Introduction
In total, ~500,000 new cases of head and neck
squamous cell carcinoma (HNSCC) are diagnosed annually worldwide
(1). The majority of these
patients suffer from locally advanced stage disease (2). The treatment options for head and
neck cancer include surgery, radiation, chemotherapy or a
combination of these modalities. Unresectable SCC formations of the
head and neck are frequently treated with concurrent chemotherapy
(3). However, 40–60% of patients
suffering from HNSCC develop loco-regional failure and distant
metastases (4). It has been
suggested that this cancer treatment failure is due to the presence
of a certain subpopulation of cancer cells, termed cancer stem
cell-like cells or cancer initiating cells (CSCs) (5,6).
Therefore, there is an increasing requirement for more cancer
cell-specific drugs. Gupta et al (7) investigated salinomycin as a selective
killer of CSCs in a high-throughput screening investigation.
Salinomycin was the most effective agent at destroying CSCs out of
a total of 16,000 compounds (7).
Salinomycin is a 751 Dalton monocarboxylic polyether antibiotic,
which is isolated from the bacterium Streptomyces albus
(8). It has a broad spectrum of
bioactivity, including antibacterial, antifungal, antiparasetic,
antimalarial and tumor cell cytotoxicity (9). It is widely used in veterinary
medicine as an antiprotozoal agent against coccidial parasites.
Salinomycin is >100-fold more effective than paclitaxel at
causing CSC death (7). However,
the complex underlying mechanisms involved remain to be fully
elucidated. A previous study by Riccioni et al (10) reported that salinomycin acts as a
potent inhibitor of p-glycoprotein.
The potential clinical treatment options of
salinomycin are restricted by neuronal, muscular and other
toxicities (11). The former is
mediated by an increased concentration of Na+, which
leads to an increase in cytosolic Ca2+ concentration via
the Na+/Ca2+ exchanger
(Na+/Ca2+-X) in the plasma membrane and the
mitochondria (11). By inhibiting
the mitochondrial Na+/Ca2+-X using the
benzodiazepine derivate, CGP37157 (CGP), Boehmerle and Endres
(11) demonstrated a significant
reduction in salinomycin neuronal toxicity. CGP may offer potential
as a novel additive in treatment with salinomycin to reduce its
toxicity and, thus enable future clinical use. The aim of the
present study was to investigate whether CGP also inhibits the
tumor toxicity of salinomycin.
Materials and methods
Culture of the human carcinoma cell
line
The FaDu HNSCC cell line (American Type Culture
Collection, Manassas, VA, USA), which is derived from a human
hypopharyngeal carcinoma, and HLaC79 clone 1 (C1), a paclitaxel
resistant clone, were used in the present study. The HLaC79 C1 was
generated in the Department of Otolaryngology, University of
Wuerzburg (Wuerzburg, Germany) (12–14).
The cells were grown in RPMI-1640 medium (Biochrom AG, Berlin,
Germany), supplemented with 10% fetal calf serum (Linaris,
Wertheim-Bettingen Germany), 100 U/ml penicillin, 100 μg/ml
streptomycin, 1% sodium pyruvate (100 mM; Biochrom AG) and 1%
non-essential amino acids (100-fold concentration; Biochrom AG),
which was termed RPMI-expansion medium (RPMI-EM). The cells were
cultured at 37°C with 5% CO2 in culture flasks. The
medium was replaced every other day and passaging was performed on
reaching 70–80% confluence by trypsinization (0.25% trypsin; Gibco
Life Technologies, Karlsruhe, Germany), washing with
phosphate-buffered saline (PBS; Roche Diagnostics GmbH, Mannheim,
Germany) and seeding into flasks or treatment wells. The subsequent
experiments were performed using cells in the exponential growth
phase.
The HNSCC cells were were seeded at a density of
6×103 cells/well and incubated for 24 h at 37°C.
Subsequently, the cells were treated either with salinomycin (1, 5
and 10 μM; Sigma-Aldrich, Schnelldorf, Germany) or with
salinomycin (1, 5 and 10 μM) + CGP (10 μM;
Sigma-Aldrich) for 24 h, in order to assess the possible
interaction between salinomycin and CGP. All experiments were
performed in triplicate.
Cell morphology and fluorescein diacetate
assay (FDA)
The cells were cultured as a monolayer and in
three-dimensional conditions (spheroids). Following the treatment
with salinomycin, an FDA (5 mg/ml FDA in acetone; Sigma-Aldrich)
was used in order to identify viable cells in the culture.
Following treatment with salinomycin and salinomycin + CGP for 24
h, the cells (6×103) were stained with a solution of 80
μg/ml fluorescein diacetate and 50 μg/ml ethidium
bromide (Sigma-Aldrich). Subsequently, the cells were observed
under a fluorescence microscope (Leica DMI 4000B Inverted
Microscope; Leica Microsystems, Wetzlar, Germany). Living cells
convert the non-fluorescent FDA into the green fluorescent
compound, fluorescein, while dead cells exhibit red-orange staining
in their nuclei.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) assay and clonogenic assay
The MTT assay (Sigma-Aldrich) is a colorimetric
staining method, according to Mosmann (15) and was used to study the viability
of cells. The plates were incubated with 100 μl MTT (1
mg/ml), followed by 5 h incubation at 37°C with 5% CO2.
