Introduction
A coma is a serious complication, which can occur
following traumatic brain injury (TBI) (1). The arousal of a patient from a coma
is a priority in improving the functional outcome of the patient,
reducing disability and increasing quality of life. Previous
evidence has indicated that the central orexinergic/hypocretinergic
systems exhibit prominent arousal promoting actions. Rejdak et
al (2) described a decreased
level of orexin-A in the cerebrospinal fluid of patients following
acute brain injury caused by a hemorrhagic stroke. The
hypocretin/orexin neuropeptide is synthesized exclusively in the
lateral hypothalamus and are involved in several brain functions,
including animal survival instincts, and the promotion and
maintenance of arousal in animals, a core process in animal
behavior (3). The orexinergic
neurons not only receive extensive innervations encoding
physiological, psychological and environmental cues, but also send
final outputs to key arousal-promoting brain areas (3). The arousal-promoting effects of
orexin-A act on the promotion of cortical activity and with other
neurons associated with wakefulness, including the histaminergic,
noradrenergic and serotonergic systems (4). However, whether orexin-A has a
wake-promoting effect on pathological conditions, including coma,
remains to be elucidated.
There are a number of therapeutic methods in the
promotion of arousal of patients in a comatose state. However, the
effects are not definite. Electrical stimulation (ES) has been used
as a therapeutic method in physical therapy for decades (5). Common types of ES involve deep brain
stimulation, cervical epidural spinal cord stimulation and
peripheral nerve stimulation. Median nerve stimulation (MNS), a
form of peripheral nerve stimulation, has been reported to be
effective, which has also been used for the treatment of
post-stroke, post-trauma, hypoxic states, and imaging and
laboratory measures, including electroencephalography (EEG),
single-photon emission computed tomography (SPECT) and
catecholamine metabolism have demonstrated clinical improvement in
patients treated with ES, compared with those without ES (6–8). The
mechanisms may involve activation of the neuro-endocrine system to
improve functioning following traumatic cerebral damage through
peripheral routes to central areas. It is hypothesized that the
peripheral stimuli connect with the ascending reticular activating
system (ARAS), which further connects with intralaminar nuclei of
the thalamus, stimulating cortical layer 1 and enhancing arousal
(7,9).
MNS may be an effective method of wake-promotion for
patients in a TBI-induced comatose state, which may act to activate
associated arousal systems, including the orexinergic system and
the upregulation of orexin-A and orexin receptor-1 (OX1R) in the
hypothalamus. The present study aimed to investigate the potential
mechanisms underlying the arousal-promoting action of MNS and
examine the key role of orexin-A in promoting arousal from a
comatose state.
Materials and methods
Animal model
A total of 100 male/female adult Sprague-Dawley rats
weighing 250–300 g were used in the present study. The rats were
obtained from the Institute of Laboratory Animals at Nanchang
University (Nanchang, China). All rats were housed in the
Laboratory Animal Center of the First Affiliated Hospital of
Nanchang University and were maintained in an air-conditioned room
with a 12-h light/dark cycle, fed a standard diet and with access
to water ad libitum. The protocol was approved by the Local
Animal Research Committee and was performed in accordance with the
Chinese Association for Accreditation of Laboratory Care and
National Institutes of Health (Bethesda, MD, USA) guidelines. The
ethical approval for the present study was obtained from the Ethics
Committee of Nanchang University. Due to a series of complications
caused by TBI, only 90 surviving rats were involved in the
experimental procedures. The animals were divided into the
following three groups, each containing 30 rats: i) Control group
without any interventions; ii) Sham-stimulated (TBI) group, in
which TBI was induced leading to a comatose state; iii) Stimulated
(TBI + MNS) group, in which rats were treated with MNS subsequent
to a TBI-induced coma. In the present study, the classic 'free fall
weight-drop model' was selected, which was developed initially by
Allne to model spinal cord injury in 1911. Subsequently, Scott
applied the method to investigate brain injury in 1940 and, in
1981, Freeney improved the technique and succeeded in generating a
cerebral cortex contusion model in rats (10). The experimental rats were
anesthetized using diethyl ether (concentration ≥99%) inhalation
and allowed to breathe air spontaneously. Whilst anesthesiatized,
conventional surgical techniques were used to expose the skull via
a 5 mm vertical incision. A cross hit point was marked using a
syringe needle at a 2 mm distance to the left midline and 1 mm
before the coronal suture. A cylindrical impact hammer, weighing
400 g, was dropped at a vertical height of 40–44 cm, along a
vertical metal bar, which hit a plastic spacer on the hit-point,
which had been marked, causing a concave fracture of the skull.
Following injury, the incision was closed, and the animals were
disinfected and transferred to a cage. After 1 h, the level of
consciousness was classified into six degrees, according to their
sensory and motor functions: i) Activities unlimited within the
cage; ii) activities decreased. iii) Activities decreased with
motor incoordination; iv) righting reflex could be elicited;
animals unable to stand. v) righting reflex disappeared; animals
able to react to pain; vi) no reaction to pain. Rats, which were
classified as degree v or vi, which lasted at least 30 min, were
deemed to be in a comatose state (11) and were used in the following
procedures.
MNS
The rats in the stimulated group were treated with
MNS following TBI when the total duration in a comatose state
reached 30 min. This was performed using a low frequency electrical
stimulator (ES-420; ITO Physiotherapy & Rehabilitation, Tokyo,
Japan). An acupuncture needle was inserted in the middle of the
wrist joint at a depth of 5 mm and was connected to the stimulator.
