Introduction
Nasopharyngeal carcinoma (NPC) is the most common
type of cancer that originates in the upper region of the pharynx,
and commonly occurs in males and females worldwide (1). Despite recent advances in the
treatment strategy, treatment failure and tumor relapse render
complete eradication of tumors difficult due to the presence of
cancer stem cells (CSCs). The recently proposed CSC theory states
that the presence of a small sub-population of cancer cells within
the tumor, termed CSCs can cause resistance to therapy and tumor
recurrence. Current conventional treatment strategies kill the
majority of the neoplastic cells; however, the CSCs are not
targeted efficiently and thus are responsible for minimal residual
disease (MRD). Therefore it is necessary to target and kill CSCs in
order to achieve the complete eradication of tumor cells and to
provide long-term disease free survival.
Cancer stem cells have been isolated and
characterized in several types of solid tumor by two different
methods. Firstly using the expression of stem cell surface markers,
such as CD133 and CD44 (2) and
secondly using the Hoechst 33342 dye exclusion technique (3). A small population of cells exhibited
a distinct FACS profile to the side of the predominant population
due to a more efficient Hoechst 33342 dye efflux and lower
fluorescent intensity signal. These cells are designated as side
population (SP) cells and have been recently well-characterized in
several types of tumor, such as small cell lung cancer, glioma,
prostate cancer, leukemia, neuroblastoma, hepatoma, nasopharyngeal
carcinoma, colorectal cancer, thyroid cancer and lung cancer
(3–11). These SP cells exhibit
characteristic features of CSCs, such as chemotherapy and apoptosis
resistance, elevated expression of ABC transporter proteins,
self-renewal capacity, expression of stem cell surface markers,
high tumorigenicity and differentiation potential (12–14).
Moreover, SP cells were highly invasive and exhibited abnormal
activation of Wnt/β-catenin signaling pathways (15). Therefore, these SP cells are
considered as enriched CSCs. Hence, isolation and characterization
of these SP cells may aid in elucidating the molecular mechanism
underlying the tumorigenesis mediated by CSCs in NPC and to target
the CSCs effectively. Consequently, the current study aimed to
isolate and characterize the CSC-like SP cells from NPC samples,
using a FACs-based Hoechst 33342 dye exclusion technique.
Materials and methods
Sample collection and cell culture
NPC samples were collected at the time of surgery
according to the ethical approval. Stage IV, infratemporal NPC
samples were taken from 15 patients. The cancer tissues were washed
extensively in phosphate-buffered saline (PBS) solution containing
antibiotics (Sigma-Aldrich, St. Louis, MO, USA) and were incubated
overnight in Dulbecco's modified Eagle's medium (DMEM/F12;
Gibco-BRL, Carlsbad, CA, USA) containing 500 U/ml penicillin, 500
μg/ml streptomycin and 1.25 μg/ml amphotericin B
(Gibco-BRL). Enzymatic digestion was performed using 1.5 mg/ml
collagenase (Gibco-BRL) and 20 μg/ml hyaluronidase
(Sigma-Aldrich) in PBS for 1 h. Cells were cultured in DMEM with
10% fetal bovine serum (FBS; Sigma-Aldrich), supplemented with
antibiotics and maintained in T-75 flasks at 37°C in a humidified
5% CO2 and 95% air atmosphere. When cells were 90%
confluent, they were removed from the culture flask using
Trypsin-EDTA (0.25%-53 mM EDTA) washed with phosphate-buffered
saline (PBS), and suspended in 10% DMEM. Cells were counted using a
hemocytometer (Z359629; Bright-Line, Sigma-Aldrich, St. Louis, MO,
USA). The present study was approved by the Ethics Committee of
Tumor Hospital of Jilin Province, (Jilin, China).
Labeling with Hoechst 33342 dye
Groups consisted of: Control, cells labeled with
Hoechst 33342 dye alone (n=9); and drug treated, cells treated with
verapamil (Sigma-Aldrich) and labeled with Hoechst 33342 dye (n=9;
Sigma-Aldrich). Cells were counted by a hemocytometer and
~106 cells/ml in 10% DMEM were labeled with Hoechst
33342 stock (sigma)-bisbenzimide (5 μl/ml) either with dye
alone or in combination with 0.8 μl/ml verapamil.
Furthermore, cells were counter stained with 2 μg/ml
propidium iodide (PI; Sigma-Aldrich). The cells were sorted using a
flow cytom eter (Attune NxT; Life Technologies, Carlsbad, CA, USA)
and the sorted cells were cultured and maintained in DMEM/F-12
supplemented with 10% FBS. The Hoechst 33342 dye was excited at 355
nm and its dual-wavelength fluorescence was analyzed with an
optical filter (blue, 450 nm and red, 675 nm; Omega Optical,
Brattleboro, VT, USA).
Immunofluorescent staining
The FACS sorted SP and main population (MP) cells
were fixed onto glass slides in ice-cold 4% paraformaldehyde (4°C
for 10 min), and blocked with 1% bovine serum albumin
(Sigma-Aldrich) for 30 min at room temperature (RT) to block
nonspecific binding of IgG. After washing in PBS, cells were
incubated with fluorescein isothiocyanate (FITC)-conjugated mouse
anti-human CD44 (cat. no. HPA005785; 1:200; Sigma-Aldrich) and
Oct-4 (cat. no. P0082; 1:200; Abcam, Cambridge, MA, USA) at 4°C for
30 min in the dark. After extensive washing in PBS, cell nuclei
were counterstained with Hoechst 33342 and viewed under a confocal
microscope.