Following the removal of MTT, 100 μl isopropanol
(Sigma-Aldrich) was added for 1 h at 37°C with 5% CO2.
The viability of the cells was quantified by measuring the
absorbance at 570 nm (Titertek Multiskan PLUS (MK II) ELISA-reader;
Labsystems, Helsinki, Finland).
To determine the long-term effects of the treatment
reagents, the cells were treated with salinomycin for 24 h at 37°C.
Subsequently, the supernatant was removed and fresh medium was
added. Following incubation for 14 days at 37°C the cell colonies
were stained with crystal violet (0.4 g/l; Sigma-Aldrich) and
counted using the Leica DMI 4000B Inverted Microscope (Leica
Microsystems, Wetzlar, Germany).
Annexin V-propidium iodide (PI)
staining
Apoptosis of the FaDu and HLaC79 C1 HNSCC cell lines
was assessed using an Apoptosis Detection kit (BD Biosciences,
Heidelberg, Germany). Following exposure to salinomycin or
salinomycin + CGP for 24 h at different concentrations (1, 5 and 10
μM) at 37°C, the cells in suspension and the adherent cells
were harvested and washed twice with cold PBS. The cells were
resuspended in 1:10 binding buffer (Sigma-Aldrich), containing 0.1
M HEPES (pH 7.4), 1.4 M NaCl and 25 mM CaCl2, at a
concentration of 1×106 cells/ml, and 100 μl
aliquots of this cell suspension (1×105 cells) were
subsequently transferred to a 5 ml culture tube. Annexin V-APC (5
μl) and PI (5 μl) were added to each aliquot
containing 1×105 cells. Following incubation for 15 min
in the dark at room temperature, the cells were resuspended in 400
μl 1:10 binding buffer. A FACSCanto flow cytometer (Beckton
Dickinson, Heidelberg, Germany) was used to analyze the samples. PI
staining is visible in cells with damaged membranes, as observed
during necrosis.
Total RNA extraction, and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The expression levels of MDR-1 in the FaDu
and HLaC79 C1 cells were investigated using RT-qPCR. The total RNA
was extracted from the cells using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA), according to the manufacturer's
