Introduction
Vascular endothelial cells (ECs) form the boundaries
between circulating blood and vascular walls; they are necessary to
maintain vascular homeostasis (1).
As such, any injury of ECs induced by reactive oxygen species (ROS)
is crucial in the development of the early stages of vascular
diseases, such as high blood pressure, high cholesterol, diabetes
and cancer. ROS can cause oxidative damage to lipids, proteins and
enzymes in ECs; as a result, cellular function is impaired. This
condition causes the apoptosis of severely damaged ECs (2–5). As
one of the most important ROS, H2O2 can
easily cross the plasma membrane and damage neighboring cells,
including H2O2-producing cells. Thus,
H2O2 has been extensively used to induce
oxidative stress in in vitro models (6–8).
Therefore, the inhibition of H2O2-induced
damage in ECs has been considered as a potential therapeutic
strategy of various cardiovascular diseases, such as
atherosclerosis (9,10).
Since ancient times, natural plant extracts have
been used to develop novel therapeutic agents. Among these agents,
the phytochemicals, flavonoids, isoflavonoids and related compounds
are the most useful as they are present in edible plants and
exhibit broad pharmacological activities (11). For centuries, soybeans and their
products have been a part of the staple diet of Asians (12). Isoflavones are major soybean
flavonoids extensively investigated due to their beneficial
properties in preventing coronary heart disease, cancer and
osteoporosis (13). The
antioxidant activity of soy isoflavones is essential for its
cardiovascular protective effect.
In this study, H2O2 was
utilized to mimic the effect of oxidative stress in human umbilical
vein endothelial cells (HUVECs) and to investigate the
pharmaceutical functions of soy isoflavones in oxidative
stress-induced vascular endothelial cell damage.
Materials and methods
Reagents
Soy isoflavones (purity, >98%) were provided by
Hubei Yuanceng Pharmaceutical Co., Ltd. (Wuhan, China).
H2O2 was purchased from the Tianjin Guangfu
Chemical Research Institute (Tianjin, China). Dimethylsulfoxide
(DMSO), 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium
bromide (MTT), bovine serum albumin (BSA), and streptomycin were
obtained from Amresco (Solon, OH, USA). Penicillin, gelatin,
glutamine, paraformaldehyde and Hoechst 33258 were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) was
obtained from Roche Diagnostics (Mannheim, Germany). The rabbit
anti-human polyclonal Bcl-2 (1:1,000; sc-492), rabbit anti-human
polyclonal Bax (1:1,000; sc-6236), goat anti-human polyclonal
Caspase-3 (1:1,000; sc-1226), goat anti-human Caspase-9 (1:1,000;
sc-22182), rabbit anti-human polyclonal Cytochrome c
(1:1,000; sc-7159) and rabbit anti-human polyclonal IκB (1:1,000;
sc-371) antibodies were purchased from Santa Cruz Biotechnology
Inc. (Santa Cruz, CA, USA). Rabbit anti-human monoclonal nuclear
factor (NF)-κB (1:1,000; SAB4502609) and rabbit anti-human
monoclonal β-actin (1:1,000; SAB5500001) antibodies were purchased
from Sigma-Aldrich (St. Louis, MO, USA). All other reagents were of
analytical chemical grade.
Cell culture
The EVC-304 HUVECs were purchased from the Cell Bank
of the Chinese Academy of Sciences (Shanghai, China) and cultured
in Dulbecco's modified Eagle's medium (Gibco Life Technologies,
Carlsbad, CA, USA) supplemented with 10% FBS, 100 U/ml penicillin
and 100 U/ml streptomycin (Life Technologies, Carlsbad, CA USA) at
37°C in a humidified atmosphere of 5% CO2 and 95% air.
Cells from the stock flask were suspended in phosphate-buffered
saline, treated with trypsin (Sigma-Aldrich) and counted using a
hemocytometer (Ningbo Biocotek Scientific Instrument Co., Ltd.,
Ningbo, China). After ~3 days from seeding, active growth of cells
began and the experiment was started only after this period.
Cell viability assay
The MTT cytotoxicity assay was used to measure cell
viability as described (14).
EVC-304 cells were grown to ~80% confluence, maintained with fresh
medium described above, and subcultured every 2–3 days. The cells
were treated with soy isoflavones (25, 50, 100 and 200 µΜ)
for 12 h prior to testing for the presence of 100 µΜ
H2O2 for another hour. Each treatment
condition was tested in 5 replicate wells. At the end of the
treatment, cells were incubated with 100 µl of 0.5 mg/ml MTT
at 37°C for 4 h. Then, 100 µl DMSO was added to each well.