For ABCG2 expression, SP and MP cells were incubated
with mouse anti-human ABCG2 antibodies (cat. no. ab95692; 1L200;
Abcam) at 4°C overnight. After washing in PBS, cells were combined
with horseradish peroxidase-conjugated goat anti-mouse IgG (cat.
no. an6789; 1;500; Abcam) and incubated for 30 min at RT. Cells
were counterstained with hematoxylin and mounted with glycerol
vinyl alcohol aqueous mounting solution (16). Under an optical microscope (BX51M;
Olympus, Tokyo, Japan), the red ABCG2-positive cells were
observed.
The immunostaining of tumor spheres generated by SP
cells was performed as described previously (17). Spheres were fixed onto glass slides
in ice-cold 4% paraformaldehyde (4°C, 10 min), and blocked with
normal serum for 30 min. The cells were then incubated with mouse
monoclonal anti-Oct-4 and -CD44 (1:200; Chemicon, Tokyo, Japan)
overnight. After washing the slides with PBS, they were incubated
with FITC-conjugated goat anti-mouse IgG overnight in a dark room.
Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI)
and viewed under a fluorescence microscope (LSM 510; Carl Zeiss
GmbH, Jena, Germany). All images were processed with Adobe
Photoshop CS5 (Adobe Systems, Inc., CA, USA).
Cell resistance assay
Approximately 1×103 cells/plate were
cultured in 96-well plates and treated with 10 μg/ml
5-fluorouracil (5-FU; Sigma-Aldrich), 20 μmol/l cisplatin
(Sigma-Aldrich), 2 μmol/l paclitaxel (Sigma-Aldrich) and 2
μg/ml docetaxel (Sigma-Aldrich). The mean value of optical
density (OD)450 obtained was presented as a graph. Cell
resistance in the two groups was calculated using the following
formula: Cell resistance rate (%) = (experimental group
OD450 value/control group OD 450 value) × 100, as
described previously (18).
Reverse transcription (RT)-quantitative
polymerase chain reaction (PCR)
Total RNA was extracted and complementary DNA was
prepared using a reverse transcriptase kit (Fermentas, Vilnius,
Lithuania). First-strand cDNA synthesis and qPCR were performed
using the PrimeScript RT-PCR kit (Takara, Otsu, Japan) and the SYBR
Premix Ex Taq II kit (Takara, Otsu, Japan), according to the
manufacturer's instructions. RT-qPCR analysis was subsequently
performed on an iCycler IQ real-time detection system (Bio-Rad,
Hercules, CA, USA), using IQ Supermix with SYBR-Green (Bio-Rad).
The sequences of human specific primers used were as follows:
Forward: CTGGCTTTGGTGAACTGTTG and reverse: AGTTGCTCACAGCCAAGACA for
AXIN2; forward: AGCACCTTGGATGGGTATTC and reverse:
CACAATCCTGAGGCACAGTCDKK1 for DKK1; forward:
TCAATCAAAGTGCTTCTTTTTTATG and reverse: TTGTGGAAGAATCACGTGGCABCG2
for ABCG2; forward: ATGTCGTGGAGTCTACTGGC and reverse:
TGACCTTGCCCACAGCCTTG for GAPDH; and forward: CTCCCAACTGGTTCGACCTT
and reverse: CGGTTTCCATATTTCTCAGT for BMI-1. The amplified products
were separated by electrophoresis on ethidium bromide-stained 1.2%
agarose gels. Band intensity was measured by Image J from two
independent experiments.
Biochemistry
For western blot analysis, proteins were extracted
from the SP and MP cells, and protein concentration was determined
using the Bradford assay (19).
Following 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (Bio-Rad, Hercules, CA, USA), proteins were
transferred to a nitrocellulose membrane (Bio-Rad), the gels were
treated with rabbit anti-human ABCG2, actin and Bcl-2 primary
antibodies, goat anti-rabbit IgG with alkaline phosphatase markers
secondary antibody and a chemiluminescence reagent. Blots were
detected and scanned using a densitometer (Bio-Rad GS-710).
Sphere formation assay
A sphere formation assay was performed exactly as
described previously (20). The
sorted SP cells and MP were placed at a density of 1,000 cells/ml,
resuspended in tumor sphere medium consisting of serum-free 1:1
mixture of Ham's F-12/DMEM (Sigma-Aldrich), N2 supplement
(Sigma-Aldrich), 10 ng/ml human recombinant bFGF and 10 ng/ml EGF
(R&D Systems, Inc., Minneapolis, MN, USA), and subsequently
cultured in ultra-low attachment plates for ~2 weeks. Sorted SP and
MP cells were seeded at a low density of 20 cells/l and the number
of generated spheres (>100 cells/ml) was counted after 7 days of
culture.
Statistical analysis
One-way analysis of variance and Student's t-test
was performed to determine the significant difference between the
treatment and control groups. Statistical analysis was performed
using SAS statistical software (SAS Institute, Inc., Cary, NC,
USA). P<0.01 was considered to indicate a statistically
significant difference.