instructions. The extracted total RNA was reverse transcribed into
cDNA using the High-Capacity RNA-to-cDNA Master Mix (Applied
Biosystems, Foster City, CA, USA). For the quantification of gene
expression, a SYBR Green PCR Master Mix kit (Applied Biosystems)
was used. The MDR-1 primer was purchased from Applied Biosystems,
with the following sequences: Forward 5′-AGAAAGCGAAGCAGTGGT TCA-3′
and reverse 5′-CGAACTGTAGACAAACGATGAG CTA-3′. PCR was performed in
duplicate using 100 ng cDNA per replicate on the StepOnePlus
Real-Time PCR system (Applied Biosystems). The amplifications for
gene quantification were as follows: 50°C for 2 min, 95°C for 10
min and 40 cycles of 95°C for 15 sec and 60°C for 1 min. RNA levels
were quantified using a photometer (BioPhotometer; Eppendorf,
Hamburg Germany) and the expression levels of GAPDH were used as an
internal control.
Statistical analysis
All data were analyzed by statistical analysis with
GraphPad Prism software, version 4.0 (GraphPad Software, Inc., La
Jolla, CA, USA). To investigate whether the cytotoxic effect of
salinomycin was dose-dependent, Friedman's test was used. The
Kruskal-Wallis test was conducted for all other tests to evaluate
statistical significance. P<0.05 was considered to indicate a
statistically significant difference.
Results
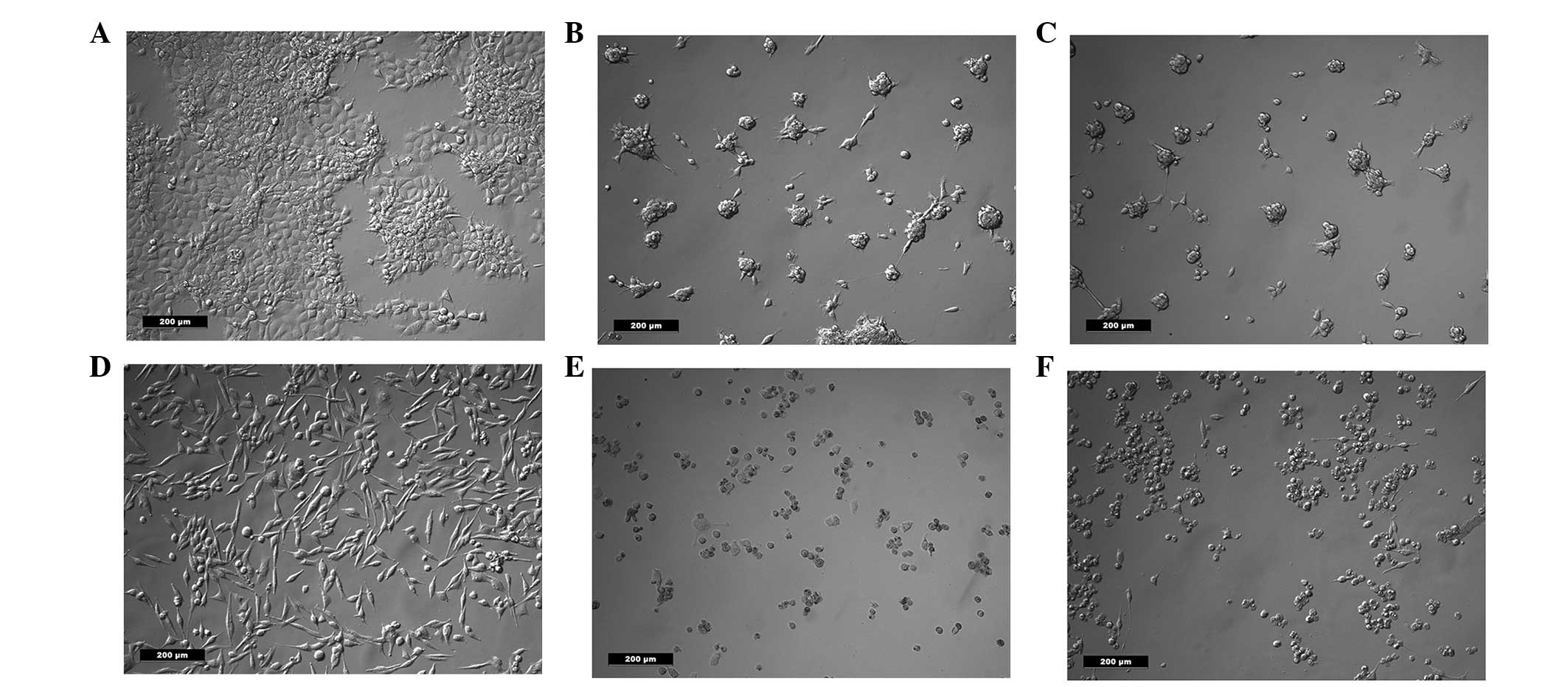
Cell morphology and FDA
Following treatment with salinomycin or salinomycin
+ CGP, the morphology of the FaDu and HLaC79 C1 cells was assessed
using an inverted microscope. No morphological differences were
observed between the cells treated with salinomycin and the cells
treated with salinomycin + CGP (Fig.
1). In each group, the cells were rounded, suggesting they were
undergoing apoptosis.
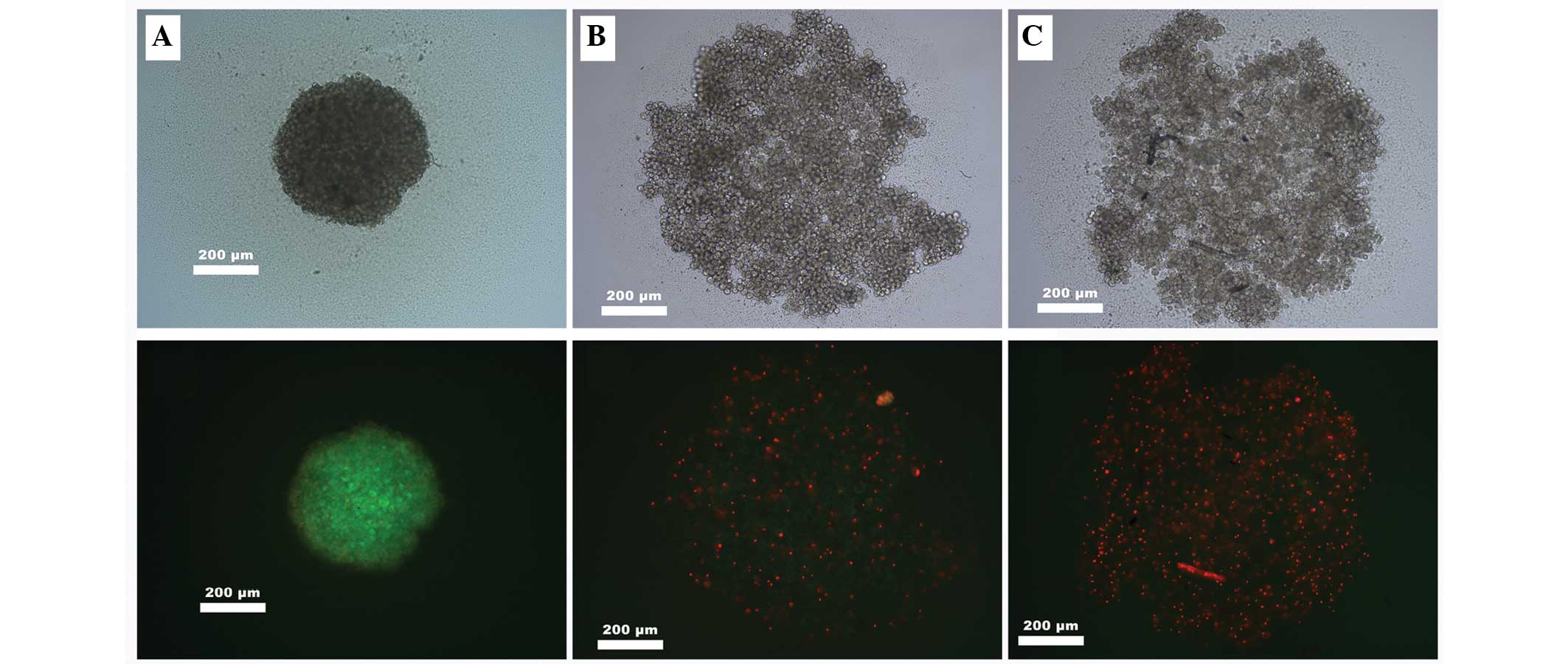
Following spheroid formation, the cells were treated
with salinomycin or salinomycin + CGP. The cells were then stained
with FDA solution. A significant attenuation of cell viability was
observed following the FDA staining, with no weakening of
salinomycin tumor toxicity caused by the addition of CGP (Fig. 2).