The parameters were as follows: Frequency of 30 Hz; pulse width of
0.5 ms; electrical current of 1.0 mA; stimulation duration of 15
min. According to the six assessment criteria of consciousness, the
behavior and consciousness of the animals were observed and
evaluated in the 1–2 h following completion of MNS. Finally, the
rats were returned back to their cages with sufficient food and
water until sacrifice.
Tissue extraction
The rats in the stimulated group were sacrificed 6,
12 or 24 h after MNS, respectively (n=10/time-point), at the same
time as the rats in the corresponding control and sham-stimulated
groups. Briefly, the rats were sacrificed using 10% chloral hydrate
i.p. (Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). The head was removed and the hypothalamic tissues were
separated on an ice box. The tissues were preserved in liquid
nitrogen (Beijing Solarbio Science & Technology Co., Ltd.). The
expression of orexin-A was detected using immunohistochemistry,
western blot analysis and ELISA.
Immunohistochemistry
Under deep anesthesia with intra-peritoneal chloral
hydrate (200 and 20 mg/kg, respectively), the rats were perfused
with phosphate-buffered saline (PBS) followed by 4% (v/v)
paraformaldehyde (Beijing Solarbio Science & Technology Co.,
Ltd.). The brains were post-fixed in the same fixative overnight,
and then cryoprotected in 20% sucrose in PBS overnight, following
which the tbrains were cut into 40-μm coronal sections using
a sliding microtome (RM2015; Leica Microsystems, Wetzlar, Germany).
For immunostaining, the sections were placed in 0.3% (w/w) hydrogen
peroxide for 30 min and then incubated overnight with the primary
antibody at room temperature. The primary antibodies included
polyclonal rabbit anti-OX1R antibody (cat. no. ab68718; Abcam, Hong
Kong, China) and peroxidase-conjugated addinipure goat anti-rabbit
IgG (H+L; cat. no. ZB-2301; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China). Immunohistochemistry was
assessed according to the average gray value and positive cell
expression intensity.
Western blotting
The tissue samples were homogenized using a tissue
protein extraction kit (cat. no. CW0891; Beijing Cangwei
Biotechnology Co., Ltd., Beijing, China). The kit contents included
25 ml tissue protein extraction reagent and 240 μl protease
inhibitors mixture. The homogenates were centrifuged at 12,000 × g
for 10 min at 4°C. The supernatants were divided into aliquots and
stored at −80°C. The concentration of proteins in each sample was
determined using a protein assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Equal quantities of the protein extract (15
μl; 7.01 μg/μl) were added to 10%
SDS-polyacrylamide gels and transferred onto a polyvinylidene
difluoride membrane (Beijing Solarbio Science & Technology Co.,
Ltd.). Western blotting was performed using a polyclonal rabbit
anti-OX1R antibody (1:200; cat. no. ab68718; Abcam) in
Tris-buffered saline with Tween-20 (TBS-T) containing 5% milk.
Following an overnight incubation at 4°C, the membranes were washed
three times with TBS-T and incubated for 1 h at room temperature
with peroxidase-conjugated addinipure goat anti-rabbit IgG (H+L;
1:3,000, cat. no. ZB-2301, Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) in TBS-T containing 5% milk. Subsequently,
the blots were treated with chemiluminescence substrate (cat. no.
32109, ECL Plus, GE Healthcare Bio-sciences, Pittsburgh, PA, USA,
containing 25 ml luminol/enhancer and 25 ml stable peroxide buffer,
and were quantified. Finally, the blots were stripped and reprobed
with anti-actin (1:400) to control for loading variations. Quantity
One software (version 4.6.2.70; Bio-Rad Laboratories, Inc.) was
used to quantify the protein bands. The results were expressed as
the mean ± standard deviation of the ratio of immunoreactivity
normalized against β-actin.
ELISA
The prepared tissue samples were assessed using an
ELISA kit for orexin-A (cat. no. cE90607; Wuhan USCN Life Science
Inc., Wuhan, China), containing a pre-coated, ready to use 96-well
strip plate, standard (liquid; x2), detection reagent A (120
μl), detection reagent B (120 μl), TMB substrate (9
ml), wash buffer (20 ml), plate sealer for 96 wells (x4), standard
diluent (20 ml), assay diluent A (6 ml), assay diluent B (6 ml) and
stop solution (6 ml). Following all reagent preparations and set-up
of wells for the diluted standard, blank and sample, the standard
and sample were diluted, respectively. When the reaction was
complete, the samples were analyzed using a microplate reader
(Model 680, Bio-Rad Laboratories, Inc.) and measured at 450 nm
immediately. The optical density value of the standard (X-axis) was
plotted against the log of the concentration of the standard
(Y-axis), and the concentration of orexin-A was calculated.
Statistical analysis
The data of orexin-A and OX1R in the hypothalamus
from the different groups and time-points are expressed as the mean
± standard deviation. The levels of orexin-A and OX1R were analyzed
statistically using a χ2 test, homoscedasticity test,
one-way analysis of variance, two-way classification analysis of
variance and a Kruskal-Wallis H test using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). For all comparisons, P<0.05 was considered to
indicate a statistically significant difference.
Results
Observation of behavior in rats following
MNS
At 1 h post-MNS, the behavior of rats were
re-evaluated using a double-blind technique. The results revealed
that only seven rats re-awaked of the 30 sham-stimulated rats (10
degree v; 13 degree vi). However, 21 rats re-awakened in the group
of 30 stimulated rats (6 degree v; 3 degree vi), with nine rats
remaining in a comatose state.