Results
Analysis of ABCG2 expression and stem
cell surface proteins in SP cells
NPC samples were analyzed for the presence of cancer
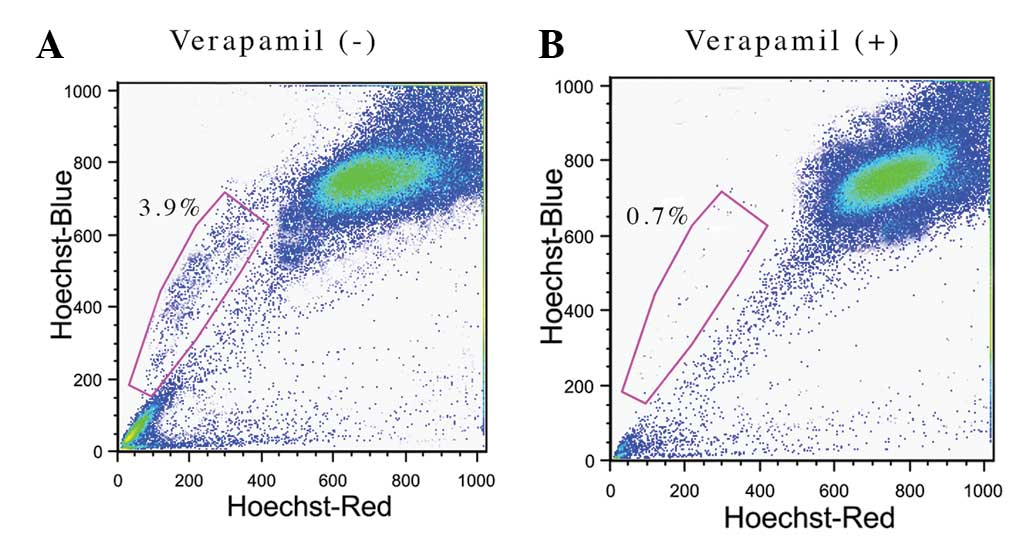
stem-like SP cells by a Hoechst 33342 dye exclusion technique.
During FACS analysis, the dead cells were excluded by PI staining
and therefore, the live cells were carefully selected. After
setting the gate for the ability of the cells to expel the Hoechst
33342 dye, ~3.9% of the total cells were identified to be SP cells
(Fig. 1A) and the remaining cells
were considered to be MP cells. The Hoechst 33342 exclusion
property of SP cells involves the overexpression of multi-drug
resistance transporter 1 (MDR1), a member of the ABC transporter
transmembrane protein family. Verapamil is a MDR1 transporter
protein inhibitor, which efficiently inhibits the drug efflux