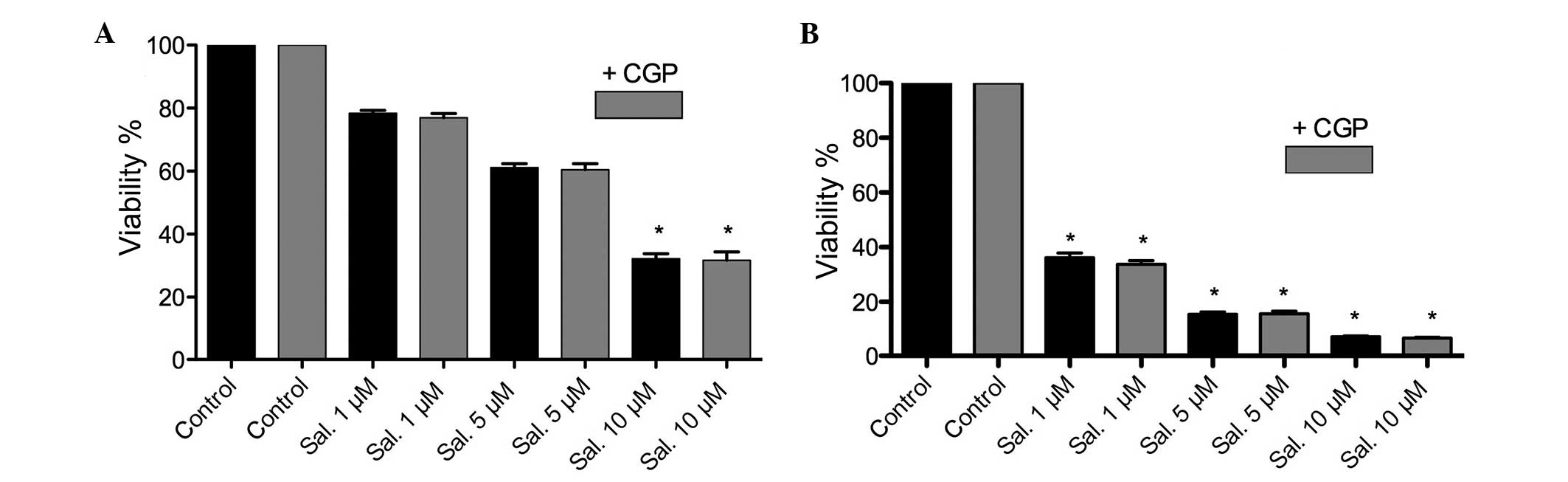
MTT assay and clonogenic assay
Following treatment with salinomycin or salinomycin
+ CGP, an MTT assay was performed. A significant (P<0.05)
dose-dependent attenuation of cell viability was observed following
treatment with salinomycin. The combination of salinomycin and CGP
revealed no inhibition of salinomycin tumor toxicity. However, a
significant difference between the FaDu and HLaC79 C1 cells was
observed in terms of the concentration of salinomycin (P<0.05).
The half maximal inhibitory concentration (IC50) of
salinomycin in the FaDu cells was calculated to be between 5 and 10
μM, whereas the IC50 of the HLaC79 C1 cells was
calculated to be between 0.5 and 1 μM. The MTT assay