Immunohistochemistry
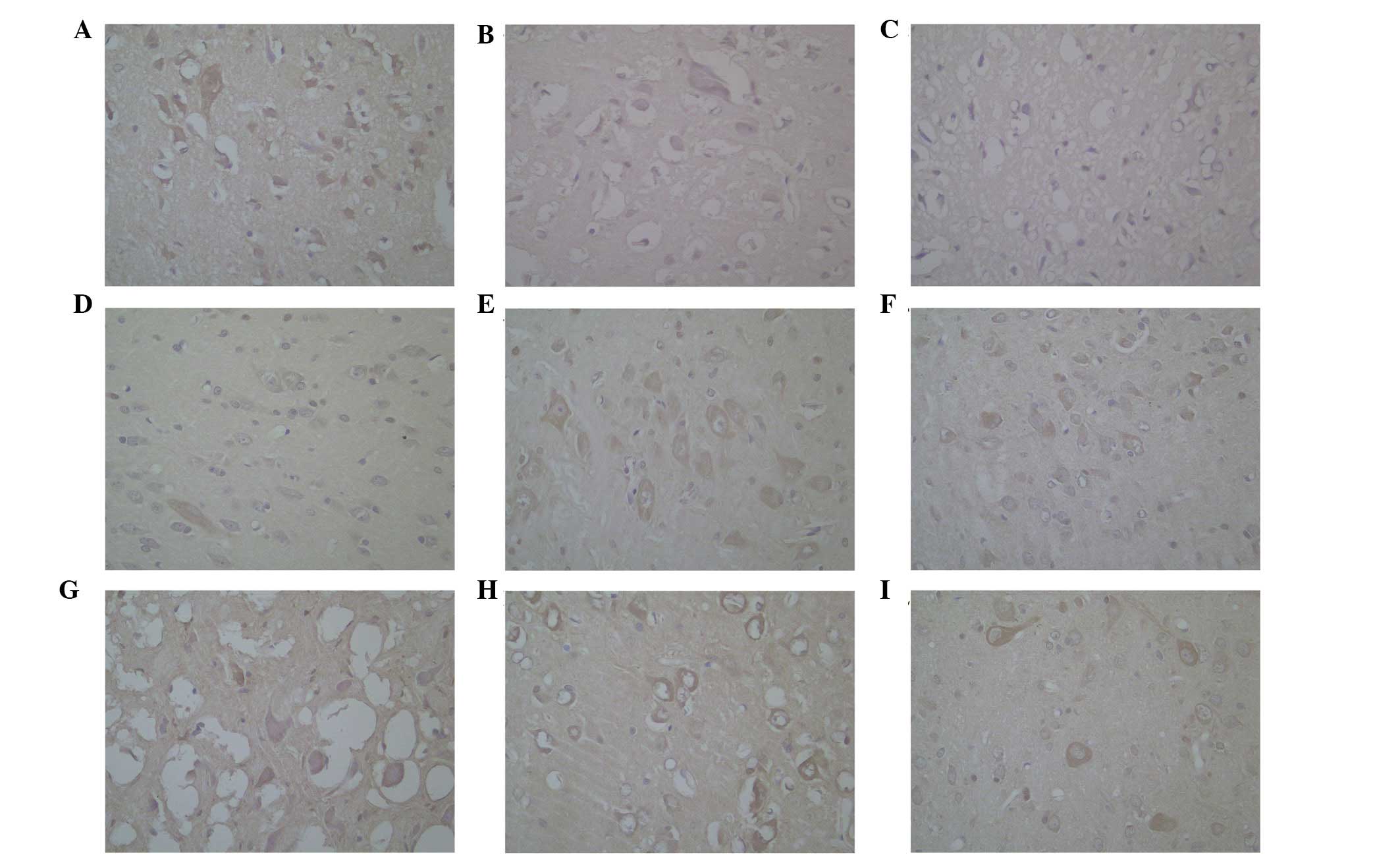
Immunoreactivity against OX1R was identified in the
cytoplasm of neurons in the hypothalamus (Fig. 1), which regularly lined the
circular profile in the cytoplasm. The histological data obtained
using the anti-OX1R (Fig. 1)
antibodies were similar in terms of the abundance of positive
cells, the localization of the positive material and the intensity
of staining.
On determining that OX1R was distributed in the
cytoplasm of neurons in the hypothalamus, the data from the groups
were analyzed using a Kruskal-Wallis H test. In the hypothalamus,
the levels of OX1R in the stimulated group (40.67) were higher,
compared with those in the control group (13.75) and
sham-stimulated groups (28.08; χ2=27.637; P<0.001).
No significant difference was observed between the levels of OX1R
in each group at the three different time-points.