action by the SP cells. Following verapamil treatment, the
percentage of SP cells was significantly reduced to 0.7% (Fig. 1B). The data confirms that the
presence of ABC transporters in SP cells are responsible for
chemotherapy resistance.
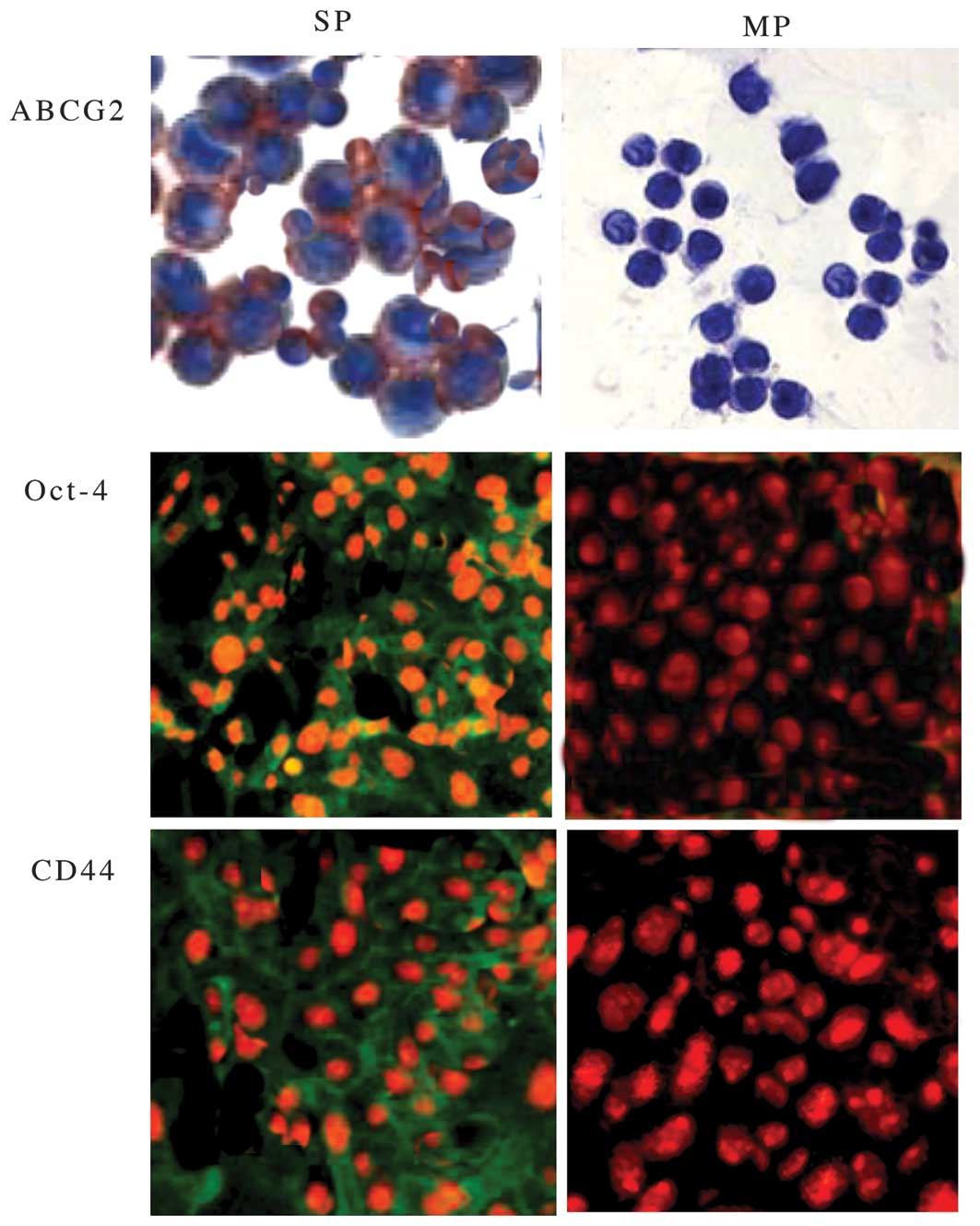
The SP and MP cells were then analyzed to compare
the expression level of ABCB2 genes and other stem cell genes, such
as OCT-4 and CD44 by fluorescence microscopy. As shown in Fig. 2, FACS-sorted SP cells showed
increased expression of ABCG2, CD44 and Oct-4 compared with MP
cells. The increased expression of CD44 and Oct-4 in SP cells
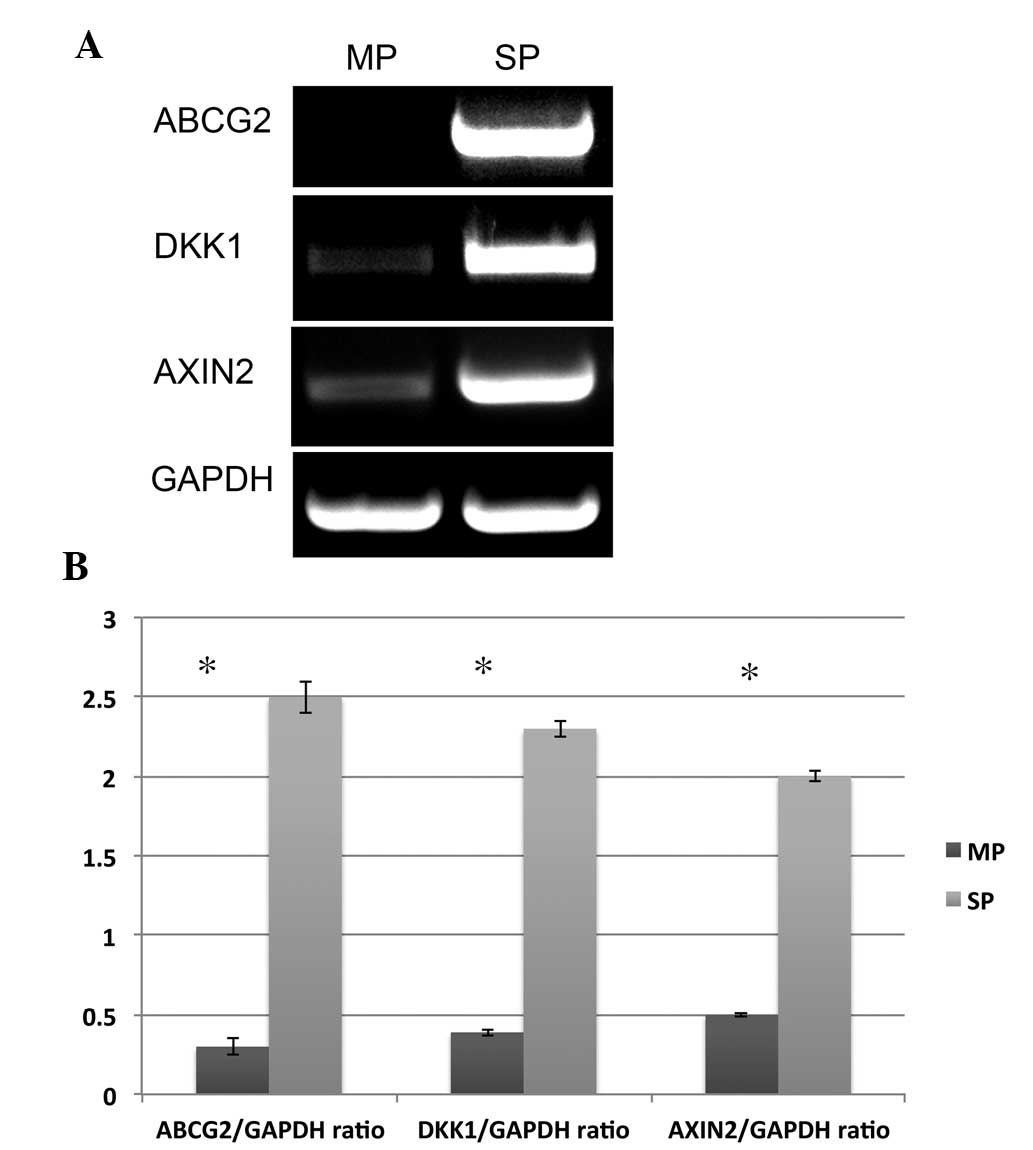
indicated that SP cells have a high capacity for self-renewal. In
addition to this data, elevated mRNA expression of ABCG2 in SP
cells was also observed (Fig. 3A).