revealed no counteraction of salinomycin tumor toxicity following
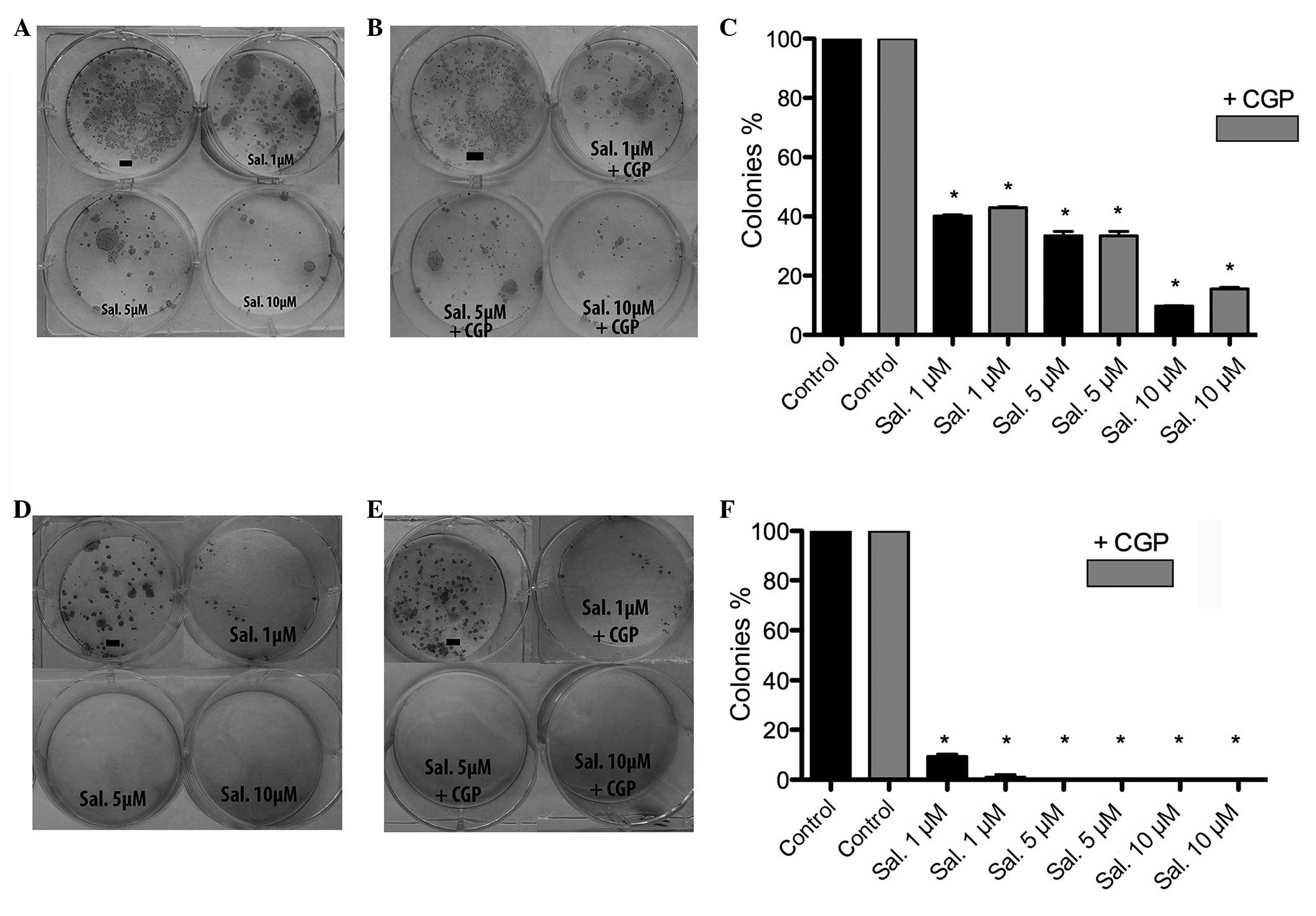
the addition of CGP. Assessment of colony formation revealed the
inhibition of colony formation following treatment with
salinomycin, which was not inhibited by the addition of CGP. The
HLaC79 C1 cells reacted more sensitively to salinomycin, compared
with the FaDu cells (Figs. 3 and
4).
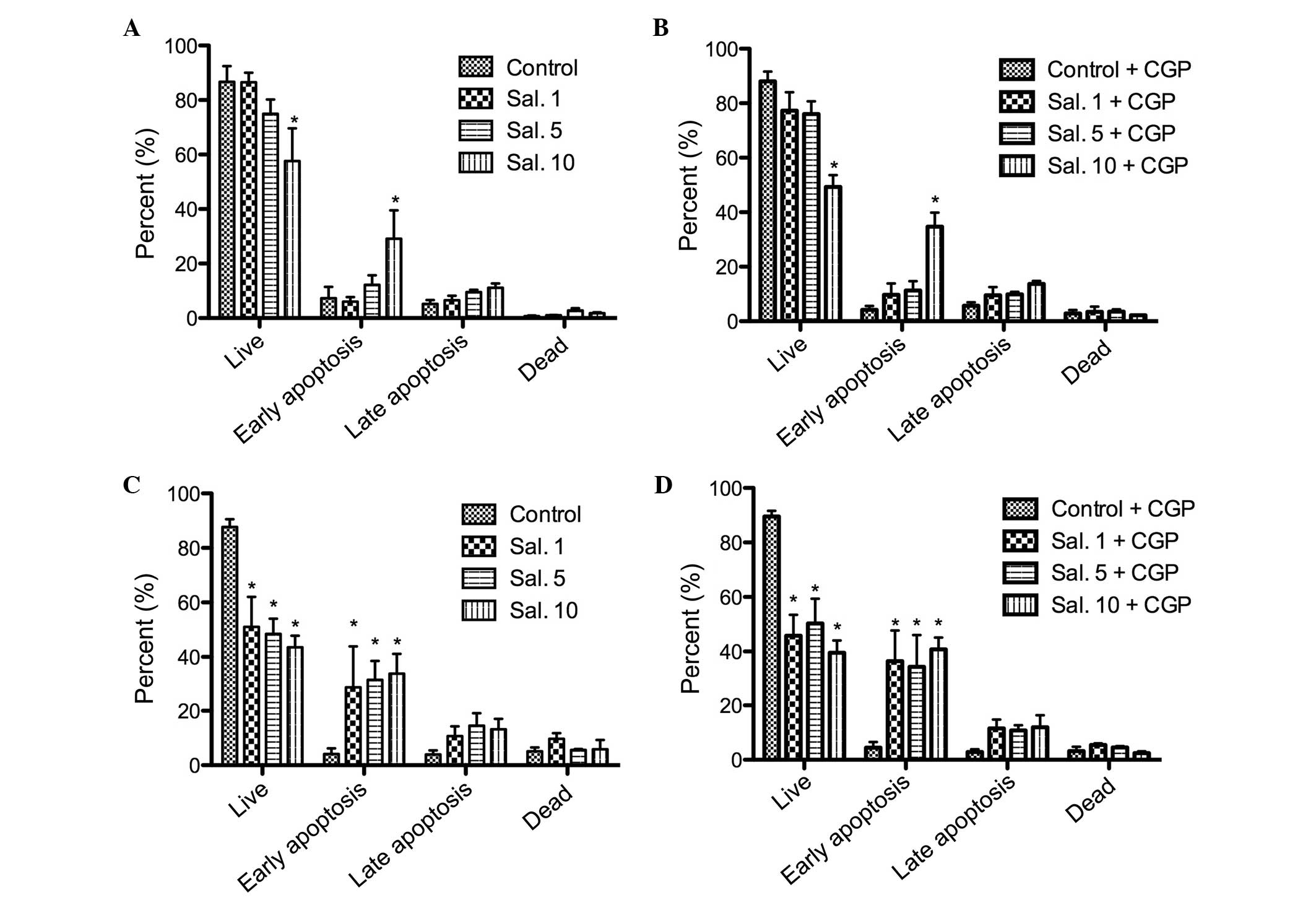
Annexin V-PI staining
The annexin V-PI staining was performed to confirm
the results of the MTT assay and to differentiate between cell
apoptosis and cell necrosis. No increase in the number of apoptotic
cells was observed following treatment with CGP. A dose-dependent
cell apoptosis response was observed following treatment with