Absorbance of each well was detected at 450 nm using a microplate
reader (Model 550; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The relative cell viability was expressed as the ratio of the
microwave-treated cells to that of control cells. All experiments
were performed in triplicate.
Morphological examination for Hoechst
33258 staining
EVC-304 cells were collected, washed with PBS and
fixed with 2% paraformaldehyde at room temperature for 15 min.
Cells were then were washed with PBS and stained with Hoechst 33258
staining solution (25 µg/ml; Sigma-Aldrich) for 30 min at
room temperature. The stained nuclei were observed using a
fluorescence photomicroscope (IX71; Olympus, Tokyo, Japan).
Flow cytometric evaluation of
apoptosis
EVC-304 cells were double stained by an Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's instructions. Samples stained with Annexin V and
propidium iodide (PI) were quantitatively analyzed at an emission
wavelength of 488 nm and an excitation wavelength of 570 nm using
the BD FACSCalibur flow cytometer (Becton Dickinson, Franklin
Lakes, NJ, USA).
Measurements of intracellular superoxide
dismutase (SOD), glutathione peroxidase (GSH-Px) and
malondialdehyde (MDA) contents
The activities of SOD, GSH-Px and MDA were all
determined using the SOD, GSH-Px and MDA Assay kits, and all the
procedures complied with the manufacturer's instructions (Jiancheng
Bioengineering Institute, Nanjing, China). The activity of the
enzymes was expressed as U/mg protein. The SOD activity assay was
based on its ability to inhibit the oxidation of hydroxylamine by
the O2− produced from the xanthine-xanthine oxidase
system. One unit of SOD activity was defined as the quantity that
reduced the absorbance at 550 nm by 50%. GSH-Px activity was
measured using the enzyme-catalyzed reaction product (reduced
glutathione) and the absorbance was recorded at 412 nm. The
activities of GSH-Px were expressed as U/mg protein. The MDA
content was measured at a wavelength of 532 nm by reaction with
thiobarbituric acid (TBA) to form a stable chromophore. The values
of the MDA level were expressed as nmol/mg protein.
Western blot analysis
For immunoblot analyses, 40 µg protein
lysates per sample were denatured in 4X SDS-PAGE sample buffer
(Tris-HCl 260 mM, pH 8.0; 40% (v/v) glycerol, 9.2% (w/v) SDS, 0.04%
bromophenol blue and 2-mercaptoethanol as a reducing agent) and
subjected to SDS-PAGE on 12% acrilamide/bisacrilamide gels (DingGuo
ChangSheng Biotechnology Co., Ltd., Beijing, China). Separated
proteins were transferred to nitrocellulose membranes (Hybond-P
PVDF; Bio-Rad Laboratories, Inc.). Residual binding sites on the
membrane were blocked by incubation in TBST (10 mM Tris, 100 mM
NaCl, 0.1% Tween 20) with 5% (w/v) non-fat milk powder overnight at
4°C. Membranes were then probed with with anti-Bcl-2, anti-Bax,
anti-Caspase-3, anti-Caspase-9, anti-Cytochrome c,
anti-NF-κB, anti-IκB and anti-β-actin antibodies at a dilution of
1:1,000 for 16 h at 4°C, after which they were incubated with the
appropriate peroxidase-linked secondary antibody with a dilution of
1:5,000 for 1 h at room temperature. Chemiluminescence signals were
visualized with an enhanced chemiluminescence ultra-sensitive
light-emitting liquid (Beyotime Institute of Biotechnology,
Shanghai, China) and quantified by Quantity One software, version
4.62 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Results are expressed as the mean ± standard error
of the mean. All data were analyzed using SPSS version 13 software
(SPSS Inc., Chicago, IL, USA). For comparisons between groups of
more than two unpaired values, one-way analysis of variance (ANOVA)
was used. If an ANOVA F-value was significant, post hoc
comparisons were performed between groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Soy isoflavones inhibit
H2O2-induced cytotoxicity in EVC-304
cells
Initially, the cytotoxicity of soy isoflavones on
EVC-304 cells was examined using the MTT assay. Soy isoflavones did
not show obvious cytotoxic effects up to 200 µM (Fig. 1A). Then it was further evaluated
whether soy isoflavones had a protective effect. EVC-304 cells were
pretreated with soy isoflavones for 12 h, then incubated with
H2O2 for 1 h and cell viability was measured
by the MTT assay. The viability of cells was decreased
significantly following treatment with 100 µM
H2O2 for 1 h (P<0.001 vs. untreated
group), while soy isoflavones protected cells from
H2O2-induced cytotoxicity in a
concentration-dependent manner (25 µM, 46.6±4.1%; 50
µM, 52.2±6.8%; 100 µM, 60±5.1%; and 200 µM,
68.3±5.8%), as shown in Fig.