Hence, the data clearly suggest that elevated expression of ABCG2,
CD44 and Oct-4 in SP cells may be crucial in drug resistance and
tumor relapse of NPC.
Abnormal activation of Wntβ-catenin
signaling and multi-drug resistance of SP cells
The Wnt/β-catenin signaling pathway has been found
to be associated with the self-renewal property of cancer cells.
Using a TOPFLASH luciferase reporter assay, it was previously
reported that activity of β-catenin-dependent transcription and
elevated expression of DKK1 and AXIN2 was found in HNSCC SP cells
compared with non-SP cells (21).
Similar to these findings, RT-qPCR results also showed that the
expression levels of two Wnt/β-catenin target genes, DKK1 and AXIN2
are significantly elevated in SP cells compared with MP cells
(Fig. 3A and B).
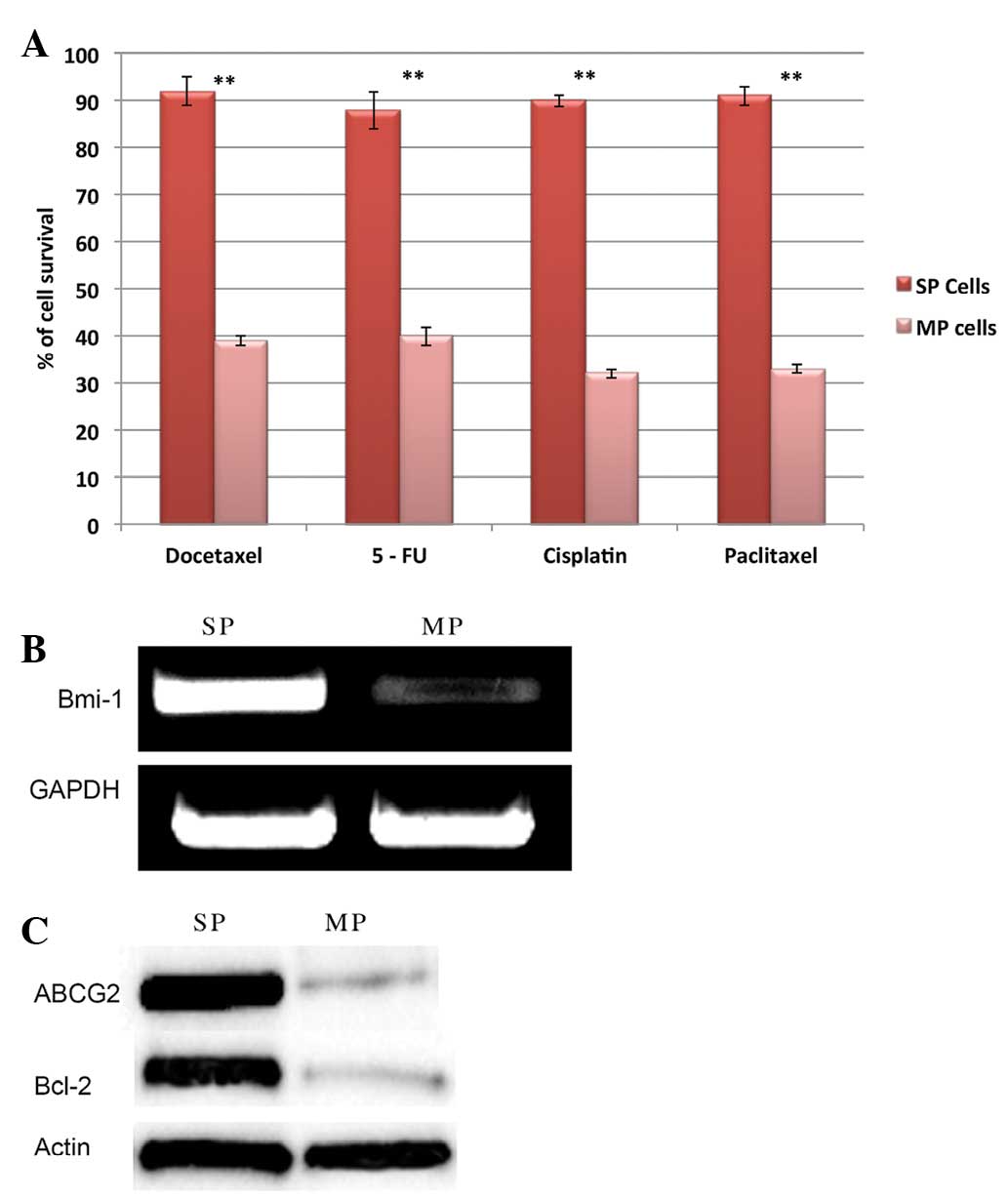
In order to characterize the SP cells, the
self-renewal potential and drug resistance of SP cells was analyzed
by sphere formation and drug resistance assays. It was observed
that SP cells are highly resistant to multiple drugs, such as
docetaxel, 5-FU, cisplatin and paclitaxel. The drug-treated SP
cells were more resistant and exhibited significantly increased
survival rates when compared with MP cells (Fig. 4A). Furthermore, RT-qPCR and western
blot analysis data revealed that SP cells exhibit elevated mRNA
