro Plus 6.0
(Media Cybernetics, Inc., Rockville, MD, USA)
Expression of AMH in the GCs surrounding
the oocyte and basement membrane of small antral follicles with
different doses of GnRHa
Additional analysis was performed to determine the
mean density from different compartments of AMH staining within
each follicle in the small antral follicles. The area of the GCs
surrounding the oocyte and basement membrane were independently
calculated using the same image analysis technique described above
for the different follicular stages.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Statistical analysis was performed and differences
between the respective treatments performed and the control were
determined using one-way analysis of variance followed by Dunnett's
multiple comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Pattern and location of the expression of
AMH during follicular development
The results of the immunohistochemistry revealed
that the expression of AMH was positive in The GCs from the primary
follicles and was confined to the cytoplasm (Fig. 1). The expression of AMH increased
with decreasing size of the follicles. The AMH immunostaining was
most marked in the preantral and small antral follicles, and was
absent in the large antral follicles. In the atretic follicles and
primordial follicles, no AMH was detected. At the early antral
stage, AMH was predominantly present in the GCs surrounding the
oocyte and in a few cells surrounding the antrum. No follicles
expressed AMH in the thecal layer. Only GCs of the primary
follicles exhibited homogeneous expression of AMH. Similar to the
physical status, GnRHa treatment did not change the pattern of
stage-specific expression of AMH or the location of expression in
any of the four treatment groups. No specific immunoreactivity was
observed in the negative-control ovaries.
 | Figure 1Immunohistochemical localization of
the expression of AMH in different follicular stages. Histological
ovarian sections (magnification, x200). Positive
immunohistochemical staining of AMH in follicles at varying stages
are shown on the left; negative control stains are shown on the
right. Primary (A and B, black arrow), small preantral (C and D,
black arrow), large preantral (E and F, black arrow), small antral
(G and H, black arrow), and large antral (I and J, black arrow)
follicles, corpus luteum (A and B, CL), atretic follicles (C and D,
red arrow). Scale bar=100 µm. AMH, anti-mullerian
hormone. |
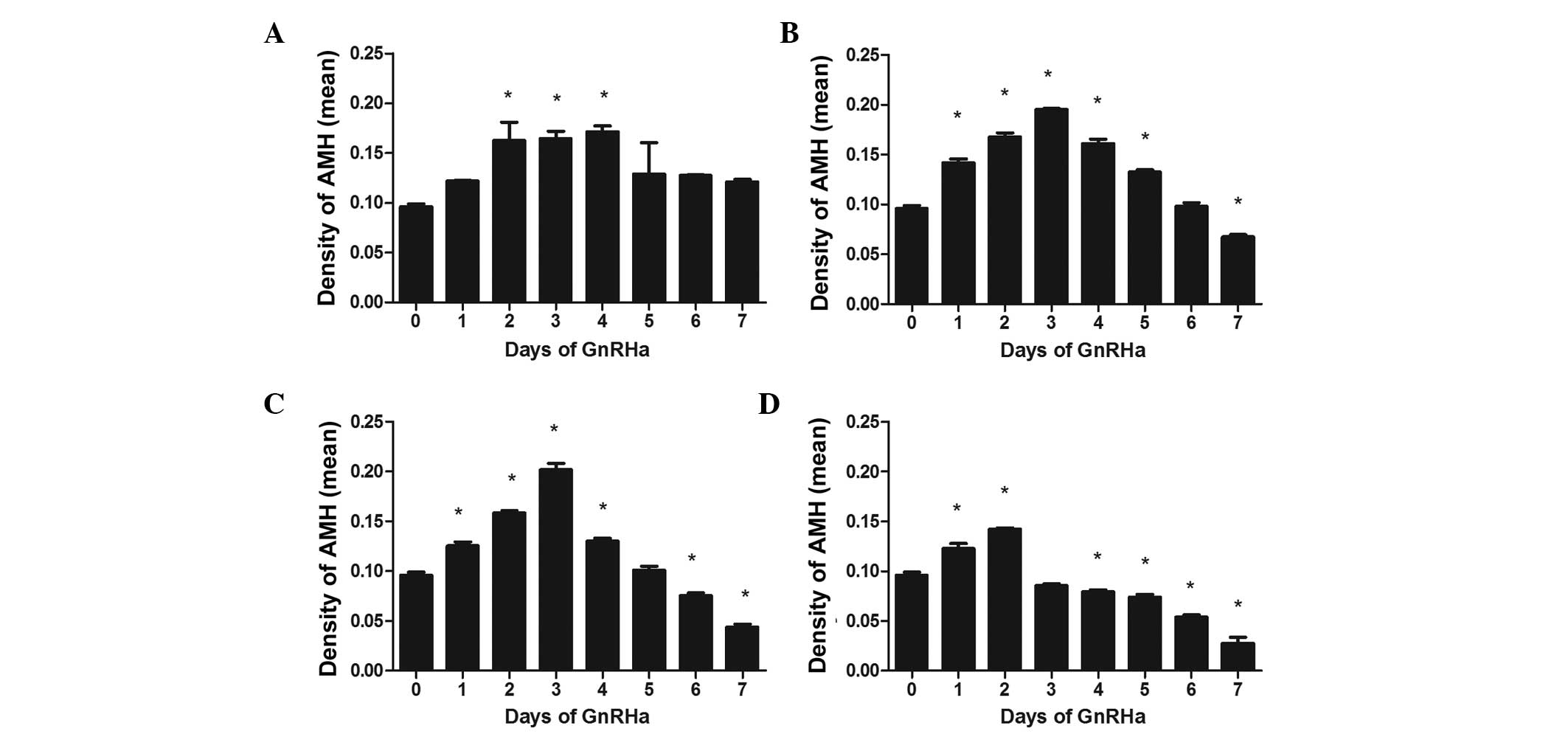
Effects of GnRHa on the expression levels
of AMH in primary follicles
Fig. 2 shows the
effect of treatment with different doses of GnRHa on the expression
of AMH in primary follicles. The expression of AMH expression
increased initially, peaking at ~day 3, and then declined
gradually. This peak moved forward from day 4 to day 2 as the dose
of GnRHa increased, with the peak AMH values all significantly
different to those on day 0 (all P<0.05). AMH decreased
gradually following the peak, with no significant difference at the
lowest dose (Fig. 2A). However,
significant differences were observed at the highest three doses on
day 7 (Fig. 2B–D).