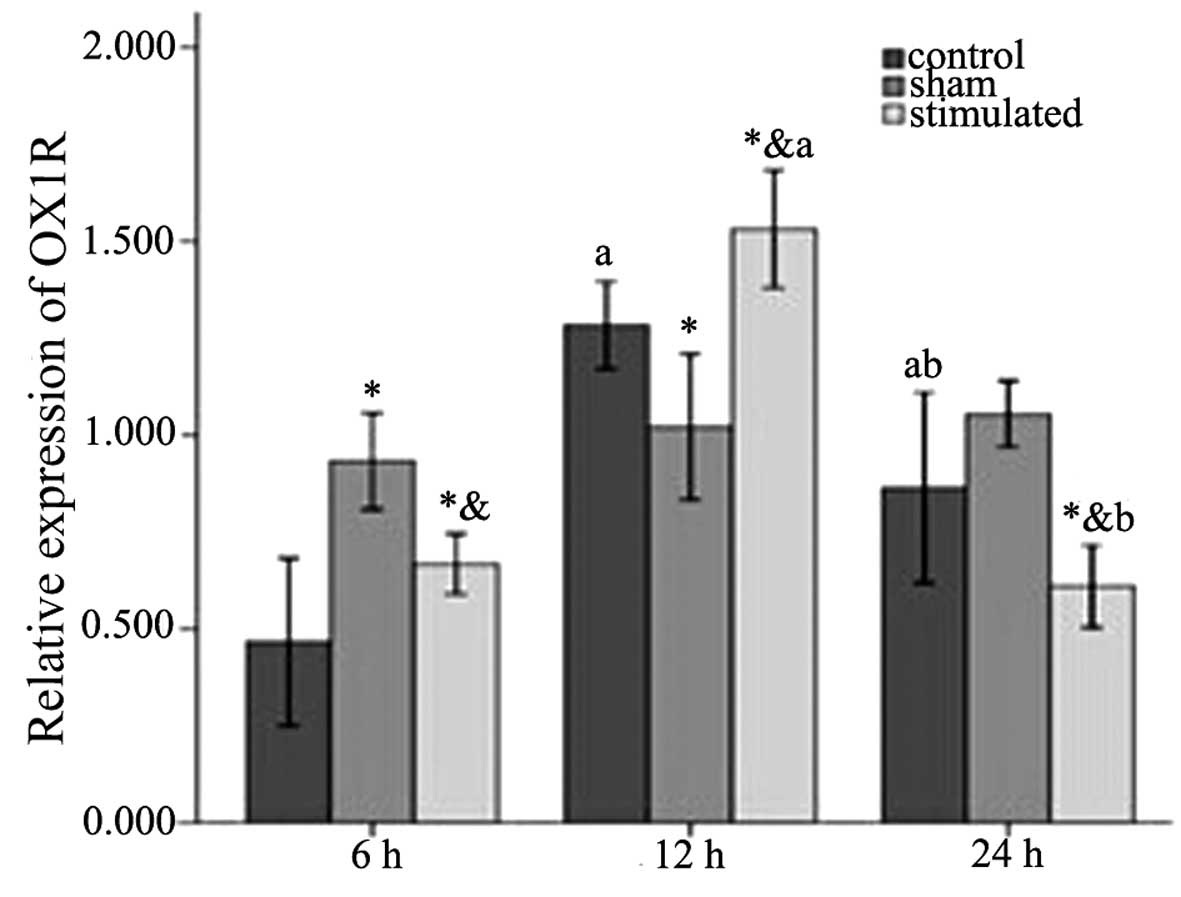
Western blotting
In the hypothalamus, OX1R exhibited statistically
significant differences among the three groups at 6 h
(control<stimulated<sham), 12 h
(sham<control<stimulated), 24 h
(stimulated<control<sham), and among the 6 h
(F2,88=10.279; P=0.001), 12 h (F2,88=11.102;
P=0.001) and 24 h (F2,88=9.347; P=0.002) time points
(Table I and Figs. 2 and 3). As for the difference within the
groups, the control group (6<24<1; F2,88=16.674;
P<0.001) and stimulated group (24<6<12;
F2,88=79.975; P<0.001) exhibited statistically
significant differences (Table I
and Fig. 3). The sham-stimulated
group exhibited an increasing trend at 6, 12 and 24 h, however, no
statistically significant difference was observed. The interaction
between the groups and time-points was further analyzed, which
revealed that there was an interacting effect between the two
factors (groups*time: F2,88=14.342;
P<0.001).
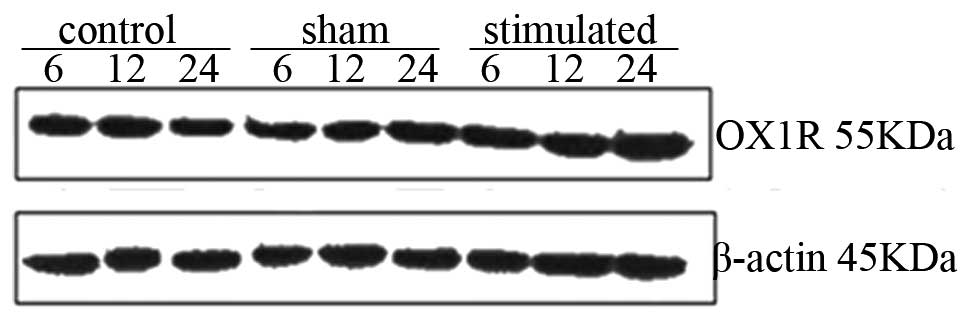
 | Figure 3Western blot analysis of OX1R in rat
hypothalamic tissues 6, 12 and 24 h. Relative protein levels of
OX1R were assessed using densitometry. Results were normalized to
values obtained for the protein expression of β-actin. The
anti-OX1R antibody detected a band of 55 kDa. The intensity of the
55 kDa band was markedly increased and the level of OX1R was
highest in the treatment group at 12 h. Statistical analyses
consisted of a one-way analysis of variance (n=10). Data are
expressed as the mean ± standard deviation. OX1R within each group
was compared at different time-points, aP<0.05, 12 h
and/or 24 h, compared with 6 h, bP<0.05, 24 h,
compared with 12 h. OX1R was compared among the three groups at 6,
12 or 24 h. *P<0.05, sham-stimulated group, compared
with control group. &P<0.05, stimulated group,
compared with sham-group. OX1R, orexin receptor-1. |
 | Table IExpression levels of OX1R and orexin-A
in the hypothalamus among the three treatment groups at each
time-point. |
Table I
Expression levels of OX1R and orexin-A
in the hypothalamus among the three treatment groups at each
time-point.