expression of the anti-apoptotic gene Bmi-1 (Fig. 4B) and increased expression of ABCG2
and Bcl-2 protein (Fig. 4C). ABCG2
and anti-apoptotic factors are crucial factors involved in drug and
apoptotic resistance of SP cells. It was also observed that
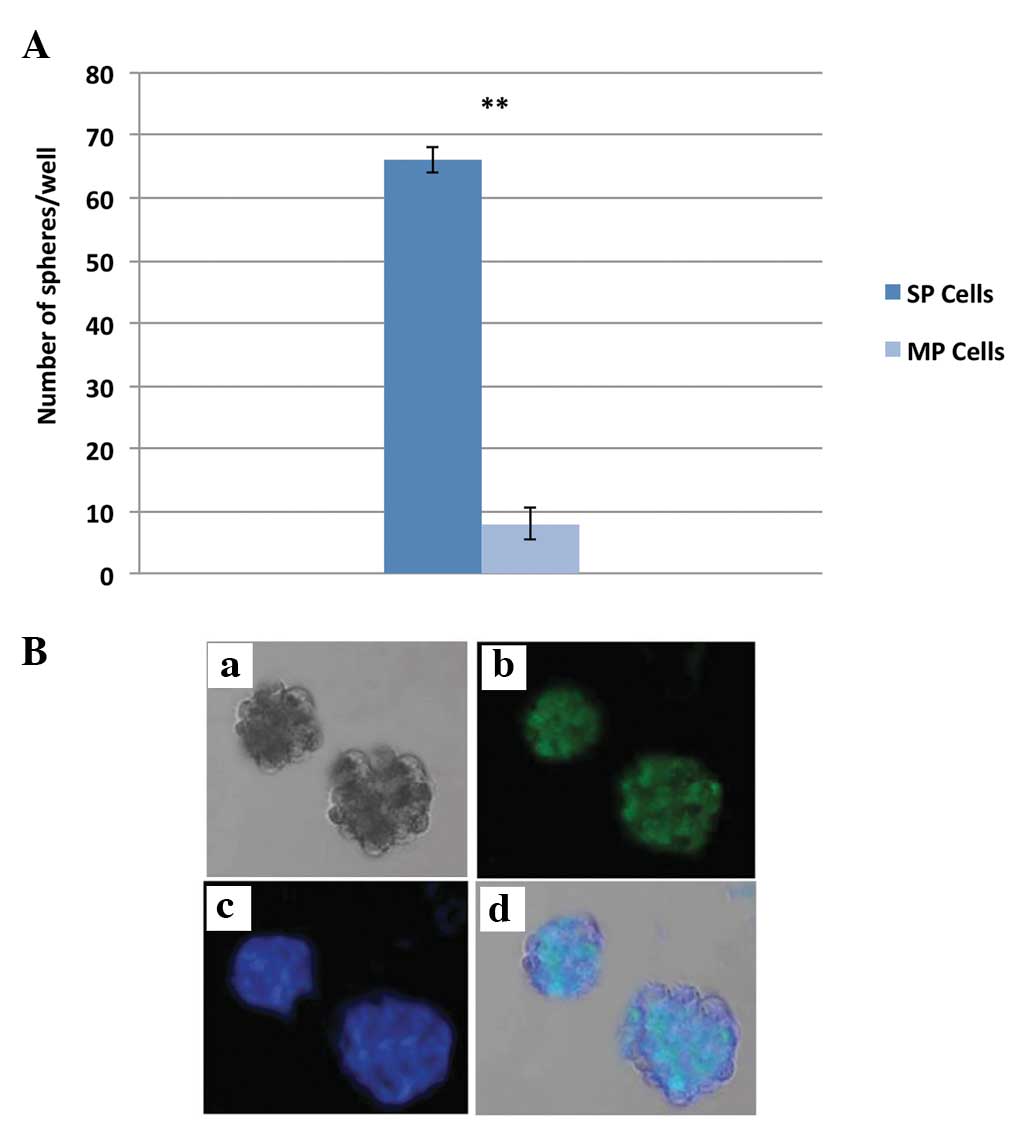
FACS-purified SP cells can generate tumor spheres rapidly and the
total number of spheres generated by NPC SP cells are significantly
higher than that by MP cells (Fig.
5A). Furthermore, the NPC SP cell generated tumor spheres are
immunopositive for CD44 (Fig. 5B).
The data clearly demonstrated that NPC SP cells are responsible for
chemotherapeutic resistance and may be involved in rapid tumor
recurrence and invasion.
Discussion
The presence of CSCs results in the failure of
treatment due to the development of MRD. Current conventional
treatment strategies efficiently kill the majority of the
neoplastic cells and but fail to kill the CSCs. According to the
CSC theory, CSCs are the predominant factor in multi-drug
resistance. These CSCs are also responsible for tumor growth,
metastasis and tumor invasion (22). It was reported that CSCs can
survive conventional anticancer therapies owing to the
overexpression of efflux pumps, which ultimately contributed to
multi-drug resistance (23).