Effects of GnRHa on the expression of AMH
in small preantral follicles
In the three lower dose treatment groups, the
expression of AMH increased on days 1 and 2 to a significant degree
(all P<0.05; Fig. 3). However,
the highest dose treatment group caused no elevation in the level
of AMH on day 1 (P>0.05), and were observed to decline on day 2,
with levels significantly lower than those observed on day 0
(P<0.05). Following the decrease on day 2, the levels of AMH
increased, and peaked on day 4. With continuous administration of
GnRHa, the expression of AMH decreased to a significantly lower
level on day 7, subsequent to the peak in all four treatment groups
(P<0.01).
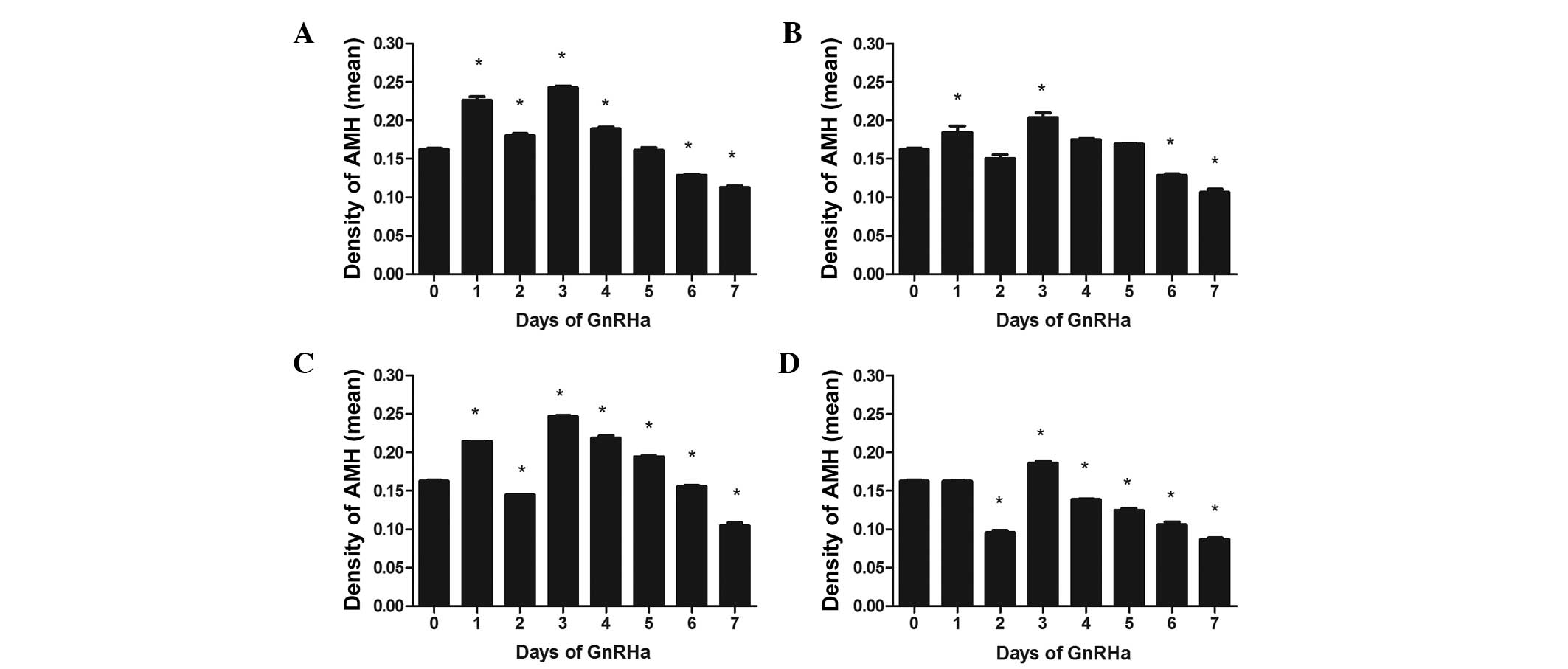
Effects of GnRHa on the expression of AMH
in large preantral follicles
Similar to the observations in the small preantral
follicles, the intensity of AMH staining was increased on day 1 in
the large preantral follicles, with a significant difference in the
three lower dose groups (P<0.01), but no significant difference
in the highest dose group (P>0.05). On day 2, the expression of
AMH in all the groups reduced, compared with those on day 1,
although the level of AMH in the lowest dose group remained
significantly higher than that at day 0 (P<0.01; Fig. 4A). The levels of AMH in the
remaining three groups were all lower than those on day 0, with
differences becoming increasingly significant with increasing GnRHa
dose. All peaks in AMH occurred on day 3. Following this peak, the
levels of AMH levels declined gradually, with all groups exhibiting
significantly lower levels by day 7, compared with day 0 (all
P<0.01).
