Introduction
Reduced glutathione (GSH) is an abundantly expressed
intracellular thiol peptide in aerobic cells, which is known to be
involved in cellular detoxification, antioxidant defense,
maintenance of thiol status, and modulation of cellular
proliferation (1,2). The concentration of intracellular GSH
is influenced by numerous factors, and reflects the balance between
rates of consumption and de novo synthesis. The synthesis of
GSH proceeds via two ATP-dependent enzymatic steps: Formation of
γ-glutamylcysteine from glutamate and cysteine, which is followed
by formation of GSH from γ-glutamylcysteine and glycine. The
initial step of GSH biosynthesis is rate-limiting and is catalyzed
by glutamate cysteine ligase (GCL), a heterodimer that consists of
a 73 kDa catalytic subunit (GCLC) and a 29 kDa modulatory subunit
(3). The second step of GSH
synthesis is catalyzed by glutathione synthetase (GSS), which is a
52 kDa homodimer. GSH metabolism has a complex role in both cancer
and anticancer therapy (4).
Furthermore, GSH is important in the detoxification of carcinogens,
and elevated levels of GSH may increase the resistance of numerous
types of tumor to chemotherapy and radiotherapy (5–7).
Various factors are associated with the initiation
and development of cancer, including genetic, environmental and
dietary influences (8). Previous
studies investigating cancer development have examined various
genes, including oncogenes, tumor suppressor genes, DNA repair
genes, and genes encoding phase I and II enzymes (9–12).
GSH levels have been shown to be elevated in numerous types of
human cancer, including bone marrow (13), breast (14,15)
and lung (16,17). Therefore, understanding the role of
GSH in the development of cancer will be a significant step towards
cancer prevention and/or attenuation. The present study examined
the expression levels of GSH and the enzymes involved in its
biosynthesis in human colon cancer tissues and cell lines.
Materials and methods
Reagents
Anti-GCLC antibody was purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Anti-GSS antibody was
purchased from Abcam (Cambridge, MA, USA). Anti-β-actin antibody
was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). All other chemicals and reagents used in the present study
were of analytical grade.
Cell culture
The Caco-2, SNU-407, SNU-1033, HCT-116, and HT-29
human colon cancer cell lines were obtained from the Korean Cell
Line Bank (Seoul, Korea). The FHC normal human colon cell line was
purchased from the American Type Culture Collection (Rockville, MD,
USA). All cells were maintained at 37°C in a humidified incubator
containing 5% CO2. The SNU-407, SNU-1033, HCT-116, and
HT-29 cells were cultured in RPMI 1640 (Gibco Life Technologies,
Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal
bovine serum (FBS; Gibco Life Technologies), 100 μg/ml
streptomycin (Gibco Life Technologies) and 100 U/ml penicillin
(Gibco Life Technologies). The Caco-2 cells were cultured in
Minimal Essential medium (Gibco Life Technologies) supplemented
with 10% heat-inactivated FBS, 100 μg/ml streptomycin, and
100 U/ml penicillin. The FHC cells were cultured in a 1:1 mixture
of Ham's F12 and Dulbecco's modified Eagle's medium (Gibco Life
Technologies) supplemented with 25 nM HEPES (Gibco Life
Technologies), 10 ng/ml cholera toxin (EMD Millipore, Billerica,
MA, USA), 5 μg/ml insulin (Sigma-Aldrich, St. Louis, MO,
USA), 5 μg/ml transferrin (Sigma-Aldrich), 100 ng/ml
hydrocortisone (Sigma-Aldrich) and 10% FBS.
Patient tissue
The colon tissue samples were obtained from 15 human
patients (3 female, 12 male; median age 66, age range, 43–86; 1
stage I, 3 stage II and 11 stage III) with colon cancer who were
treated at the Jeju National University Hospital (Jeju, Korea).
Normal and cancerous sections of tissue (~0.5–1 cm) were collected
from each patient during the operation. The present study was
approved by the institutional review board for ethics of the Jeju
National University Hospital (IRB:2011-38), and written informed
consent was obtained from the patients.