Therefore, it is essential to eliminate the CSCs in order to
completely eradicate the tumor and prevent MRD.
The proposed method to identify the CSCs is the
FACS-based Hoechst 33342-dye exclusion technique in which a small
population of cancer cells efflux this dye and are termed SP cells
(22). These SP cells exhibit the
features of CSCs, such as drug resistance, self-renewal capacity,
high differentiation potential, expression of stem cell surface
markers and apoptosis resistance. Hence, isolation and further
characterization of SP cells aids in the development of novel
anticancer drugs, which could efficiently target and kill the
CSCs.
In the present study, 3.9% of the total cells were
observed to be SP cells in the NPC sample using the Hoechst 33342
dye exclusion method. Upon treatment with verapamil, the prevalence
of SP cells was reduced to 0.7%, which confirms the existence of
the ABC transporter protein. In addition, gene and protein
expression studies as well as immunofluorescence clearly showed
that SP cells overexpressed the ABC transporter protein, ABCG2.
Hence, these findings suggest that chemotherapy resistance of SP
cells from NPC actively involves the overexpression of ABCG2.
The OCT-4 gene was shown to be involved in cell
proliferation (24) and the
survival of CSCs is partly mediated through the Oct-4/Tcl1/Akt1
pathway (25). Similarly, the cell
surface proteoglycans, such as glycoprotein CD44, are important in
tumor invasion and metastasis in several types of tumor (26). Furthermore, the overexpression of
CD44 was shown to be involved in chemo- and radioresistance
(27). Also, CSCs are highly
resistant to death-inducing signals due to the enhanced expression
level of anti-apoptotic proteins and high levels of drug
transporters (7,18). It was previously reported that NPC
samples showed increased expression of CD44 positive cells, which
may be responsible for high levels of tumorigenesis and metastasis
(2). In line with these findings,
it was also demonstrated that NPC SP cells have increased
expression of CD44, Oct-4, Bcl-2 and Bmi-1 compared with MP cells.
Therefore, there may be significant functional interaction between
ABCG2, CD44, Oct-4, Bmi-1 and Bcl-2 in the contribution of
chemotherapy/apoptosis resistance, tumorigenesis and invasion of
NPC cells. In addition, it was also demonstrated that FACs purified
SP cells showed high resistance to the chemotherapeutic agents and
have increased cell survival rates compared with MP cells. These
results clearly confirm that overexpression of efflux pumps (ABCG2)
and increased expression of anti-apoptotic factor Bcl-2 are the
predominant factors, which confer the properties of multidrug
resistance and treatment failure in NPC. Furthermore, the SP cells
exhibit self-renewal capacity as they generate more tumor spheres
than non-SP cells. Recently, it was shown that secretion of certain
interleukins, such as IL-3 and IL-4, promotes enhanced survival
rates by altering the rate of apoptosis (28–30).
Therefore, it was also hypothesized that secretion of interleukins
in NPC SP cells may be involved in apoptosis resistance.
Notably, it was found that the Wnt/β-catenin pathway
is highly activated in NPC SP cells. Abnormal activation of
Wnt/β-catenin in HNSCC was shown to be involved in chemotherapy
resistance and invasive growth of cancer cells (31–33).
Similarly, enhanced Wnt/β-catenin pathway activation is the
phenotype of NPC SP cells and may also contribute to multi-drug
resistance and tumor invasion (31). However, the detailed mechanism of
altered Wnt/β-catenin pathway signaling requires detailed
investigation. In conclusion, the data suggest that isolation and
characterization of SP cells may provide valuable information to
improve cancer treatment and to design a novel anticancer drug that
effectively kills CSCs and prevents MRD in NPC.
Acknowledgments
The authors would like to thank Dr Wanshan Li,
Department of Oral and Maxillofacial Surgery, Chongqing Medical
University for sharing the FACS protocol by personal
communication.
References
|
1
|
Kam MK, Wong FC, Kwong DL, Sze HC and Lee
AW: Current controversies in radiotherapy for nasopharyngeal
carcinoma (NPC). Oral Oncol. 50:907–912. 2014. View Article : Google Scholar
|
|
2
|
Su J, Xu XH, Huang Q, Lu MQ, et al:
Identification of cancer stem-like CDZ cells in human
nasopharyngeal carcinoma cell line. Arch Med Res. 42:15–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goodell MA, Brose K, Paradis G, Conner AS
and Mulligan RC: Isolation and functional properties of murine
hematopoietic stem cells that are replicating in vivo. J Exp Med.
183:1797–1806. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salcido CD, Larochelle A, Taylor BJ,
Dunbar CE and Varticovski L: Molecular characterisation of side
population cells with cancer stem cell-like characteristics in
small-cell lung cancer. Br J Cancer. 102:1636–1644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yin B, Yang Y, Zhao Z, Zeng Y, Mooney SM,
et al: Arachidonate 12-lipoxygenase may serve as a potential marker
and therapeutic target for prostate cancer stem cells. Int J Oncol.
38:1041–1046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabuse M, Ohta S, Ohashi Y, Fukaya R, et
al: Functional analysis of HOXD9 in human gliomas and glioma cancer
stem cells. Mol Cancer. 10:602011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feuring-Buske M and Hogge DE: Hoechst
33342 efflux identifies a subpopulation of cytogenetically normal
CD34(+) CD38(-) progenitor cells from patients with acute myeloid
leukemia. Blood. 97:3882–3889. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirschmann-Jax C, Foster AE, Wulf GG,
Nuchtern JG, et al: A distinct 'side population' of cells with high
drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA.