Effects of GnRHa on the expression of AMH
expression in small antral follicles
The AMH staining observed in the GCs of the small
antral follicles increased in all GnRHa treatment groups on day 1,
however, only the increase in the highest GnRHa treatment group was
statistically significant to that on day 0 (P<0.01; Fig. 5). All treatment groups exhibited
reduced expression levels of AMH on day 2, compared with day 0,
with the difference being significant in the two highest dose
groups (P<0.05). The peak in expression occurred on day 4 in the
three lower dose groups, and on day 3 in the highest dose group.
The expression levels of AMH declined gradually following the peak
in all treatment groups. The decrease on day 7 was statistically
significant, compared with day 0, in the three highest dose groups
(P<0.05), while no difference was observed in the lowest dose
group (P>0.05).
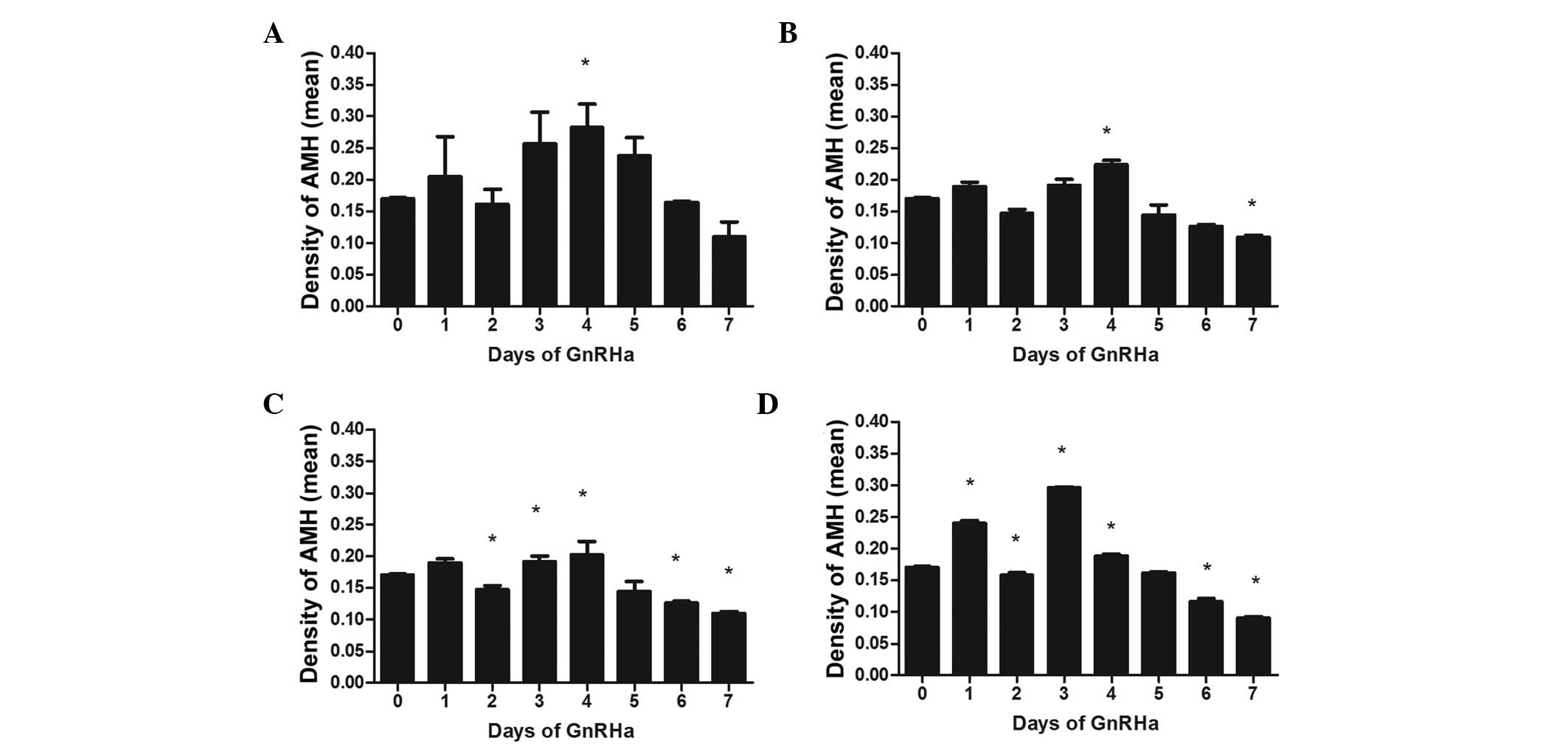
Effects of GnRHa on the expression of AMH
in the GCs surrounding the oocyte and basement membrane of the
small antral follicles
Fig. 6 shows the
changes in the expression of AMH in the GCs surrounding the oocyte
and basement membrane. In the GCs surrounding the oocyte, a
significant increase in the expression of AMH was observed on day 1
in the highest dose group only (P<0.01; Fig. 6D). On day 2, the intensity of AMH
staining decreased in all groups, compared with day 1, however, the
expression of AMH in the highest dose group was significantly
higher than that on day 0 (P<0.01). Peak levels occurred on day
3 in all groups but the lowest dose group, which peaked on day 4,
following which the levels of AMH slowly declined. The levels of
AMH in the two lowest dose groups returned to basal levels on day 7
(P>0.05). However, a rapid decrease resulting in a significantly
lower level was observed on day 7, compared with day 0, in the two
highest dose (all P<0.01).
Regarding the GCs surrounding the basement membrane,
no significant changes in AMH were observed in any group on day 1.
However, the two highest dose groups exhibited significant
decreases in the expression levels of AMH on day 2 (P<0.05). The
AMH peaks in the GCs surrounding the basement membrane were
synchronous with those of the GCs surrounding the oocyte in all
groups (P<0.05). In all groups, the expression of AMH declined
following this peak. In the two lowest dose groups, no significant
difference was observed in the AMH levels on day 7, compared with
day 0 (P>0.05). In the two highest dose groups, significantly
lower levels were observed on day 7, compared with those on day 0
(P<0.05).