salinomycin. The HLaC79 C1 cells were more sensitive to
salinomycin, compared with the FaDu cells (Fig. 5). The rate of early apoptosis
increased significantly (P<0.05) in a dose-dependent manner in
the FaDu cells. No improvements in the rates of late apoptosis and
necrosis were observed as the concentration of salinomycin
increased (Fig. 5). The addition
of CGP did not counteract the tumor toxicity of salinomycin (data
not shown).
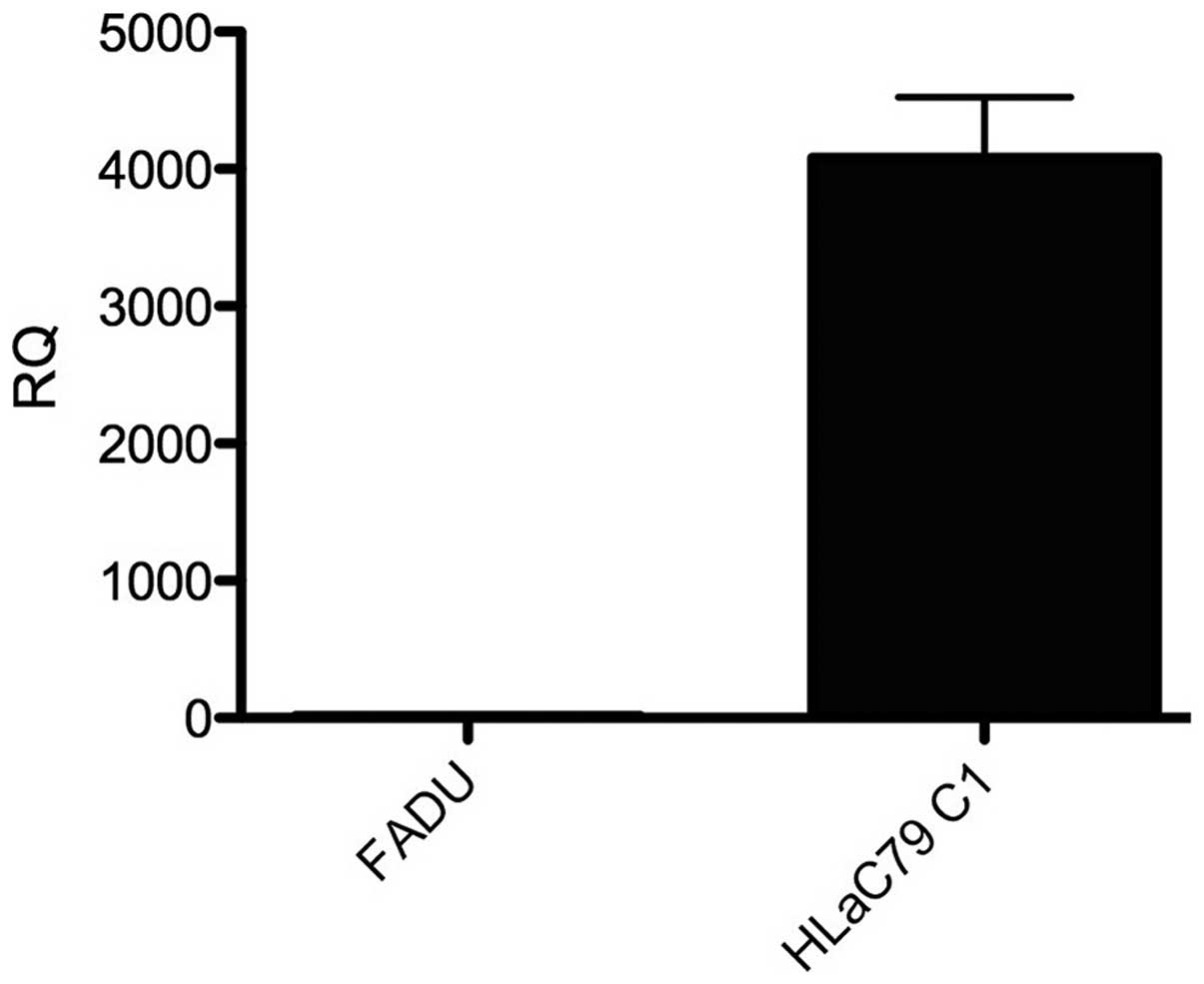
RT-qPCR. The expression levels of MDR-1 in
the FaDu and HLaC79 C1 cancer cell lines were assessed using
RT-qPCR. The HLaC79 C1 cell line was observed to exhibit positive
expression of MDR-1, however no expression of MDR-1 was detected in
the FaDu cells (Fig. 6).