Intracellular GSH measurement
The intracellular GSH content was measured using the
GSH-400 Colorimetric Assay kit (Oxis Biotech Inc., Portland, OR,
USA). The cells and tissues were lysed with lysis buffer and were
harvested, homogenized in a metaphosphoric working solution, and
subsequently centrifuged at 15,000 × g for 10 min. The obtained
supernatant was mixed with an equal amount of trichloroacetic acid
(a precipitation reagent provided by the GSH-400 assay kit) and
further centrifuged at 15,000 × g for 5 min. A total of 50
μl R1 solution (from the GSH-400 kit), supplemented with
chromogenic reagent in HCl was added to 900 μl supernatant,
prior to being gently centrifuged at 15,000 × g for 5 min. A total
of 50 μl R2 solution (30% NaOH; from the GSH-400 kit) was
then added to the solution, and the mixtures were incubated at
25±3°C for 10 min. Following centrifugation at 15,000 × g for 5
min, the absorbance of the clear supernatant was measured at 400 nm
using a Scanning Multi-Well Spectrophotometer (Sunrise; Tecan
Group, Ltd., Salzburg, Austria).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cells using the
easy-BLUE™ Total RNA Extraction kit (Intron Biotechnology Inc.,
Seongnam, Korea) and template RNA was amplified using 1 μl
RT reaction buffer, along with primers (Bioneer Corporation,
Daejeon, Korea), dNTPs, and 0.5 U Taq DNA polymerase (Invitrogen
Life Technologies, Carlsbad, CA, USA), in a final volume of 25
μl. The PCR conditions were as follows: Initial denaturation
at 94°C for 5 min; followed by 35 cycles of 94°C for 30 sec, 60°C
for 30 sec, 72°C for 30 sec, and a final elongation step at 72°C
for 7 min. The following primers were used to amplify the GCLC and
GSS cDNA: GCLC forward, 5′-AGTTCAATACAGTTGAGG-3′, reverse,
5′-TACTGATCCTATAGTTAT-3′ (350 bp); GSS forward,
5′-CTGGAGCGGCTGAAGGACA-3′, reverse, 5′-AGCTCTGAGATGCACTGGACA-3′
(806 bp); and GAPDH forward, 5′-GTGGGCCGCCCTAGGCACCAGG-3′; and
reverse, 5′-GGAGGAAGAGGATGCGGCAGTG-3′ (868 bp). Amplifications were
performed on a MyCycler Thermocycler (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The amplified products were separated by
electrophoresis on a 1% agarose gel, and stained with RedSafe™
nucleic acid staining solution (Intron Biotechnology Inc.), prior
to gel image capture under UV light using Image Quant™ TL analysis
software (GE Healthcare Life Sciences, Chalfont, UK).
Immunoblot analysis
The cells were lysed on ice for 30 min in 100
μl lysis buffer, (120 mM NaCl, 40 mM Tris pH 8.0, and 0.1%
NP 40) prior to being centrifuged at 13,000 × g for 15 min. The
supernatants were collected and the protein concentration was
determined using the Bradford method (Bio-Rad Laboratories, Inc.).
Aliquots containing 40 μg protein were boiled for 5 min
prior to being separated by 10% SDS-PAGE. The proteins were then
transferred onto nitrocellulose membranes (EMD Millipore) and the
blots were blocked with 1% bovine serum albumin (Sigma-Aldrich) in
Tris-buffered saline with 1% Tween-20 for 1 h. The blots were then
incubated with GCLC (PA5-19702; 1:1,000), GSS (ab91591; 1:1,000),
or β-actin (sc-47778; 1:2,000) antibodies at 4°C overnight. The
membranes were subsequently incubated with horseradish
peroxidase-conjugated secondary immunoglobulin G antibodies (Pierce
Biotechnology Inc., Rockford, IL, USA). The protein bands were
detected using an Enhanced Chemiluminescence Western Blotting
Detection kit (GE Healthcare Life Sciences). Blots were quantified
using ImageJ software, version 1.47 (National Institutes of Health,
Bethesda, MD, USA)
Immunohistochemistry
The colon tissue specimens were fixed in 10%
buffered formalin and embedded in paraffin. The tissue blocks were
cut into 3 μm sections and mounted onto Superfrost
Plus-coated slides (Thermo Fisher Scientific, Inc.). The sections
were then deparaffinized in xylene and rehydrated through a series
of graded ethanol: 2 min in 100% twice, 2 min in 95%, 2 min in 70%
and 2 min in 50%. The staining was performed using a BenchMark XT
Immunostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA),
according to the manufacturer's instructions. Antigen retrieval was
carried out on the immunostainer at 100°C in EDTA buffer for 30
min. The slides were subsequently incubated with anti-GCLC and
anti-GSS antibodies (1:1,000) at 37°C for 32 min, and
3,3′-diaminobenzidine was used as a chromogen. The slides were
counterstained with hematoxylin (Sigma-Aldrich) prior to mounting,
and were evaluated using a light microscope (Olympus BX51; Olympus,
Center Valley, PA, USA) and interpreted by a pathologist.