Discussion
Serum AMH is affected by the expression of AMH in
various follicular stages, however, the way in which AMH levels in
the serum change during the process of downregulation remains to be
fully elucidated, and reports on whether the expression of AMH in
the GCs of follicles at different growing stages is affected by
GnRHa in vivo are lacking. In the present study, it was
demonstrated for the first time, to the best of our knowledge, that
the expression of AMH in follicles at various stages change
dynamically following treatment with various doses of GnRHa in
vivo, during the process of downregulation.
In the mouse ovary, the present study demonstrated
that the expression of AMH in follicular GCs was stage-specific.
AMH protein was produced at low levels by the columnar GCs of the
primary follicles, and was most abundant in the GCs of the
preantral and small antral follicles. The expression of AMH was
terminated in the large antral follicles and the corpora lutea, and
was absent in the primordial and atretic follicles. No AMH was
observed in the thecal or interstitial cells. These results are
consistent with previous findings (4,24).
In the present study, treatment with GnRHa in vivo dids not
affect the stage-specific location of the expression of AMH. In
addition, it was also observed that the intensity of the expression
of AMH was homogeneous in the primary follicle GCs, but
heterogeneous in the preantral and small antral follicles. In
primary follicles, apoptosis seldom occurs in human ovarian tissue
sections (25). Similar
homogeneity in the expression of AMH in all the GnRHa groups in the
present study is indirectly supported by investigations performed
in hypophysectomized, estrogen-treated immature rats, which
suggested that GnRHa does not induce apoptosis in the GCs of
primary follicles (26). However,
in follicles between the small preantral and small antral stages,
physical apoptosis, along with the proliferation of granulosa cells
and GnRHa induce pharmacological apoptosis by binding GnRH
receptors.
In the present study, the expression of AMH
expressions exhibited a similar dynamic change in all follicular
stages all treatment groups, with the exception of the decrease
observed on day 2 from the small preantral stages onward. The
levels of AMH were observed to initally increase, and then
gradually decrease. The peak of AMH occured on ~day 3. GnRHa
decreased the levels of AMH below their initial levels by day 7,
which occurred in a dose-dependent manner. Furthermore, from the
peak onwards, the expression of AMH declined in a time-dependent
manner. In general, the AMH profile in the GCs of the small antral
follicles was similar to that of preantral the follicles. It is
evident that antrum formation physically demarcates the former
preantral GCs into mural and cumulus GC populations (27). Notably, the present study
demonstrated that the time-dependent dynamic changes in the
expression of AMH in the GCs from the two different regions were
different. Firstly, the AMH profile in GCs surrounding the oocyte
was similar to that of the preantral follicles, while a marked
difference was observed in the GCs surrounding the basement
membrane, particularly on the first day of GnRHa administration,
without a visible increase. This result confirmed that of a
previous finding that cumulus cells are more closer associated with
preantral GCs, compared with mural granulosa cells (28). Secondly, the expression of AMH in
the small antral follicles was more abundant in the GCs surrounding
the oocyte than in the GCs within the basement membrane, which was
consistent with previous findings (5,24).
The difference in AMH intensity indirectly demonstrated functional
differences between the GCs in different locations.
Follicle-stimulating hormone receptor (FSHR) is highly expressed in
cumulus cells. By binding FSHR, FSH can induce the expansion of
cumulus cells and is involved in oocyte maturation (29). In addition, it has been reported
that the expression levels of FSHR and AMHR2 in cumulus cells is
associated with the expression of AMH (30). Therefore, the response to FSH is
more marked in GCs surrounding the oocyte than in the basement
membrane, which partly explains their more visible ascent on day 1
in all groups surrounding the oocyte.
During the first few days of the downregulation,
daily injection GnRHa causes an initial flare-up of gonadotropin
concentration by acting at the level of the hypothalamo-hypophysis
axis. Following this increase, the pituitary gland is downregulated
to a hypogonadotropic state (31).
Therefore, under the effects of GnRHa, gonadotropins first increase
and then slowly decrease. Previous studies have demonstrated this
dynamic change in FSH, LH and E2, and observed an increasing
tendency on day 3 when GnRHa was administrated to rats (32). In the present study, a similar
increasing tendency of AMH around day 3 was observed. Accordingly,
the present study hypothesized that the expression of AMH is, at
least partially, gonadotropin-dependent. This hypothesis is
supported further by previous in vitro findings
demonstrating the stimulation of AMH production in GCs following
the addition of FSH (33).
Similarly, experiments performed in primates also support a
possible positive role for gonadotropins in the regulation of AMH
(34). By contrast, other studies
have revealed that FSH may downregulate the expression of AMH
(19,24). Therefore, controversies exist
regarding the effect of gonadotropins on the expression of AMH,
although early investigations have reported that the expression of
AMH is gonadotropin-independent (35,36).
Indirectly, the results of the present study
suggested that the production of AMH in primary follicles is
partially gonadotropin-responsive. Supporting this hypothesis are
previous findings that FSH and LH receptor mRNAs exist in primary
follicles (37,38). The time-dependent changes observed
in the expression of AMH in preantral and small antral follicles
were not entirely in accordance with the trend of gonadotropins,
particularly on day 2, therefore, the production of AMH was not
only positively regulated by gonadotropins, but also negatively
regulated by other factors.
GnRHa acts predominantly on the
hypothalamus-pituitary-gonad axis by inducing a hypogonadotropic
milieu from continuous exposure to the agent. Apart from its
pituitary actions, several studies have demonstrated that GnRHa can
exert effects on the ovary in an autocrine-paracrine manner, as an
intra-ovarian regulatory factor, particularly by affecting
follicular development and steroidogenesis (39,40).