101:14228–14233. 2004. View Article : Google Scholar
|
|
9
|
Mitsutake N, Iwao A, Nagai K, Namba H, et
al: Characterization of side population in thyroid cancer cell
lines: cancer stem-like cells are enriched partly but not
exclusively. Endocrinology. 148:1797–1803. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haraguchi N, Utsunomiya T, Inoue H, Tanaka
F, Mimori K, Barnard GF and Mori M: Characterization of a side
population of cancer cells from human gastrointestinal system. Stem
Cells. 24:506–513. 2006. View Article : Google Scholar
|
|
11
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bourguignon LY, Peyrollier K, Xia W and
Gilad E: Hyaluronan-CD44 interaction activates stem cell marker
Nanog, Stat-3-mediated MDR1 gene expression and ankyrin-regulated
multidrug efflux in breast and ovarian tumor cells. J Biol Chem.
283:17635–17651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao AC, Lou W, Sleeman JP and Isaacs JT:
Metastasis suppression by the standard CD44 isoform does not
require the binding of prostate cancer cells to hyaluronate. Cancer
Res. 58:2350–2352. 1998.PubMed/NCBI
|
|
14
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reya T and Clevers H: Wnt signaling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Liu W, Feng X, Wang L, Jiang X,
et al: Identification of ABCG2+ cells in nasopharyngeal carcinoma
cells. Oncol Rep. 27:1177–1187. 2012.PubMed/NCBI
|
|
17
|
Ma L, Lai D, Liu T, et al: Cancer
stem-like cells can be isolated with drug selection in human
ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin.
42:593–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He QZ, Luo XZ, Wang K, et al: Isolation
and characterization of cancer stem cells from high-grade serous
ovarian carcinomas. Cell Physiol Biochem. 33:173–184. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
20
|
Yanamoto S, Kawasaki G, Yamada S,
Yoshitomi I, Kawano T, et al: Isolation and characterization of
cancer stem-like side population cells in human oral cancer cells.
Oral Oncol. 47:855–860. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song J, Chang I, Chen Z, Kang M and Wang
CY: Characterization of side populations in HNSCC, highly invasive,
chemoresistant and abnormal wnt signaling. PLoS One. 5:e114562010.
View Article : Google Scholar
|
|
22
|
Gil J, Stembalska A, Pesz KA and Sasiadek
MM: Cancer stem cells: the theory and perspectives in cancer
therapy. J Appl Genet. 49:193–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komuro H, Saihara R, Shinya M, Takita J,
et al: Identification of side population cells (stem-like cell
population) in pediatric solid tumor cell lines. J Pediatr Surg.
42:2040–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Campbell PA, Perez-Iratxeta C,
Andrade-Navarro MA and Rudnicki MA: Oct4 targets regulatory nodes
to modulate stem cell function. PLoS One. 2:e5532007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu T, Liu S, Breiter DR, Wang F, Tang Y
and Sun S: Octamer 4 small interfering RNA results in cancer stem
cell-like cell apoptosis. Cancer Res. 68:6533–6540. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ponta H, Sherman L and Herrlich PA: CD44:
From adhesion molecules to signalling regulators. Nat Rew Mol Cell
Bio. 4:33–45. 2003. View
Article : Google Scholar
|
|
27
|
Honeth G, Bendahl PO, Ringnér M, Saal LH,
Gruvberger-Saal SK, et al: The CD44+/CD24−
phenotype is enriched in basal-like breast tumors. Breast Cancer
Res. 10:R532008. View
Article : Google Scholar
|
|
28
|
Dancescu M, Rubio-Trujillo M, Biron G,
Bron D, Delespesse G and Sarfati M: Interleukin 4 protects chronic
lymphocytic leukemic B cells from death by apoptosis and
upregulates Bcl-2 expression. J Exp Med. 176:1319–1326. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kieslinger M, Woldman I, Moriggl R, et al:
Antiapoptotic activity of Stat5 required during terminal stages of
myeloid differentiation. Genes Dev. 14:232–244. 2000.PubMed/NCBI
|
|
30
|
Prokopchuk O, Liu Y, Henne-Bruns D and
Kornmann M: Interleukin-4 enhances proliferation of human
pancreatic cancer cells: Evidence for autocrine and paracrine
actions. Br J Cancer. 92:921–928. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J and Wang CY: TBL1-TBLR1 and
beta-catenin recruit each other to Wnt target-gene promoter for
transcription activation and oncogenesis. Nat Cell Biol.
10:160–169. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang F, Zeng Q, Yu G, Li S and Wang CY:
Wnt/beta-catenin signaling inhibits death receptor-mediated
apoptosis and promotes invasive growth of HNSCC. Cell Signal.
18:679–687. 2006. View Article : Google Scholar
|
|
33
|
Chen S, Guttridge DC, You Z, Zhang Z,
Fribley A, et al: Wnt-1 signaling inhibits apoptosis by activating
b-catenin/Tcf-mediated transcription. J Cell Biol. 152:87–96. 2011.
View Article : Google Scholar
|