| Method | 6 h
| 12 h
| 24 h
|
|---|
| Control | Sham | Stimulated | Control | Sham | Stimulated | Control | Sham | Stimulated |
|---|
| WB | 0.467±0.265 | 0.933±0.151c | 0.668±0.117c,d | 1.283±0.137c | 1.021±0.230c | 1.531±0.215a,c | 0.864±0.301a,b | 1.054±0.104 | 0.610±0.158b,c,d |
| ELISA | 56.600±4.488 |
69.942±6.384c |
72.214±4.706c |
69.142±6.076c | 72.128±6.384 |
80.400±7.718a,c,d |
76.200±12.602a | 76.800±10.935 |
83.771±5.253a |
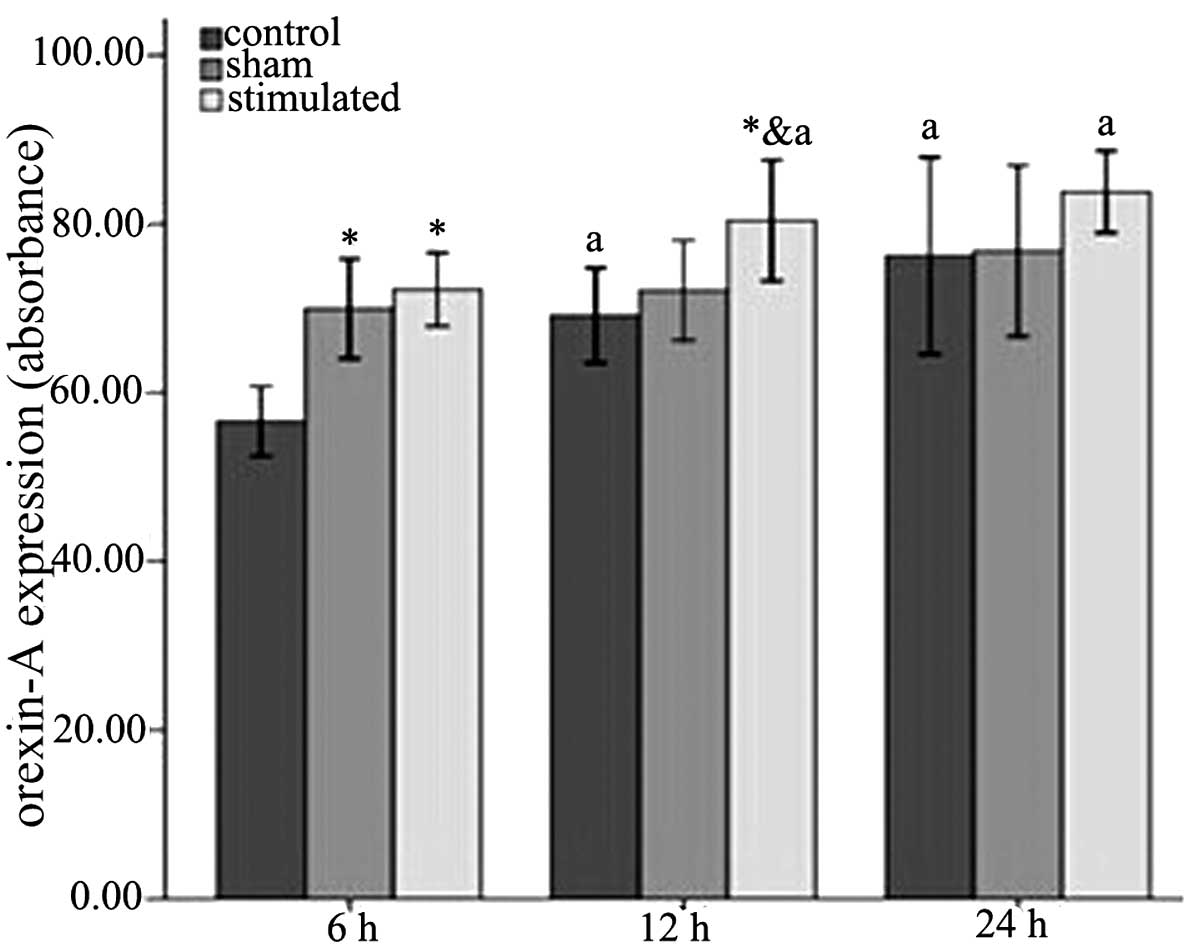
ELISA
The levels of orexin-A in the different groups and
time-points were detected using an ELISA. The level of orexin-A
level exhibited a significant increase among the three groups
(control<sham-stimulated<stimulated) at 6 h (P<0.001) and
12 h (P<0.05; Table I and
Fig. 4). This increasing trend in
the three groups was also observed at 24 h, however, without
statistical significance. As for the difference of orexin-A within
the groups, the three groups all exhibited the same trend
(6<12<24). However, only the control group exhibited
statistically significant differences (P<0.05; Table I and Fig. 4).
Discussion
Orexin-A is involved in a variety of physiological
functions, including feeding behaviors (12,13),
energy homeo-stasis (14–16), the sleep-wake cycle (17), and the activation of central
sympathetic outflow (18). A
previous study demonstrated the wake-promoting effects of orexin-A
and MNS (4). In addition, orexin-A
has been identified as important in the regulation of arousal.
Although the numbers of clinical studies investigating the actions
of MNS have increased, the exact mechanism remains to be
elucidated. Therefore, the present study hypothesized that MNS can
be used to promote wakefulness in patients in a comatose state, and
that one of the mechanisms was due to its positive effect on
orexin-A. There were four aspects arising from this hypothesis,
which the present study aimed to investigate. The first involved
the association between orexins and wakefulness, the second was
associated with the wake-promoting actions of MNS, the third was
associated with the mechanisms of MNS and, finally, the interacting
effects were examined.