This direct GnRHa effect on the ovary is supported by increasing
evidence that GnRH receptors exist in ovarian tissue (39,41-46).
One of the direct actions on the ovary is inhibition of the
expression of gonadotropin receptors in granulosa cells (47). These findings suggest that the
anti-gonadotropic effect by GnRHa contributes to the reduction of
AMH. The results of a previous study revealed that lower levels of
AMH in intrafollicular fluid are found in females treated with
GnRHa (21). According to previous
studies, GnRHa directly inhibits proliferative activity and induces
apoptosis in GCs from preantral and small antral follicles
(21,26,48),
and the incidence of apoptosis increases in a dose-dependent manner
(49). Other studies have reported
that the expression of AMH is associated with the proliferation of
GCs (5). The present study
hypothesized that the downregulation of the expression of AMH by
GnRHa results from an inhibitory effect of mitotic activity and
induction of apoptosis, although the mechanisms remain to be
elucidated.
In the present study, the protein expression pattern
of AMH in primary follicles on day 2 was different from that of the
preantral and small antral follicles. The discrepancy in the
density of GnRH receptors may, at least in part, explain this
difference, GnRH receptors have been reported to localize in
granulosa cells of the preantral and small antral follicles,
whereas no GnRH receptors exist in primary follicles (45). The present study hypothesized that
the positive effects of FSH on the expression of AMH expression
were partially counteracted by the negative effects of GnRHa
through acting on GnRH receptors, leading to a significant decrease
in the levels of AMH on day 2 in the preantral and antral
follicles. In preovulatory rat GCs, GnRH induces an increase in the
receptor levels in a dose-dependent manner (50). This may partly explain the finding
that the expression of AMH in the preantral and small antral
follicles was lowest at the end of downregulation in the group
treated with the highest dose of GnRHa. As for the degree of
decrease in the expression of AMH in the GCs surrounding the oocyte
and basement membrane of the small antral follicles, GnRH receptors
may explain this discrepancy. An increased density of GnRH
receptors exist in the cumulus cells surrounding the oocyte,
compared with mural GCs (45).
Therefore, inhibition of AMH by GnRHa may be higher in GCs
surrounding the oocyte.
During the process of downregulation to induce a
hypogonadotropic state, GnRHa causes an initial 'flare-up' effect,
resulting in a transitory increase of gonadotrophins. This effect
accelerates follicle recruitment (51). In addition, it is now evident that
AMH is involved in mouse primordial follicle selection (9,52),
and growing follicle cyclic recruitment in humans (8) and mice (53). AMH inhibits FSH-stimulated follicle
growth by decreasing the responsiveness of the follicle to FSH
(53,54). The inhibitory effect of AMH on the
expression of aromatase activity and LH receptors by cultured GCs
is also in agreement with the inhibitory effect on follicle growth
(55). Therefore, it was suggested
that the decrease of AMH observed in the present study on day 2
accelerated follicle recruitment, while the increase of AMH around
the peak day restricted further follicular development in an
autocrine-paracrine manner. Following the the 'flare-up,' FSH
decreases gradually. In the hypogonadotropic state, follicle
development is relatively static. The low expression levels of AMH
on day 7 may have been associated with increased sensitivity to
FSH, allowing follicles to be selected synchronously for continued
growth by exogenous application of gonadotrophins. This suggested
that AMH may be one of the factors involved in follicular
synchronicity.
Follicular development is a process not only
regulated by the hypothalamic-pituitary-ovarian axis, but is also
affected by autocrine/paracrine factors of the ovaries (51). A bidirectional communication
between oocytes and GCs contributes to follicular development
(56). The oocyte itself has
effects on gene expression and protein synthesis in the GCs by
secreting paracrine factors, and GCs regulate oocyte developmental
competence (57). Treatment of
GnRHa in vivo may affect the communication between the
oocyte and GCs, by either changing the function of the GCs or
modulating the response of GCs to paracrine factors secreted by the
oocyte, leading to changes in the expression of AMH. The expression
and secretion of AMH by cumulus GCs is correlated with oocyte
maturity, with an inverse association between the expression of AMH
and oocyte maturity (58). This
suppression of oocyte maturation by AMH has been demonstrated
previously (59). In addition,
observations from the rat model also support this inhibitory role
of AMH (60). Therefore, the
downregulation of AMH observed on day 7 in the presents study may
be a marker of oocyte quality, and it was hypothesized that
appropriate treatment with GnRHa in vivo may improve oocyte
quality. Consistent with this hypothesis, a previous study
performed in the mice demonstrated that administrating GnRH in
addition to the standard pregnant mare serum gonadotropin (PMSG)
and human chorionic gonadotropin (hCG) treatments improves IVF
fertility rate, compared with standard treatments (61). This further supports the
possibility that GnRHa in vivo may regulate follicle
development and improve oocyte quality through changes in the
expression of AMH during the process of downregulation.
The present study demonstrated that treatment of
GnRHa in vivo affects the expression of AMH in follicles at
different stages in the ovaries of cycling mice during the process
of downregulation, however it has no affect on its expression
pattern. The effect of GnRHa on the expression of AMH was
dose-dependent. The dynamic changes observed in the expression of
AMH in the present study may contribute to the use of GnRHa in
regulating follicle development and improving oocyte quality during
downregulation in assisted reproductive technology. However, the
potential mechanisms underlying the daily fuctuations in the
expression of AMH caused by GnRHa in vivo requires further
investigation.