1B.
Soy isoflavones improved morphological
changes of EVC-304 cells
The uniform shape of EVC-304 nuclei and
well-distributed deep blue fluorescence were revealed by Hoechst
33258 staining. Hoechst 33258 was used as an apoptosis marker,
which detected apoptotic nuclei with condensed and/or fragmented
DNA. The majority of nuclei in the control groups had uniform blue
chromatin with an organized structure (Fig. 2A). Following treatment with 100
µM H2O2 for 1 h, EVC-304 cells
exhibited typical features of apoptosis, as shown in Fig. 2B. Soy isoflavone pretreatment
reduced H2O2-induced apoptosis, demonstrated
by few apoptotic nuclei (Fig.
2C–E), similar to that of the control conditions. The data
showed that the apoptotic index increased markedly in cells
stimulated with H2O2; however soy isoflavone
pretreatment reduced the level of EVC-304 cells induced by
H2O2.
Soy isoflavones reduce EVC-304 cell
apoptosis induced by H2O2
In order to quantitatively gain insight into the
anti-apoptotic effects of soy isoflavones in
H2O2-induced EVC-304 cells, the apoptosis
rate of EVC304 cells after treatment with 100 µM
H2O2 for 1 h, was measured by Annexin-V/PI
staining. As shown in Fig. 3A and
B, the apoptosis rate increased from 3.26±0.4 to 41.13±2.2%. By
contrast, increased doses of soy isoflavones could evidently
attenuate the apoptosis of EVC-304 cells to 32.75±2.4, 21.04±2.5
and 9.48±2.8%, respectively (Fig.
3C–E).
Soy isoflavones reduce oxidative stress
in EVC-304 cells
Major antioxidant defenses include antioxidant
scavengers, such as SOD and GSH-Px. An increase in MDA, which is a
lipid peroxidation end-product, indicates reduced antioxidant
capacity. Total SOD, GSH-Px and MDA activity in EVC-304 cells was
measured. After treating the cells with H2O2
for 1 h, the SOD and GSH-Px levels decreased respectively,
(Fig. 4A; P<0.001 vs. untreated
group). However, incubation with soy isoflavones significantly
attenuated the changes in the content of SOD and GSH-Px (Fig. 4A and B; P<0.01 vs. the
H2O2 group). In addition, cells treated with
H2O2 for 1 h showed increasing intracellular
MDA release, (P<0.001 vs. untreated group); however, incubation
with soy isoflavones produced a marked decrease in the
intracellular level of MDA (Fig.
