Spandidos Publications style
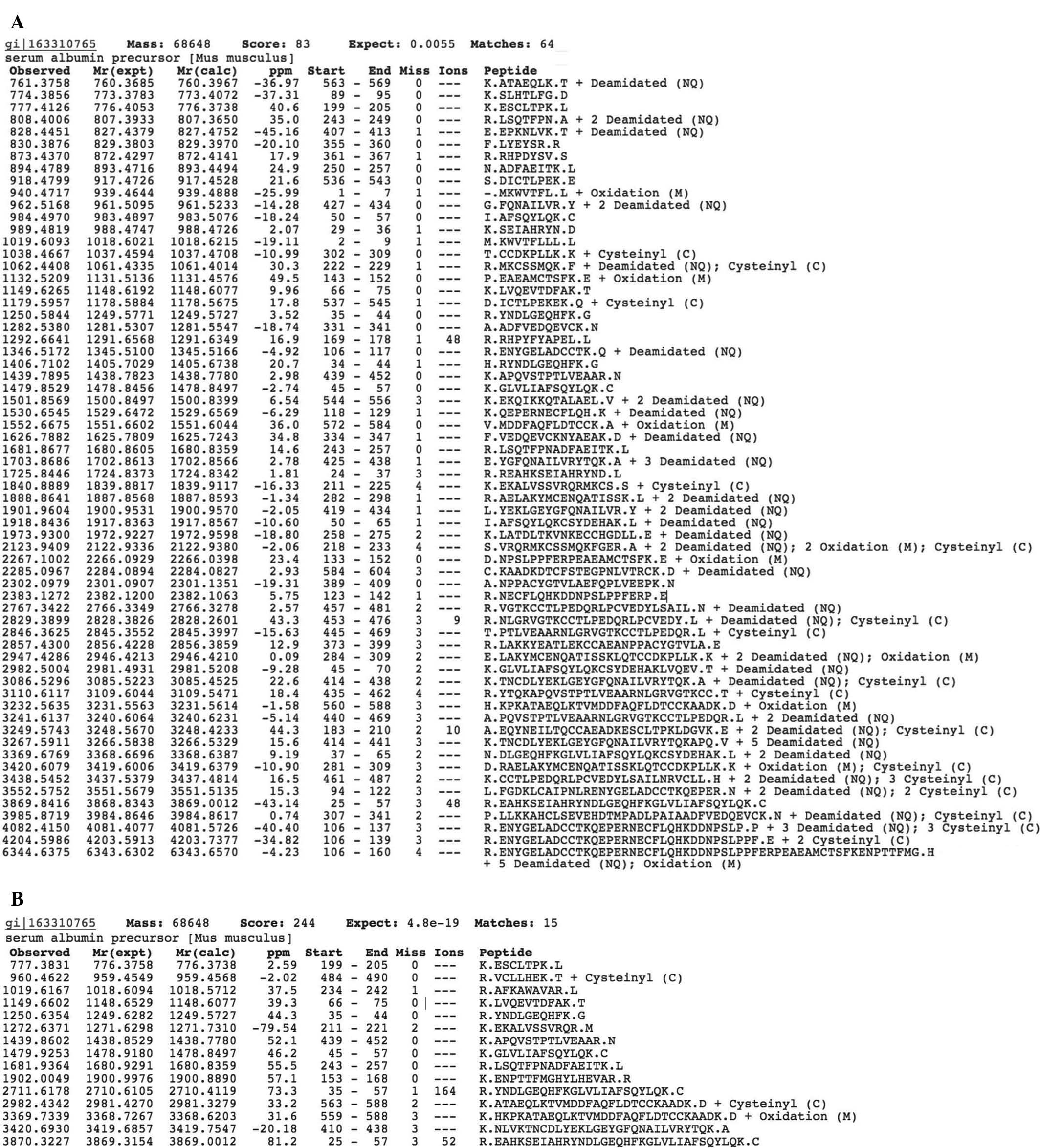