Statistical analysis
Statistical significance was determined using
analysis of variance and Tukey's tests with SigmaStat software,
version 3.0 (Systat Software, Inc., San Jose, CA, USA). All values
are presented as the mean ± standard error. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression levels of GSH and GSH
metabolizing enzymes in human colon cancer cell lines
The intracellular GSH levels were assessed in a
normal human colon cell line (FHC) and five human colon cancer cell
lines (Caco-2, SNU-407, SNU-1033, HCT-116, and HT-29) using a
commercially available colorimetric assay kit. The intracellular
GSH levels were significantly higher in all five of the colon
cancer cell lines, as compared with the normal colon cell line
(Fig. 1). Concordant with these
results, RT-qPCR and immunoblot analyses revealed that the mRNA and
protein expression levels of the GSH synthetic enzymes: GCLC and
GSS, were also higher in the cancer cell lines, as compared with
the normal colon cell line (Fig.
2A and 2B). Although the
expression levels of GSH and GSH synthetic enzymes differed among
the colon cancer cell lines, the results of the present study
suggest that the expression levels of GSH and GSH metabolizing
enzymes may serve as useful biomarkers for the detection of colon
cancer.
Expression levels of GSH and GSH
metabolizing enzymes in normal and colon carcinoma tissue
samples
The expression levels of GSH were then examined in
the normal and cancerous colon tissue samples of 15 patients
undergoing treatment at the Jeju National University Hospital. In
nine out of 15 patients, GSH expression levels were higher in the
tumor tissue samples, as compared with the corresponding normal
tissue samples (Fig. 3).
Immunoblot analyses showed that the protein expression levels of
GCLC were higher in tumor tissue samples, as compared with the
corresponding normal tissue samples in 53% (8/15) of the patients
with colon cancer (Fig. 4A).
Similarly, the protein expression levels of GSS were higher in
tumor tissue samples, as compared with the corresponding normal
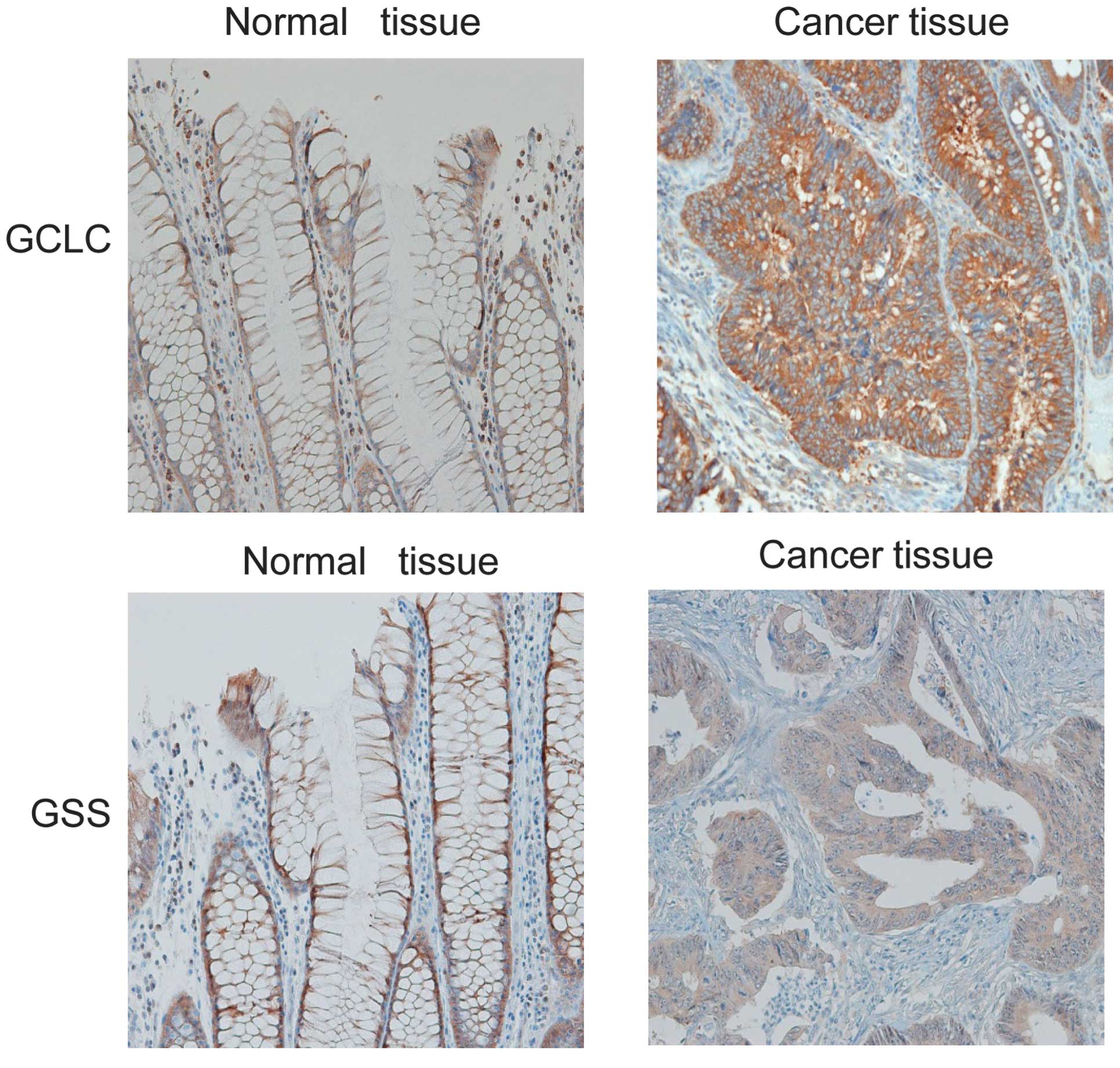
tissue samples in 67% (10/15) of the patients (Fig. 4B). Furthermore, immunohistochemical
analyses of the colon tissue sections revealed low protein
expression levels of GCLC and GSS in normal mucosa, whereas diffuse
and high expression levels of these proteins were detected in the
cancerous tissue (Fig. 5).
Discussion
GSH is a major cellular antioxidant and is crucial
for maintaining the balance between oxidation and antioxidation
(2,18). GSH is also important in cellular