Abbreviations:
|
AMH
|
anti-mullerian hormone
|
|
AMHRII
|
anti-mullerian hormone type II
receptor
|
|
ANOVA
|
analysis of variance
|
|
ART
|
assisted reproductive technology
|
|
COH
|
controlled ovarian
hyperstimulation
|
|
FSH
|
follicle stimulating hormone
|
|
FSHR
|
FSH receptor
|
|
GCs
|
granulosa cells
|
|
GnRHa
|
gonadotrophin-releasing hormone
agonist
|
|
hGCs
|
human granulosa cells
|
|
HGL5
|
human granulosa cell line
|
|
IVF
|
in vitro fertilization
|
|
LH
|
luteinizing hormone
|
|
MIS
|
mullerian inhibiting substance
|
|
PBS
|
phosphate buffer saline
|
|
PMSG
|
pregnant mare serum gonadotropin
|
Acknowledgments
This study was sponsored by the Shantou University
Medical College Clinical Research Enhancement Initiative and the
National Natural Science Foundation of China (grant. nos. 81070542
and 30872771). The authors would like to thank Dr Xin Zhang for
their advice, and the faculty and staff from the Laboratory of
Molecular Cardiology of the First Affliated Hospital of Shantou
University Medical College, for their assistance with histological
examination.
References
|
1
|
Cate RL, Hession C, Tizard R, Farber NM,
Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EP and Mattaliano RJ:
Isolation of the bovine and human genes for Müllerian inhibiting
substance and expression of the human gene in animal cells. Cell.
45:685–698. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Massague J: The transforming growth
factor-beta family. Annu Rev Cell Biol. 6:597–641. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vigier B, Picard JY, Tran D, Legeai L and
Josso N: Production of anti-Müllerian hormone: another homology
between Sertoli and granulosa cells. Endocrinology. 114:1315–1320.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Munsterberg A and Lovell-Badge R:
Expression of the mouse anti-müllerian hormone gene suggests a role
in both male and female sexual differentiation. Development.
113:613–624. 1991.
|
|
5
|
Hirobe S, He WW, Lee MM and Donahoe PK:
Mullerian inhibiting substance messenger ribonucleic acid
expression in granulosa and sertoli cells coincides with their
mitotic activity. Endocrinology. 131:854–862. 1992.PubMed/NCBI
|
|
6
|
Ueno S, Takahashi M, Manganaro TF, Ragin
RC and Donahoe PK: Cellular localization of müllerian inhibiting
substance in the developing rat ovary. Endocrinology.
124:1000–1006. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bézard J, Vigier B, Tran D, Mauléon P and
Josso N: Anti-müllerian hormone in sheep follicles. Reprod Nutr
Dev. 28:1105–1112. 1988. View Article : Google Scholar
|
|
8
|
Weenen C, Laven JS, Von Bergh AR,
Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC and Themmen
AP: Anti-Müllerian hormone expression pattern in the human ovary:
Potential implications for initial and cyclic follicle recruitment.
Mol Hum Reprod. 10:77–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Durlinger AL, Gruijters MJ, Kramer P,
Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA
and Themmen AP: Anti-Mullerian hormone inhibits initiation of
primordial follicle growth in the mouse ovary. Endocrinology.
143:1076–1084. 2002.PubMed/NCBI
|
|
10
|
Durlinger AL, Kramer P, Karels B, de Jong
FH, Uilenbroek JT, Grootegoed JA and Themmen AP: Control of
primordial follicle recruitment by anti-Müllerian hormone in the
mouse ovary. Endocrinology. 140:5789–5796. 1999.PubMed/NCBI
|
|
11
|
Ha TU, Segev DL, Barbie D, Masiakos PT,
Tran TT, Dombkowski D, Glander M, Clarke TR, Lorenzo HK, Donahoe
PK, et al: Mullerian inhibiting substance inhibits ovarian cell
growth through an Rb-independent mechanism. J Biol Chem.
275:37101–37109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
di Clemente N, Ghaffari S, Pepinsky R,
Pieau C, Josso N, Cate RL and Vigier B: A quantitative and
interspecific test for biological activity of anti-mullerian
hormone: The fetal ovary aromatase assay. Development. 114:721–727.
1992.PubMed/NCBI
|
|
13
|
Teixeira J, Maheswaran S and Donahoe PK:
Müllerian inhibiting substance: An instructive developmental
hormone with diagnostic and possible therapeutic applications.
Endocr Rev. 22:657–674. 2001.PubMed/NCBI
|
|
14
|
Visser JA, de Jong FH, Laven JS and
Themmen AP: Anti-Müllerian hormone: A new marker for ovarian
function. Reproduction. 131:1–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Metallinou C, Asimakopoulos B, Schröer A
and Nikolettos N: Gonadotropin-releasing hormone in the ovary.
Reprod Sci. 14:737–749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmutzler R, Reichert C, Diedrich K,
Wildt L, Diedrich C, Al-Hasani S, van der Ven H and Krebs D:
Combined GnRH-agonist/gonadotrophin stimulation for in-vitro
fertilization. Hum Reprod. 3:29–33. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mohamed KA, Davies WA and Lashen H:
Antimüllerian hormone and pituitary gland activity after prolonged
down-regulation with goserelin acetate. Fertil Steril.
86:1515–1517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hagen CP, Sørensen K, Anderson RA and Juul
A: Serum levels of antimüllerian hormone in early maturing girls
before, during and after suppression with GnRH agonist. Fertil
Steril. 98:1326–1330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anderson RA, Themmen AP, Al-Qahtani A,
Groome NP and Cameron DA: The effects of chemotherapy and long-term
gonadotrophin suppression on the ovarian reserve in premenopausal
women with breast cancer. Hum Reprod. 21. pp. 2583–2592. 2006,
View Article : Google Scholar
|
|
20
|
Winkler N, Bukulmez O, Hardy DB and Carr
BR: Gonadotropin releasing hormone antagonists suppress aromatase
and anti-Müllerian hormone expression in human granulosa cells.