Orexins, including orexin-A and orexin-B are two
excitatory hypothalamic neuropeptides, which are implicated in
narcolepsy (19). They are
synthesized by neurons situated within the caudal hypothalamus,
which projects to the locus coeruleus and other nuclei involved in
the regulation of sleep and wakefulness (20). Hypocretin selectively increases
dopaminergic neurotransmission within the prefrontal cortex and the
shell subregion of the nucleus accumbens, which is also important
in cognitive and affective processes (21). Orexin neurons receive abundant
input from the limbic system (22,23)
and enhance arousal signals in this area. In addition, orexin
neurons provide a link between energy homeostasis and vigilance
states (24). The data from the
present study revealed that higher levels of orexin-A and OX1R
following MNS led to arousal behavior in rats. Thus, it was
concluded that orexin is an essential neurotransmitter with an
arousal-promoting effect. The fluctuation of orexin-A may exert
effects on sleep-wakefulness and/or comatose states.
In 1996, peripheral nerve electrical stimulation in
the treatment of persistent vegetative states was reported by
Yokoyama et al (6). MNS is
a form of peripheral nerve electrical stimulation, which is a
non-invasive, convenient and economical form of therapy (25). Due to these characteristics, MNS
has gradually increased in popularity clinically. It has been
reported that right MNS, administered to patients in a comatose
state appears to accelerate awakening from a deep coma (9,26,27).
Similar to transcutaneous electrical nerve stimulation
investigations in Alzheimer's disease, the rationale underlying
investigations of coma treatment is that right MNS stimulates the
ARAS (9,28). Right MNS has provided promising
results in post-treatment improvements, including significantly
increased cerebral blood flow and improved electroencephalography
(6,7), improved Glasgow Coma Scale scores
(9) and, in certain cases,
significant or even complete arousal and consciousness (5,9,26).
Comparison of pre- and post-treatment SPECT imaging for a series of
patients revealed 29 patients (71%) with increased cerebral
perfusion following MNS, while cerebral perfusion was unchanged in
nine patients (22%) and decreased from baseline in three patients
(7%) (29). It follows that MNS
may promote patients in a comatose state to re-awaken and stimulate
the central nervous system. In the present study, eight rats in the
stimulated group exhibited behavioral actions following MNS,
including righting reflex, reactions to pain stimuli and locomotor
behavior. However, only one rat recovered motor function with the
ability to react to outside stimuli. This was considered a
spontaneous recovery, which may have been associated with the
degree of injury, individual resistance against damage or somatic
function. These results inferred that MNS re-awaked animals from an
induced comatose state. By contrast, the levels of orexin-A and
OX1R were significantly increasing following MNS, which suggested
there may be specific connections between MNS, orexin-A and
re-awakening from a comatose state.
Although previous investigations have described
substantial clinical studies regarding MNS, the exact mechanism of
the wake-promoting action of MNS remains to be fully elucidated.
The present study aimed to examine the molecular mechanism
associated with the neurotransmitter, orexin-A, in the
wake-promoting actions of MNS via examining alterations in the
levels of orexin-A and OX1R. Several potential mechanisms have been
suggested. MNS administration evokes an arousal-promoting effect,
possibly through increasing cerebral blood flow and raising levels
of dopamine, improving blood supply in ischemic regions, reducing
the number of necrotic neurons and protecting neuronal repair and
regeneration. Secondly, MNS enhances activities on EEG and
electro-neurophysiology assessments. Peripheral electrical signal
inputs excite the ARAS and cerebral cortex directly. The ARAS
originates in the brain stem reticular formation, more specifically
the locus coeruleus and the dorsal raphe nucleus, including the
noradrenergic and serotonergic neurotransmitter systems,
respectively. In addition, it also has connections with the nucleus
basalis of Meynert/cholinergic system (28,30,31).
Thirdly, the levels of important neurotransmitters change following
MNS. This may be beneficial to relieve patient symptoms and restore
corresponding neural functions, including consciousness, mobility
and speaking ability. MNS induces the release of acetylcholine from
the ARAS, activating the intralaminar nuclei in the thalamus, which
have a general excitatory effect on cortical layer 1, and the locus
coeruleus, which is located close to the ARAS and releases
norepinephrine leading to stimulation of cortical layer 1. Based on
these findings, MNS has been regarded as a 'portal' between the
periphery and the central nervous system (28). It has also been reported that
feedback from the periphery may contribute to the splitting of
circadian rhythms in hamsters (32). In the present study, the expression
levels of orexin-A and OX1R exhibited an increasing trend following
MNS in the hypothalamus (P<0.05). As for the three time-points,
the levels of OX1R also increased significantly (P<0.05). The
ELISA demonstrated that the expression level of orexin-A in the
stimulated group was higher, compared with those in the other two
treatment groups and revealed a significant increasing trend
between 6, 12 and 24 h (P<0.05). However, the difference between
the control group and the sham-stimulated group was not
statistically significant. Thus, the hypothesis that orexin-A and
OX1R are upregulated following MNS in TBI-induced-comatose rats was
confirmed. This suggested that one of the mechanisms underlying the
effect of MNS on comatose states may be attributed to its effect on
associated neurotransmitters, including orexin-A.
The results of the present study revealed that, in
the hypothalamus, the expression of OX1R was significantly
different among three groups at 6 h
(control<stimulated<sham), 12 h
(sham<control<stimulated), 24 h
(stimulated<control<sham) and between the three time-points
(P<0.05). Within the groups, the control (6<24<12 h;
P<0.001) and stimulated group (24<6<12 h; P<0.001)
exhibited statistically significant differences. The
sham-stimulated group exhibited an increasing trend at 6, 12 and 24
h, however, no statistically significant difference was observed.