detoxification and is associated with numerous aspects of the
immune response (2). Enhanced
resistance to chemotherapeutic drugs and radiotherapy is associated
with GSH-conjugation and detoxification (5–7).
Previous studies have reported that the expression levels of GSH
are elevated in numerous types of human cancer tissue, and
increased expression levels of GCLC have also been identified in
lung, breast, liver, and other types of cancer (19,20).
In addition, GCLC and GSS were highly expressed in the mammary
tumors and can be used as prognostic markers for mammary neoplasm
(21). Furthermore, a previous
study demonstrated that drug-resistance is associated not only with
elevated GSH levels, but also with increased activity of GCLC
(22).
The results of the present study demonstrated that
the GSH levels, and the mRNA and protein expression levels of GCLC
and GSS were higher in five human colorectal cancer cell lines, as
compared with normal human colon cell lines. Similar results were
also observed in cancerous and normal tissue samples, which were
collected from 15 patients with colon cancer. The relatively high
expression levels of GCLC and GSS in colon cancer tissue, as
compared with normal tissue, were confirmed by an
immunohistochemical analysis.
In conclusion, the results of the present study
suggest that the expression levels of GSH and GSH metabolizing
enzymes may serve as clinically useful biomarkers of colon cancer,
as well as potential targets for anticancer drugs. Further study is
required to elucidate the mechanisms of the GSH metabolizing enzyme
upregulation in human colorectal cancer cell lines and tissues.
Acknowledgments
The biospecimens and data used in the present study
were provided by the Biobank of Jeju National University Hospital.
The present study was supported by a grant from the National
R&D Program for Cancer Control, Ministry for Health and
Welfare, Korea (grant no. 1120340).
References
|
1
|
Lu SC: Regulation of glutathione
synthesis. Mol Aspects Med. 30:42–59. 2009. View Article : Google Scholar :
|
|
2
|
Lu SC: Glutathione synthesis. Biochim
Biophys Acta. 1830:3143–3153. 2013. View Article : Google Scholar :
|
|
3
|
Huang HC, Nguyen T and Pickett CB:
Regulation of the antioxidant response element by protein kinase
C-mediated phosphorylation of NF-E2-related factor 2. Proc Natl
Acad Sci USA. 97:12475–12480. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuo MT: Redox regulation of multidrug
resistance in cancer chemotherapy: Molecular mechanisms and
therapeutic opportunities. Antioxid Redox Signal. 11:99–133. 2009.