Fertil Steril. 94:1832–1839. 2010. View Article : Google Scholar
|
|
21
|
Seifer DB, MacLaughlin DT, Penzias AS,
Behrman HR, Asmundson L, Donahoe PK, Haning RV Jr and Flynn SD:
Gonadotropin-releasing hormone agonist-induced differences in
granulosa cell cycle kinetics are associated with alterations in
follicular fuid müllerian-inhibiting substance and androgen
content. J Clin Endocrinol Metab. 76:711–714. 1993.PubMed/NCBI
|
|
22
|
Britt KL, Saunders PK, McPherson SJ, Misso
ML, Simpson ER and Findlay JK: Estrogen actions on follicle
formation and early follicle development. Biol Reprod.
71:1712–1723. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pelusi C, Ikeda Y, Zubair M and Parker KL:
Impaired follicle development and infertility in female mice
lacking steroidogenic factor 1 in ovarian granulosa cells. Biol
Reprod. 79:1074–1083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baarends WM, Uilenbroek JT, Kramer P,
Hoogerbrugge JW, van Leeuwen EC, Themmen AP and Grootegoed JA:
Anti-müllerian hormone and anti-müllerian hormone type II receptor
messenger ribonucleic acid expression in rat ovaries during
postnatal development, the estrous cycle and gonadotropin-induced
follicle growth. Endocrinology. 136:4951–4962. 1995.PubMed/NCBI
|
|
25
|
Takekida S, Matsuo H and Maruo T: GnRH
agonist action on granulosa cells at varying follicular stages. Mol
Cell Endocrinol. 202:155–164. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Billig H, Furuta I and Hsueh AJ:
Gonadotropin-releasing hormone directly induces apoptotic cell
death in the rat ovary: Biochemical and in situ detection of
deoxyribonucleic acid fragmentation in granulosa cells.
Endocrinology. 134:245–252. 1994.PubMed/NCBI
|
|
27
|
Diaz FJ, Wigglesworth K and Eppig JJ:
Oocytes are required for the preantral granulosa cell to cumulus
cell transition in mice. Dev Biol. 305:300–311. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Erickson GF, Hofeditz C, Unger M, Allen Wr
and Dulbecco R: A monoclonal antibody to a mammary cell Line
recognizes two distinct subtypes of ovarian granulosa
cells*. Endocrinology. 117:1490–1499. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawashima I, Okazaki T, Noma N, Nishibori
M, Yamashita Y and Shimada M: Sequential exposure of porcine
cumulus cells to FSH and/or LH is critical for appropriate
expression of steroidogenic and ovulation-related genes that impact
oocyte maturation in vivo and in vitro Reproduction. 136:9–21.
2008.
|
|
30
|
Grøndahl ML, Nielsen ME, Dal Canto MB,
Fadini R, Rasmussen IA, Westergaard LG, Kristensen SG and Yding
Andersen C: Anti-Müllerian hormone remains highly expressed in
human cumulus cells during the final stages of folliculogenesis.
Reprod Biomed Online. 22:389–398. 2011. View Article : Google Scholar
|
|
31
|
Albuquerque LE, Tso LO, Saconato H,
Albuquerque MC and Macedo CR: Depot versus daily administration of
gonadotrophin-releasing hormone agonist protocols for pituitary
down regulation in assisted reproduction cycles. Cochrane Database
Syst Rev. 1:CD0028082013.PubMed/NCBI
|
|
32
|
Li X, Kang X, Deng Q, Cai J and Wang Z:
Combination of a GnRH agonist with an antagonist prevents flare-up
effects and protects primordial ovarian follicles in the rat ovary
from cisplatin-induced toxicity: A controlled experimental animal
study. Reprod Biol Endocrinol. 11:162013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taieb J, Grynberg M, Pierre A, Arouche N,
Massart P, Belville C, Hesters L, Frydman R, Catteau-Jonard S,
Fanchin R, et al: FSH and its second messenger cAMP stimulate the
transcription of human anti-Müllerian hormone in cultured granulosa
cells. Mol Endocrinol. 25:645–655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thomas FH, Telfer EE and Fraser HM:
Expression of anti-Mullerian hormone protein during early
follicular development in the primate ovary in vivo is influenced
by suppression of gonadotropin secretion and inhibition of vascular
endothelial growth factor. Endocrinology. 148:2273–2281. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fanchin R, Taieb J, Lozano DH, Ducot B,
Frydman R and Bouyer J: High reproducibility of serum
anti-Müllerian hormone measurements suggests a multi-staged
follicular secretion and strengthens its role in the assessment of
ovarian follicular status. Hum Reprod. 20:923–927. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fanchin R, Schonäuer LM, Righini C,
Frydman N, Frydman R and Taieb J: Serum anti-Müllerian hormone
dynamics during controlled ovarian hyperstimulation. Hum Reprod.
18:328–332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Patsoula E, Loutradis D, Drakakis P,
Michalas L, Bletsa R and Michalas S: Messenger RNA expression for
the follicle-stimulating hormone receptor and luteinizing hormone
receptor in human oocytes and preimplantation-stage embryos. Fertil
Steril. 79:1187–1193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oktay K, Briggs D and Gosden RG: Ontogeny
of follicle-stimulating hormone receptor gene expression in
isolated human ovarian follicles 1. J Clin Endocrinol Metab.
82:3748–3751. 1997.PubMed/NCBI
|
|
39
|
Janssens RM, Brus L, Cahill DJ, Huirne JA,
Schoemaker J and Lambalk CB: Direct ovarian effects and safety
aspects of GnRH agonists and antagonists. Hum Reprod Update.