4C; P<0.01 vs. the H2O2 group).
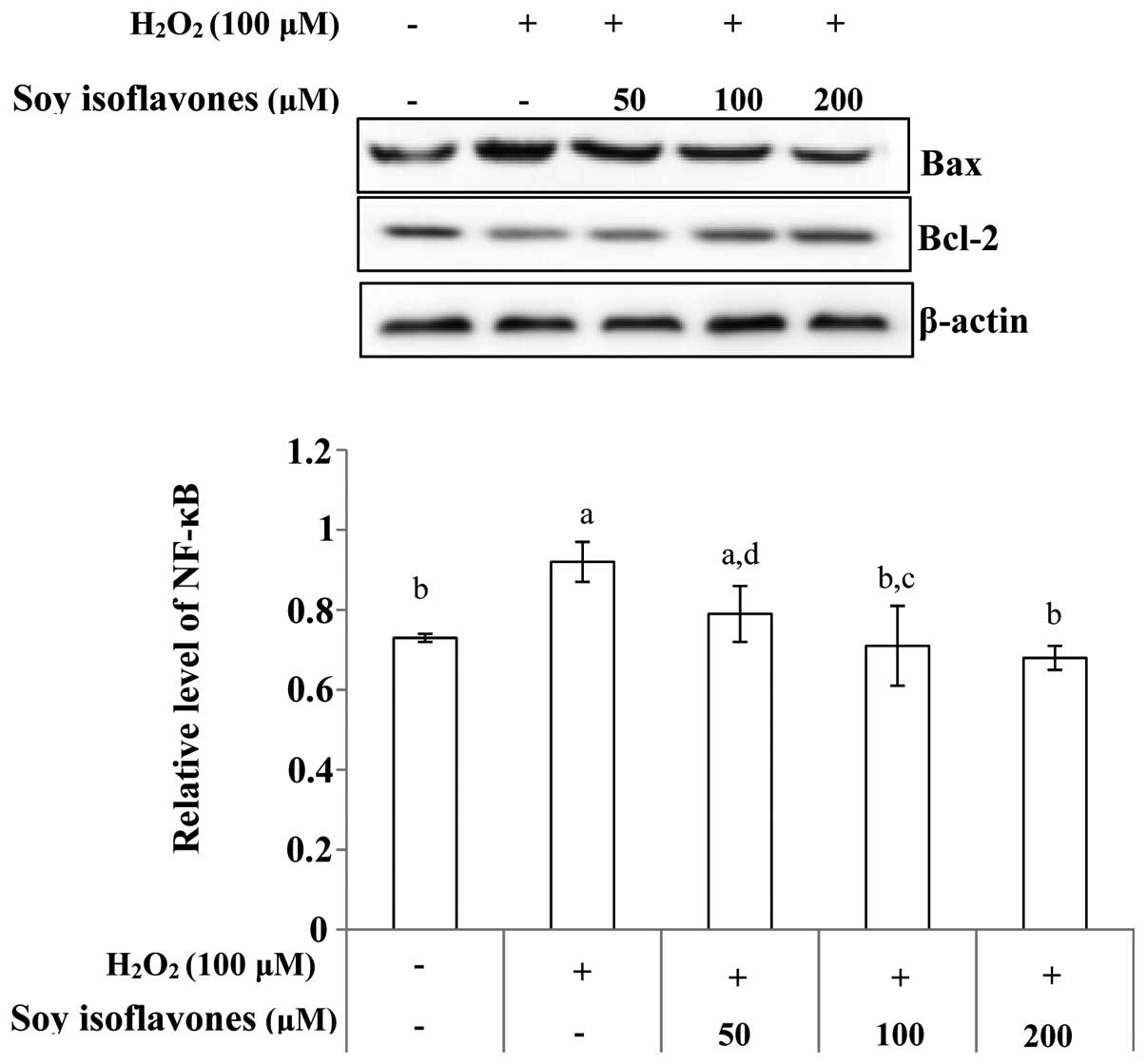
Effect of soy isoflavones on the
expression of H2O2-induced apoptosis-related
proteins
To investigate whether soy isoflavones exhibit an
effect on the apoptosis-related protein expression in EVC-304
cells, the expression of apoptosis-related proteins were analyzed
by western blot analysis. The ratio of Bax/Bcl-2 was analyzed,
which is crucial for the activation of the cell apoptosis. The
level of Bax increased while the Bcl-2 level showed marginal
decrease when the EVC-304 cells were treated with
H2O2, but pretreatment with soy isoflavones
decreased the ratio (Fig. 5). The
protein expression of cytochrome c release, and caspase-3
and caspase-9 protein levels were also investigated. As expected,
western blot analysis showed the protein levels were significantly
higher in the H2O2 group than that in the
control group; however, this effect was inhibited by soy
isoflavones (Fig. 6). Thus,
treatment with soy isoflavones decreased the protein expression
induced by H2O2, and it was indicated that
this occurred via inhibition of the mitochondria-mediated apoptosis
signaling pathway.
Effects of soy isoflavones on
H2O2-induced NF-κB expression
As noted, degradation of IκB allows the nuclear
localization of NF-κB and subsequent transcriptional activation of
target genes. Western blot analysis indicated that treatment with
H2O2 resulted in a degradation of the IκB
protein compared with that in control cells. The
H2O2-induced degradation of IκB was inhibited
following pretreatment with soy isoflavones. Western blot analysis
also indicated that treatment with soy isoflavones prior to
H2O2 treatment markedly abrogated
H2O2-induced activation of NF-κB (Fig. 7).
Discussion
In the present study, the effects of soy isoflavones
on H2O2-induced damage in EVC-304 cells in
vitro were examined. Possible mechanisms underlying these
effects were also investigated. Data indicated that soy isoflavones
could protect EVC-304 cells from H2O2-induced
damage. The underlying mechanisms may involve soy isoflavones
functioning as a potent inhibitor of oxidative stress. Soy
isoflavones could also modulate the activation of NF-κB and the
mitochondria-mediated apoptosis signaling pathway in HUVECs.