By contrast, the level of orexin-A differed significantly among the
three groups (control<sham-s timulated<stimulated) at 6 h
(P<0.001) and 12 h (P<0.05). This increasing trend in the
three groups was also observed at 24 h, but without statistical
significance. In addition, the difference in orexin-A within the
three groups exhibited the same trend (6<12<24 h). However,
only the control group exhibited a statistically significant
difference (P<0.05). Based on these findings, the interactive
effect between the groups and time-points was analyzed to determine
whether these two factors interacted, and an interactive effect was
identified between the groups and time-points (P<0.001). This
may be due to its own rhythm of hypothalamic and/or orexin neurons.
Lateral hypothalamic orexin-A neurons are rhythmic, innervated by
suprachias-matic nucleus (SCN) efferents and are important
components of the reproductive and arousal systems (32). As has been established, the
hypothalamus is associated with circadian rhythm. Neurons in the
SCN function as the master circadian clock in the brain and are
important for hypothalamic rhythm. The SCN regulates orexin neurons
so that they are more active during the circadian night, compared
with the circadian day. Orexinergic innervation and the expression
of genes encoding orexin receptors (OX1 and
OX2) has also been reported in the mouse SCN, with
OX1 being upregulated at dusk (33). Hypocretin deficiency can disturb
the circadian control of melatonin release and its temporal
association with sleep (34). An
investigation of the orange-spotted grouper (Epinephelus
coioides) demonstrated that hypothalamic mRNA levels of
prepro-orexin were higher in the light phase, compared with the
dark phase, and increased significantly at feeding times (35). These observations indicated that
orexinergic neurons exhibit a rhythmic activity, and may be active
in the day and inactive at night, which are important components of
the reproductive and arousal systems (32). Therefore, the present study
hypothesized that the rhythm of orexinergic neurons caused the
observed interactive effects between the groups and time-points.
This may explain why the levels of orexin-A and OX1R changed in
each group 6, 12 and 24 h after the experiment.
It is important to note that the present study
examined only orexin-A and OX1R in the hypothalamic region. Whether
other brain regions are involved in wake-promotion or alterations
in the levels of orexin-A and its receptor 24 h after TBI-induced
coma, and how MNS elicits orexin-A increase, remains to be
elucidated and requires further investigation. In addition, the
present study did not demonstrate whether MNS exerted a direct or
indirect effect on orexin-A and OX1R. However, the present study
suggested that the level of orexin-A is important in the
wake-promoting process, and that the wake-promoting action of MNS
was, at least partially, due to the upregulation of orexin-A and
OX1R. To overcome these limitations, other brain regions and
neurotransmitters associated with orexin-A and/or wakefulness may
be considered to determine whether orexin-A acts as an arousal
'switch'. In addition, orexin-A−/− knock-out animal
investigations and the corresponding pathways in ORX neurons
require consideration in further investigations to clarify the
mechanisms of orexin-A and MNS.
In conclusion, the present study demonstrated that
MNS enhanced the expression levels of orexin-A and OX1R in the
hypothalamus, leading to wakefulness from a TBI-induced comatose
state, and that orexin-A was important in arousal-promotion. These
results suggested that the wake-promoting action of MNS may act on
stimulating arousal-associated neurotransmitters, which are
released in the hypothalamic region, including orexin-A.
Acknowledgments
This study was supported by a grant from the Natural
Science Foundation of China (grant no. 81260295) and the Natural
Science Foundation of Jiangxi Province (grant no.
2013BAB205063).
References
|
1
|
Harvey HH: Reducing traumatic brain
injuries in youth sports: youth sports traumatic brain injury state
laws, January 2009–December 2012. Am J Public Health.
103:1249–1254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rejdak K, Petzold A, Lin L, Smith M,
Kitchen N and Thompson E: Decreased CSF hypocretin-1 (orexin-A)
after acute haemorrhagic brain injury. J Neurol Neurosurg
Psychiatyr. 76:597–598. 2005. View Article : Google Scholar
|
|
3
|
Gao XB and Wang AH: Experience-dependent
plasticity in hypocretin/orexin neurons re-setting arousal
threshold. Acta Physiol (Oxf). 198:251–262. 2010. View Article : Google Scholar
|
|
4
|
de Lecea L: A decade of hypocretins: past,
present and future of the neurobiology of arousal. Acta phyisiol
(Oxf). 198:203–208. 2010. View Article : Google Scholar
|
|
5
|
Peri CV, Shaffrey ME, Farace E, Cooper E,
Alves WM, Cooper JB, Young JS, et al: Pilot study of electrical
stimulation on median nerve in comatose severe brain injured
patients: 3-month outcome. Brain Inj. 15:903–910. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokoyama T, Kamei T and Kanno T: Right
median nerve stimulation for comatose patients. Soc Treat Coma.
5:117–125. 1996.
|
|
7
|
Suzuki A, Nishimura H, Yoshioka K, et al:
Electrical stimulation of median nerve in patients of prolonged
coma. Soc Treat Coma. 3:75–85. 1994.
|
|
8
|
Moriya T, et al: New therapeutic
strategies for patients with unconsciousness and neurological
deficits in acute stage with median nerve stimulation. Soc Treat
Coma. 7:65–69. 1998.
|
|
9
|
Cooper JB, Jane JA, Alves WM and Cooper
EB: Right median nerve electrical stimulation to hasten awakening
from coma. Brain Inj. 13:261–267. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feeney DM, Boyeson MG, Linn RT, Murray HM
and Dail WG: Responses to cortical injury: I. Methodology and local
effects of contusions in the rat. Brain Res. 211:67–77. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stephens JR and Levy RH: Effects of
valproate and citrulline on ammoniuminduced encephalopathy.