View Article : Google Scholar :
|
|
5
|
Nguyen LN, Munshi A, Hobbs ML, Story MD
and Meyn RD Jr: Paclitaxel restores radiation-induced apoptosis in
a bcl-2-expressing, radiation-resistant lymphoma cell line. Int J
Radiat Oncol Biol Phys. 49:1127–1132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vukovic V, Nicklee T and Hedley DW:
Microregional heterogeneity of non-protein thiols in cervical
carcinomas assessed by combined use of HPLC and fluorescence image
analysis. Clin Cancer Res. 6:1826–1832. 2000.PubMed/NCBI
|
|
7
|
Vukovic V, Nicklee T and Hedley DW:
Differential effects of buthionine sulphoximine in hypoxic and
non-hypoxic regions of human cervical carcinoma xenografts.
Radiother Oncol. 60:69–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herceg Z: Epigenetics and cancer: Towards
an evaluation of the impact of environmental and dietary factors.
Mutagenesis. 22:91–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arvelo F, Sojo F and Cotte C: Biology of
colorectal cancer. Ecancermedicalscience. 9:5202015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh S: Cytoprotective and regulatory
functions of glutathione S-transferases in cancer cell
proliferation and cell death. Cancer Chemother Pharmacol. 75:1–15.
2015. View Article : Google Scholar
|
|
11
|
Dizdaroglu M: Oxidatively induced DNA
damage and its repair in cancer. Mutat Res Rev Mutat Res.
763:212–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laborde E: Glutathione transferases as
mediators of signaling pathways involved in cell proliferation and
cell death. Cell Death Differ. 17:1373–1380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joncourt F, Oberli-Schrämmli AE, Stadler
M, Buser K, Franscini L, Fey MF and Cerny T: Patterns of drug
resistance parameters in adult leukemia. Leuk Lymphoma. 17:101–109.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Perry RR, Mazetta JA, Levin M and Barranco
SC: Glutathione levels and variability in breast tumors and normal
tissue. Cancer. 72:783–787. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jardim BV, Moschetta MG, Leonel C,
Gelaleti GB, Regiani VR, Ferreira LC, Lopes JR and Zuccari DA:
Glutathione and glutathione peroxidase expression in breast cancer:
An immunohistochemical and molecular study. Oncol Rep.
30:1119–1128. 2013.PubMed/NCBI
|
|
16
|
Cook JA, Pass HI, Iype SN, Friedman N,
DeGraff W, Russo A and Mitchell JB: Cellular glutathione and thiol
measurements from surgically resected human lung tumor and normal
lung tissue. Cancer Res. 51:4287–4294. 1991.PubMed/NCBI
|
|
17
|
Oberli-Schrämmli AE, Joncourt F, Stadler
M, et al: Parallel assessment of glutathione-based detoxifying
enzymes, O6-alkylguanine-DNA alkyltransferase and P-glycoprotein as
indicators of drug resistance in tumor and normal lung of patients
with lung cancer. Int J Cancer. 59:629–636. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sies H: Glutathione and its role in
cellular functions. Free Radic Biol Med. 27:916–921. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soini Y, Karihtala P, Mäntyniemi A,
Turunen N, Pääkko P and Kinnula V: Glutamate-L-cysteine ligase in
breast carcinomas. Histopathology. 44:129–135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mougiakakos D, Okita R, Ando T, et al:
High expression of GCLC is associated with malignant melanoma of
low oxidative phenotype and predicts a better prognosis. J Mol Med
(Berl). 90:935–944. 2012. View Article : Google Scholar
|
|
21
|
Leonel C, Gelaleti GB, Jardim BV, et al:
Expression of glutathione, glutathione peroxidase and glutathione
S-transferase pi in canine mammary tumors. BMC Vet Res. 10:492014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bailey HH, Gipp JJ, Ripple M, Wilding G
and Mulcahy RT: Increase in gamma-glutamylcysteine synthetase
activity and steady-state messenger RNA levels in
melphalan-resistant DU-145 human prostate carcinoma cells
expressing elevated glutathione levels. Cancer Res. 52:5115–5118.
1992.PubMed/NCBI
|