Under physiological conditions, ROS are generated at
low levels and involved in signaling and metabolic pathways.
However, an increase in oxidative stress induced by different
vascular risk factors is a key mechanism of EC injury (15). In the present study, the viability
of EVC-304 cells was significantly decreased and cell apoptosis was
induced when the cells were exposed to H2O2.
However, cell viability and apoptosis were notably improved when
soy isoflavones were added to the culture medium 12 h prior to
H2O2 administration. To verify whether or not
EC injury is correlated with oxidative stress, the activity of
antioxidant enzymes was determined. The results showed that
pre-treatment with soy isoflavones could reduce MDA content and
enhance SOD and GSH-PX activity. Therefore, soy isoflavones could
protect EVC-304 cells from cellular injury induced by oxidative
stress.
Oxidative stress can directly induce EC apoptosis,
which accelerates EC injury. EC apoptosis corresponds to an
important process in the pathogenesis of vascular diseases
(16). Pre-treatment with soy
isoflavones could effectively alleviate
H2O2-induced EC apoptosis. If the exact
mechanism of the anti-apoptotic effect of soy isoflavone in
oxidative stress-induced ECs can be identified, vascular diseases
can be prevented or alleviated. A previous study suggested that
changes in mitochondrial membrane potential can mediate committed
cells to undergo apoptosis with oxidative stress (17). Apoptosis can be initiated via two
pathways; the extrinsic and intrinsic pathways. The intrinsic
pathway is mitochondrial dependent and involves caspases and the
Bcl-2 protein family (18). Among
various caspases, caspase-9 and -3 are important in cell apoptosis.
These proteins are also the upstream regulators of mitochondrial
membrane potential, inducing the release of cytochrome c
into the cytosol. The translocation of cytochrome c from the
mitochondria to the cytosol is required for activation of the
apoptotic machinery in various cell death models (19). The Bax/Bcl-2 ratio determines
whether or not a cell survives when it is exposed to an apoptotic
stimulus (20,21). The pro-apoptotic protein Bax can
promote the release of cytochrome c, where as the
pro-survival protein Bcl-2 elicits anti-apoptotic effects. The
mitochondria are also important in apoptosis or the programmed cell
death pathway. The results indicated that exposure to
H2O2 could upregulate the ratio of Bax/Bcl-2,
enhance the release of cytochrome c, and activate the
cleavage of caspase-3 and -9. However, pre-treatment with soy
isoflavones could decrease the Bax/Bcl-2 ratio and prevent the
translocation of cytochrome c, thereby inhibiting the
cleavage of caspase-3 and -9.
The NF-κB family of transcription factors regulates
multiple biological functions. For example, NF-κB is vital in
inflammatory and innate immune responses. It is commonly activated
by various agents, such as ROS (22). Conversely, agents that scavenge ROS
can inhibit the activation of NF-κB. In this study, in vitro
soy isoflavone treatment prevented the
H2O2-induced generation of ROS in EVC-304
cells. To verify whether or not this protective effect is
correlated with the inhibitory activation of NF-κB, NF-κB and IκB
protein expression levels were detected by western blot analysis.
The results showed that NF-κB was activated by
H2O2-induced HUVEC injury. However, this
activation was effectively inhibited by pre-treatment with soy
isoflavones. H2O2-induced HUVEC injury also
resulted in IκB protein degradation; however, the pre-treatment of
soy isoflavones prevented this degradation.
Therefore, soy isoflavones elicited protective
effects against H2O2-induced cytotoxicity and
apoptosis of EVC-304 cells. Soy isoflavones could elicit
anti-apoptotic effects by inhibiting ROS generation and modulating
NF-κB and the mitochondria-mediated apoptosis signaling pathway
activation. The current study suggests that soy isoflavone is a
potential candidate for therapeutic application against
cardiovascular diseases, however the precise mechanism of its
action still requires further investigation.
Acknowledgments
This study was supported by Department of Education
of Jilin Province (grant no. 2013 [479]), Project Agreement for
Science & Technology Development, Jilin Province (no.
20140204002YY).