Epilepsia. 35:164–171. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakurai T, Amemiya A, Ishii M, Matsuzaki
I, Chemelli RM, Tanaka H, Williams SC, et al: Orexins and orexin
receptors: a family of hypothalamic neuropeptides and G
protein-coupled receptors that regulate feeding behavior. Cell.
92:573–585. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Randeva HS, Karteris E, Grammatopoulos D
and Hillhouse EW: Expression of orexin-A and functional orexin type
2 receptors in the human adult adrenals: implications for adrenal
function and energy homeostasis. J Clin Endocrinol Metab.
86:4808–4813. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams G, Bing C, Cai XJ, Harrold JA,
King PJ and Liu XH: The hypothalamus and the control of energy
homeostasis: different circuits, different purposes. Physiol Behav.
74:683–701. 2001. View Article : Google Scholar
|
|
15
|
Smart D and Jerman J: The physiology and
pharmacology of the orexins. Pharmacol Ther. 94:51–61. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kukkonen JP, Holmqvist T, Ammoun S and
Akerman KE: Functions of the orexigenic/hypocretinergic system. Am
J Physiol Cell Physiol. 283:C1567–C1591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taheri S, Ward H, Ghatei M and Bloom S:
Role of orexins in sleep and arousal mechanisms. Lancet.
355:8472000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Samson WK, Gosnell B, Chang JK, Resch ZT
and Murphy TC: Cardiovascular regulatory actions of the hypocretins
in brain. Brain Res. 831:248–253. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moreno-Balandrán E, Garzón M, Bódalo C,
Reinoso-Suárez F and de Andrés I: Sleep-wakefulness effects after
microinjections of hypocretin 1 (orexin A) in cholinoceptive areas
of the cat oral pontine tegmentum. Eur J Neurosci. 28:331–341.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peyron C, Tighe DK, van den Pol AN, de
Lecea L, Heller HC, Sutcliffe JG and Kilduff TS: Neurons containing
hypocretin (orexin) project to multiple neuronal systems. J
Neurosci. 18:9996–10015. 1998.PubMed/NCBI
|
|
21
|
Vittoz NM, Schmeichel B and Berridge CW:
Hypocretin/orexin preferentially activates caudomedial ventral
tegmental area dopamine neurons. Eur J Neurosci. 28:1629–1640.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakurai T, Nagata R, Yamanaka A, Kawamura
H, Tsujino N, Muraki Y, Kageyama H, et al: Input of
orexin/hypocretin neurons revealed by a genetically encoded tracer
in mice. Neuron. 46:297–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshida K, McCormack S, España RA, Crocker
A and Scammell TE: Afferents to the orexin neurons of the rat
brain. J Comp Neurol. 494:845–861. 2006. View Article : Google Scholar
|
|
24
|
Yamanaka A, Beuckmann CT, Willie JT, Hara
J, Tsujino N, Mieda M, Tominaga M, et al: Hypothalamic orexin
neurons regulate arousal according to energy balance in mice.
Neuron. 38:701–713. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cossu G: Therapeutic options to enhance
coma arousal after traumatic brain injury: State of the art of
current treatments to improve coma recovery. Br J Neurosurg.
28:187–198. 2014. View Article : Google Scholar
|
|
26
|
Cooper EB and Cooper JB: Electrical
treatment of coma via the median nerve. Acta Neurochir Suppl.
87:7–10. 2003.PubMed/NCBI
|
|
27
|
Liu JT, Wang CH, Chou IC, Sun SS, Koa CH
and Cooper E: Regaining consciousness for prolonged comatose
patients with right median nerve stimulation. Acta Neurochir Suppl.
87:11–14. 2003.PubMed/NCBI
|
|
28
|
Cooper EB, Scherder EJ and Cooper JB:
Electrical treatment of reduced consciousness: Experience with coma
and Alzheimer's disease. Neuropsychol Rehabili. 15:389–405. 2005.
View Article : Google Scholar
|
|
29
|
Liu JT, Lee JK, Tyan YS, Liu CY and Lin
TB: Change in cerebral perfusion of patients with coma after
treatment with right median nerve stimulation and hyperbaric
oxygen. Neuromodulation. 11:296–301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sauvage M and Steckler T: Detection of
corticotrophin-releasing hormone receptor 1 immunoreactivity in
cholinergic, dopami-nergic and noradrenergic neurons of the murine
basal forebrain and brainstem nuclei-potential implication for
arousal and attention. Neuroscience. 104:643–652. 2001. View Article : Google Scholar
|
|
31
|
Kayama Y and Koyama Y: Brainstem neural
mechanisms of sleep and wakefulness. Eur Urol. 33:12–15. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Butler MP, Rainbow MN, Rodriguez E, Lyon
SM and Silver R: Twelve-hour days in the brain and behavior of
split hamsters. Eur J Neurosci. 36:2556–2566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Belle MD, Hughes AT, Bechtold DA,
Cunningham P, Pierucci M, Burdakov D and Piggins HD: Acute
suppressive and long-term phase modulation actions of orexin on the
mammalian circadian clock. J Neurosci. 34:3607–3621. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Donjacour CE, Kalsbeek A, Overeem S,
Lammers GJ, Pévet P, Bothorel B, Pijl H and Aziz NA: Altered
circadian rhythm of melatonin concentrations in
hypocretin-deficient men. Chronobio Int. 29:356–362. 2012.
View Article : Google Scholar
|
|
35
|
Yan A, Zhang L, Tang Z, Zhang Y, Qin C, Li
B, Li W and Lin H: Orange-spotted grouper (Epinephelus coioides)
orexin: molecular cloning, tissue expression, ontogeny, daily
rhythm and regulation of NPY gene expression. Peptides.
32:1363–1370. 2011. View Article : Google Scholar : PubMed/NCBI
|