6:505–518. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang SK, Cheng KW, Nathwani PS, Choi K-C
and Leung PC: Autocrine role of gonadotropin-releasing hormone and
its receptor in ovarian cancer cell growth. Endocrine. 13:297–304.
2000. View Article : Google Scholar
|
|
41
|
Stojilkovic SS, Reinhart J and Catt KJ:
Gonadotropin-releasing hormone receptors: Structure and signal
transduction pathways. Endocr Rev. 15:462–499. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsueh AJ and Jones PB: Extrapituitary
actions of gonadotropin-releasing hormone. Endocr Rev. 2:437–461.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schirman-Hildesheim TD, Ben-Aroya N and
Koch Y: Daily GnRH and GnRH-receptor mRNA expression in the
ovariectomized and intact rat. Mol Cell Endocrinol. 252:120–125.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schirman-Hildesheim TD, Bar T, Ben-Aroya N
and Koch Y: Differential gonadotropin-releasing hormone (GnRH) and
GnRH receptor messenger ribonucleic acid expression patterns in
different tissues of the female rat across the estrous cycle.
Endocrinology. 146:3401–3408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Singh P, K rishna A, Sridaran R and
Tsutsui K: Immunohistochemical localization of GnRH and
RFamide-related peptide-3 in the ovaries of mice during the estrous
cycle. J Mol Histol. 42:371–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Torrealday S, Lalioti MD,
Guzeloglu-Kayisli O and Seli E: Characterization of the
gonadotropin releasing hormone receptor (GnRHR) expression and
activity in the female mouse ovary. Endocrinology. 154:3877–3887.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ranta T, Knecht M, Baukal AJ, Korhonen M
and Catt KJ: GnRH agonist-induced inhibitory and stimulatory
effects during ovarian follicular maturation. Mol Cell Endocrinol.
35:55–63. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Parborell F, Pecci A, Gonzalez O, Vitale A
and Tesone M: Effects of a gonadotropin-releasing hormone agonist
on rat ovarian follicle apoptosis: Regulation by epidermal growth
factor and the expression of Bcl-2-related genes. Biol Reprod.
67:481–486. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park EJ, Shin JW, Seo YS, Kim DW, Hong SY,
Park WI and Kang BM: Gonadotropin-releasing hormone-agonist induces
apoptosis of human granulosa-luteal cells via caspase-8, -9 and -3
and poly-(ADP-ribose)-polymerase cleavage. Biosci Trends.
5:120–128. 2011. View Article : Google Scholar
|
|
50
|
Yu B, Ruman J and Christman G: The role of
peripheral gonadotropin-releasing hormone receptors in female
reproduction. Fertil Steril. 95:465–473. 2011. View Article : Google Scholar
|
|
51
|
Dong M, Huang L, Wang W, Du M, He Z, Mo Y
and Yang D: Regulation of AMH and SCF expression in human granulosa
cells by GnRH agonist and antagonist. Pharmazie. 66:436–439.
2011.PubMed/NCBI
|
|
52
|
McGee EA and Hsueh AJ: Initial and cyclic
recruitment of ovarian follicles. Endocr Rev. 21:200–214.
2000.PubMed/NCBI
|
|
53
|
Durlinger AL, Kramer P, Karels B, Kumar
TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA,
Themmen AP, et al: Anti-Müllerian hormone attenuates the effects of
FSH on follicle development in the mouse ovary. Endocrinology.
142:4891–4899. 2001.PubMed/NCBI
|
|
54
|
Pellatt L, Rice S, Dilaver N, Heshri A,
Galea R, Brincat M, Brown K, Simpson ER and Mason HD:
Anti-Müllerian hormone reduces follicle sensitivity to
follicle-stimulating hormone in human granulosa cells. Fertil
Steril. 96:1246–1251. 2011. View Article : Google Scholar
|
|
55
|
di Clemente N, Goxe B, Remy JJ, Cate RL,
Josso N, Vigier B and Salesse R: Inhibitory effect of AMH upon the
expression of aromatase activity and LH receptors by cultured GCsof
rat and porcine immature ovaries. Endocrine. 2:553–558. 1994.
|
|
56
|
Eppig JJ: Oocyte control of ovarian
follicular development and function in mammals. Reproduction
(Cambridge, England). 122:829–838. 2001. View Article : Google Scholar
|
|
57
|
Devjak R1, Fon Tacer K, Juvan P, Virant
Klun I, Rozman D and Bokal E: Cumulus cells gene expression
profiling in terms of oocyte maturity in controlled ovarian
hyperstimulation using GnRH agonist or GnRH antagonist. PLoS One.
7:e471062012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kedem-Dickman A, Maman E, Yung Y, Yer
ushalmi GM, Hemi R, Hanochi M, Dor J and Hourvitz A: Anti-Müllerian
hormone is highly expressed and secreted from cumulus granulosa
cells of stimulated preovulatory immature and atretic oocytes.
Reprod Biomed Online. 24:540–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cook CL, Siow Y, Taylor S and Fallat ME:
Serum müllerian-inhibiting substance levels during normal menstrual
cycles. Fertil Steril. 73:859–861. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee MM and Donahoe PK: Mullerian
inhibiting substance: A gonadal hormone with multiple functions.
Endocr Rev. 14:152–164. 1993.PubMed/NCBI
|
|
61
|
Vasudevan K and Sztein JM: In vitro
fertility rate of 129 strain is improved by buserelin
(gonadotropin-releasing hormone) administration prior to
superovulation. Lab Anim. 46:299–303. 2012. View Article : Google Scholar : PubMed/NCBI
|