References
|
1
|
Rask-Madsen C, Li Q, Freund B, Feather D,
Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J,
Sotiropoulos KB, et al: Loss of insulin signaling in vascular
endothelial cells accelerates atherosclerosis in apolipoprotein E
null mice. Cell Metab. 11:379–389. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oeseburg H, de Boer RA, Buikema H, van der
Harst P, van Gilst WH and Silljé HH: Glucagon-like peptide 1
prevents reactive oxygen species-induced endothelial cell
senescence through the activation of protein kinase A. Arterioscler
Throm Vasc Biol. 30:1407–1414. 2010. View Article : Google Scholar
|
|
3
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dranka BP, Hill BG and Darley-Usmar VM:
Mitochondrial reserve capacity in endothelial cells: The impact of
nitric oxide and reactive oxygen species. Free Radical Bio Med.
48:905–914. 2010. View Article : Google Scholar
|
|
5
|
Fatehi-Hassanabad Z, Chan CB and Furman
BL: Reactive oxygen species and endothelial function in diabetes.
Eur J Pharmacol. 636:8–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loo AE and Halliwell B: Effects of
hydrogen peroxide in a keratinocyte-fibroblast co-culture model of
wound healing. Biochem Bioph Res Commun. 423:253–258. 2012.
View Article : Google Scholar
|
|
7
|
Simůnek T, Stérba M, Popelová O, Adamcová
M, Hrdina R and Gersl V: Anthracycline-induced cardiotoxicity:
Overview of studies examining the roles of oxidative stress and
free cellular iron. Pharmacol Rep. 61:154–171. 2009. View Article : Google Scholar
|
|
8
|
Kukidome D, Nishikawa T, Sonoda K, Imoto
K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T and
Araki E: Activation of AMP-activated protein kinase reduces
hyperglycemia-induced mitochondrial reactive oxygen species
production and promotes mitochondrial biogenesis in human umbilical
vein endothelial cells. Diabetes. 55:120–127. 2006. View Article : Google Scholar
|
|
9
|
Lang Y, Chen D, Li D, Zhu M, Xu T, Zhang
T, Qian W and Luo Y: Luteolin inhibited hydrogen peroxide-induced
vascular smooth muscle cells proliferation and migration by
suppressing the Src and Akt signalling pathways. J Pharm Pharmacol.
64:597–603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun GB, Qin M, Ye JX, Pan RL, Meng XB,
Wang M, Luo Y, Li ZY, Wang HW and Sun XB: Inhibitory effects of
myricitrin on oxidative stress-induced endothelial damage and early
atherosclerosis in ApoE-/-mice. Toxicol Appl Pharmacol.
271:114–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Birt DF, Hendrich S and Wang W: Dietary
agents in cancer prevention: Flavonoids and isoflavonoids.
Pharmacol Ther. 90:157–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Messina MJ, Persky V, Setchell KD and
Barnes S: Soy intake and cancer risk: A review of the in vitro and
in vivo data. Nutr Cancer. 21:113–131. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown NM, Belles CA, Lindley SL,
Zimmer-Nechemias L, Witte DP, Kim MO and Setchell KD: Mammary gland
differentiation by early life exposure to enantiomers of the soy
isoflavone metabolite equol. Food Chem Toxicol. 48:3042–3050. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ciofani G, Danti S, D'Alessandro D,
Moscato S and Menciassi A: Assessing cytotoxicity of boron nitride
nanotubes: Interference with the MTT assay. Biochem Biophys Res
Commun. 394:405–411. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cabiscol E, Tamarit J and Ros J: Oxidative
stress in bacteria and protein damage by reactive oxygen species.
Int Microbiol. 3:3–8. 2000.PubMed/NCBI
|
|
16
|
Ross R: Atherosclerosis - an inflammatory
disease. New Engl J Med. 340:115–126. 1999. View Article : Google Scholar
|
|
17
|
Kannan K and Jain SK: Oxidative stress and
apoptosis. Pathophysiology. 7:153–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hyman BT and Yuan J: Apoptotic and
non-apoptotic roles of caspases in neuronal physiology and
pathophysiology. Nat Rev Neuro. 13:395–406. 2012. View Article : Google Scholar
|
|
20
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu F, Zang J, Chen D, Zhang T, Zhan H, Lu
M and Zhuge H: Neohesperidin induces cellular apoptosis in human
breast adenocarcinoma MDA-MB-231 cells via activating the
Bcl-2/Bax-mediated signaling pathway. Nat Prod Commun. 7:1475–1478.
2012.
|
|
22
|
Ghosh G, van Duyne G, Ghosh S and Sigler
PB: Structure of NF-kB p50 homodimer bound to a kappa B site.
Nature. 373:303–310. 1995. View
Article : Google Scholar : PubMed/NCBI
|