Discussion
The antitumor activity of salinomycin has been
demonstrated in different types of CSCs, including breast CSCs
(7), leukemia stem cells (16) and pancreatic CSCs (17). The mechanisms involved in
tsalinomycin-induced cancer stem cell death remain to be fully
elucidated. Lu et al (18)
identified salinomycin as an inhibitor of the Wnt/β-catenin
signaling pathway. Salinomycin also inhibits the phosphorylation of
Wnt-induced lipoprotein receptor related protein 6 (LRP6). This
causes degradation of LRP6 and leads to the apoptosis of CSCs
(18). In a previous study, Arafat
et al (19) demonstrated
that salinomycin caused concentration- and time-dependent
reductions in the viability of HNSCC cell lines through a caspase
3/7-associated cell death pathway (19). In the present study, salinomycin
induced dose-dependent apoptosis in the HNSCC cells. The
sensitivities of the two cell lines differed significantly, with
the HLaC79 C1 cells being more sensitive to salinomycin, compared
with the FaDu cells. At a concentration of 1 μM, cell death
was observed in ~60% of the HLaC79 C1 cells, which is a salinomycin
concentration ~10 times lower than the concentration required to
achieve the same effect in the FaDu cells. The divergence in
sensitivity between each cell line was also demonstrated in the
annexin and colony assays. Following the addition of CGP, no
alterations in salinomycin tumor toxicity were observed. One reason
may be due to the expression of an MDR-1 receptor. Riccioni et
al (10) demonstrated that
salinomycin acted as a potent inhibitor of MDR-1. This was achieved
through a conformational change in the adenosine triphosphate
transporter (10). The present
study revealed, using RT-qPCR, that MDR-1 was highly expressed in
the HLaC79 C1 cells.
Overexpression of MDR-1 in cancer cells is
associated with a poor clinical outcome due to its resistance to a
majority of the drugs used (20),
therefore, salinomycin may be a promising candidate in these cases.
The major obstacle to using salinomycin is its cytotoxicity. The
mechanism of salinomycin in dealing with rumen microflora and
coccidia is well known (21),
however, the toxic effect in mammals remains to be fully
elucidated. Notably, the toxic effects of salinomycin depend on the
species, for example, it exhibits low toxicity in poultry and
cattle, and high toxicity in horses and dogs (9). Although the available data on the
toxicity of salinomycin in humans is limited, there is one case
report on human poisoning by salinomycin (22). In this case, a farmer accidently
ingested salinomycin at an unknown concentration, which resulted in
life-threatening neuropathia, rhabdomyolysis and hospitalization
for 6 weeks. The concentration of salinomycin in the plasma
remained undetermined, however, it was estimated that 1 mg/kg body
weight was ingested. In our previous study (23), the effects of salinomycin on human
mesenchymal stem cells (hMSCs) was investigated. The essential
functional properties of hMSC were unaffected by treatment with
salinomycin, however, dose-dependent cytotoxicity effects were
observed (23). Minati et
al (24) examined the effects
of sali-nomycin on alkali cation transport and membrane functions
in rat liver mitochondria, the results of which demonstrated that
salinomycin inhibits mitochondrial functions by acting as a mobile
carrier for alkali cations through membranes (24). Boehmerle and Endres (11) reported that salinomycin neuronal
toxicity is mediated by an increase in the concentration of
Na+, which causes an increase in cytosolic
Ca2+ concentration via Na+/Ca2+-X
in the plasma membrane and mitochondria (11). This effect was significantly
inhibited following the addition of the CGP benzodiazepine
derivate.
Additives in tumor therapy are required to reduce
the toxicity of anticancer drugs. Human serum albumin-thioredoxin-1
has been used as an effective additive for preventing cisplatin
nephrotoxicity (25). Kojouharov
et al (26) demonstrated
the reduction of 5-fluorouracil (5-FU) toxicity by the toll-like
receptor 5 (TLR5) agonist, entolimod, in an in vivo model.
TLR5 did not reduce the antitumor efficacy of 5-FU, however, the
major potential concern of using entolimod was its
protective/regenerative effects, which potentially reduced the
antitumor efficacy of the chemotherapy (26). The most important fact for
additives in tumor therapy is that they do not interact with the
anticancer drug. In the present study, CGP did not counteract the
tumor toxicity of salinomycin, nor did it exhibit any antitumor
activity.
In conclusion, salinomycin may be a promising drug
in future anticancer therapy, with CGP as a potential additive to
reduce its toxicity. However, further investigations are required
to examine the toxicological aspects of salinomycin in human cells.
Healthy organs, which express MDR-1, including the liver, kidney,
small intestine and colon, may be highly sensitive to salinomycin.
Therefore, toxicological investigations of healthy cells, healthy
cells expressing MDR-1, and animal experiments are required to
identify critical organs in salinomycin treatment.
Acknowledgments
This study was supported by the Rudolf Bartling
Stiftung (Rudolf Bartling Foundation), Hannover, Germany (grant no.
II/92/2006).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seiwert TY and Cohen EE: State-of-the-art
management of locally advanced head and neck cancer. Br J Cancer.
92:1341–1348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al EORTC 24971/TAX 323 Study Group: Cisplatin, fluorouracil,
and docetaxel in unresectable head and neck cancer. N Engl J Med.
357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prestwich RJ, Öksüz DÇ, Dyker K, Coyle C
and Şen M: Feasibility and efficacy of induction docetaxel,
cisplatin, and 5-fluorouracil chemotherapy combined with cisplatin
concurrent chemoradiotherapy for nonmetastatic Stage IV
head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol
Phys. 81:e237–e243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boman BM and Wicha MS: Cancer stem cells:
A step toward the cure. J Clin Oncol. 26:2795–2799. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davis SJ, Divi V, Owen JH, Bradford CR,
Carey TE, Papagerakis S and Prince ME: Metastatic potential of
cancer stem cells in head and neck squamous cell carcinoma. Arch
Otolaryngol Head Neck Surg. 136:1260–1266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitani M, Yamanishi T and Miyazaki Y:
Salinomycin: A new monovalent cation ionophore. Biochem Biophys Res
Commun. 66:1231–1236. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huczynski A: Salinomycin: A new cancer
drug candidate. Chem Biol Drug Des. 79:235–238. 2012. View Article : Google Scholar
|
|
10
|
Riccioni R, Dupuis ML, Bernabei M,
Petrucci E, Pasquini L, Mariani G, Cianfriglia M and Testa U: The
cancer stem cell selective inhibitor salinomycin is a
p-glycoprotein inhibitor. Blood Cells Mol Dis. 45:86–92. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boehmerle W and Endres M: Salinomycin
induces calpain and cytochrome c-mediated neuronal cell death. Cell
Death Dis. 2:e1682011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rangan SR: A new human cell line (FaDu)
from a hypopharyngeal carcinoma. Cancer. 29:117–121. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt M, Polednik C, Gruensfelder P,
Roller J and Hagen R: The effects of PC-Spes on chemosensitive and
chemoresistant head and neck cancer cells and primary mucosal
keratinocytes. Oncol Rep. 21:1297–1305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zenner HP, Lehner W and Herrmann IF:
Establishment of carcinoma cell lines from larynx and submandibular
gland. Arch Otorhinolaryngol. 225:269–277. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fuchs D, Heinold A, Opelz G, Daniel V and
Naujokat C: Salinomycin induces apoptosis and overcomes apoptosis
resistance in human cancer cells. Biochem Biophys Res Commun.
390:743–749. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang GN, Liang Y, Zhou LJ, Chen SP, Chen
G, Zhang TP, Kang T and Zhao YP: Combination of salinomycin and
gemcitabine eliminates pancreatic cancer cells. Cancer Lett.
313:137–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ
and Carson DA: Salinomycin inhibits Wnt signaling and selectively
induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl
Acad Sci USA. 108:13253–13257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arafat K, Iratni R, Takahashi T, Parekh K,
Al Dhaheri Y, Adrian TE and Attoub S: Inhibitory Effects of
Salinomycin on Cell Survival, Colony Growth, Migration, and
Invasion of Human Non-Small Cell Lung Cancer A549 and LNM35:
Involvement of NAG-1. PLoS One. 8:e669312013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou S, Wang F, Wong ET, Fonkem E, Hsieh
TC, Wu JM and Wu E: Salinomycin: A novel anti-cancer agent with
known anti-coccidial activities. Curr Med Chem. 20:4095–4101. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Story P and Doube A: A case of human
poisoning by salinomycin, an agricultural antibiotic. N Z Med J.
117:U7992004.PubMed/NCBI
|
|
23
|
Scherzed A, Hackenberg S, Froelich K, Rak
K, Technau A, Radeloff A, Nöth U, Koehler C, Hagen R and
Kleinsasser N: Effects of salinomycin on human bone marrow-derived
mesenchymal stem cells in vitro. Toxicol Lett. 218:207–214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mitani M, Yamanishi T, Miyazaki Y and
Otake N: Salinomycin effects on mitochondrial ion translocation and
respiration. Antimicrob Agents Chemother. 9:655–660. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kodama A, Watanabe H, Tanaka R, Kondo M,
Chuang VT, Wu Q, Endo M, Ishima Y, Fukagawa M, Otagiri M, et al:
Albumin fusion renders thioredoxin an effective anti-oxidative and
anti-inflammatory agent for preventing cisplatin-induced
nephrotoxicity. Biochim Biophys Acta. 1840:1152–1162. 2014.
View Article : Google Scholar
|
|
26
|
Kojouharov BM, Brackett CM, Veith JM,
Johnson CP, Gitlin II, Toshkov IA, Gleiberman AS, Gudkov AV and
Burdelya LG: Toll-like receptor-5 agonist Entolimod broadens the
therapeutic window of 5-fluorouracil by reducing its toxicity to
normal tissues in mice. Oncotarget. 5:802–814. 2014.PubMed/NCBI
|