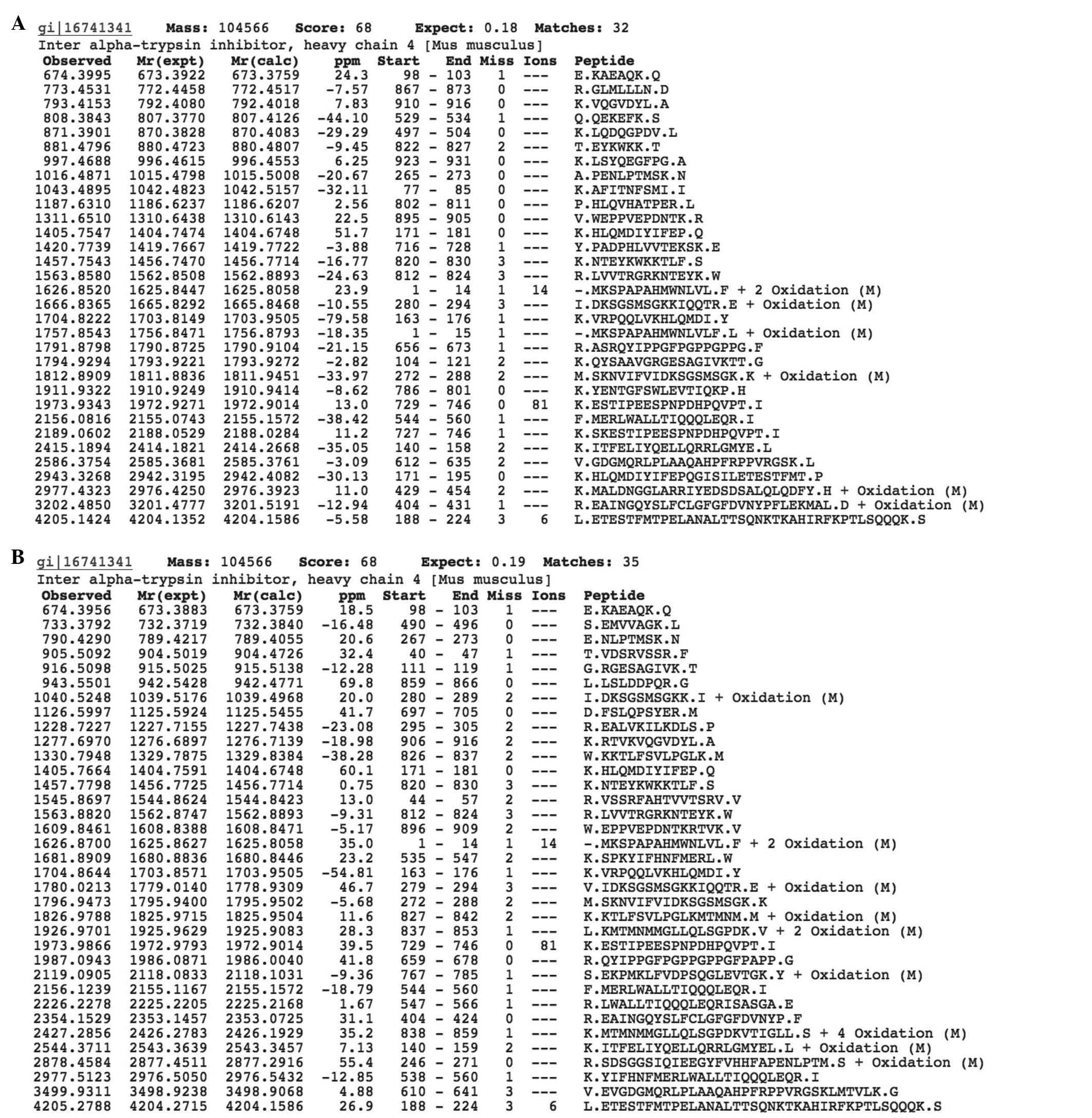
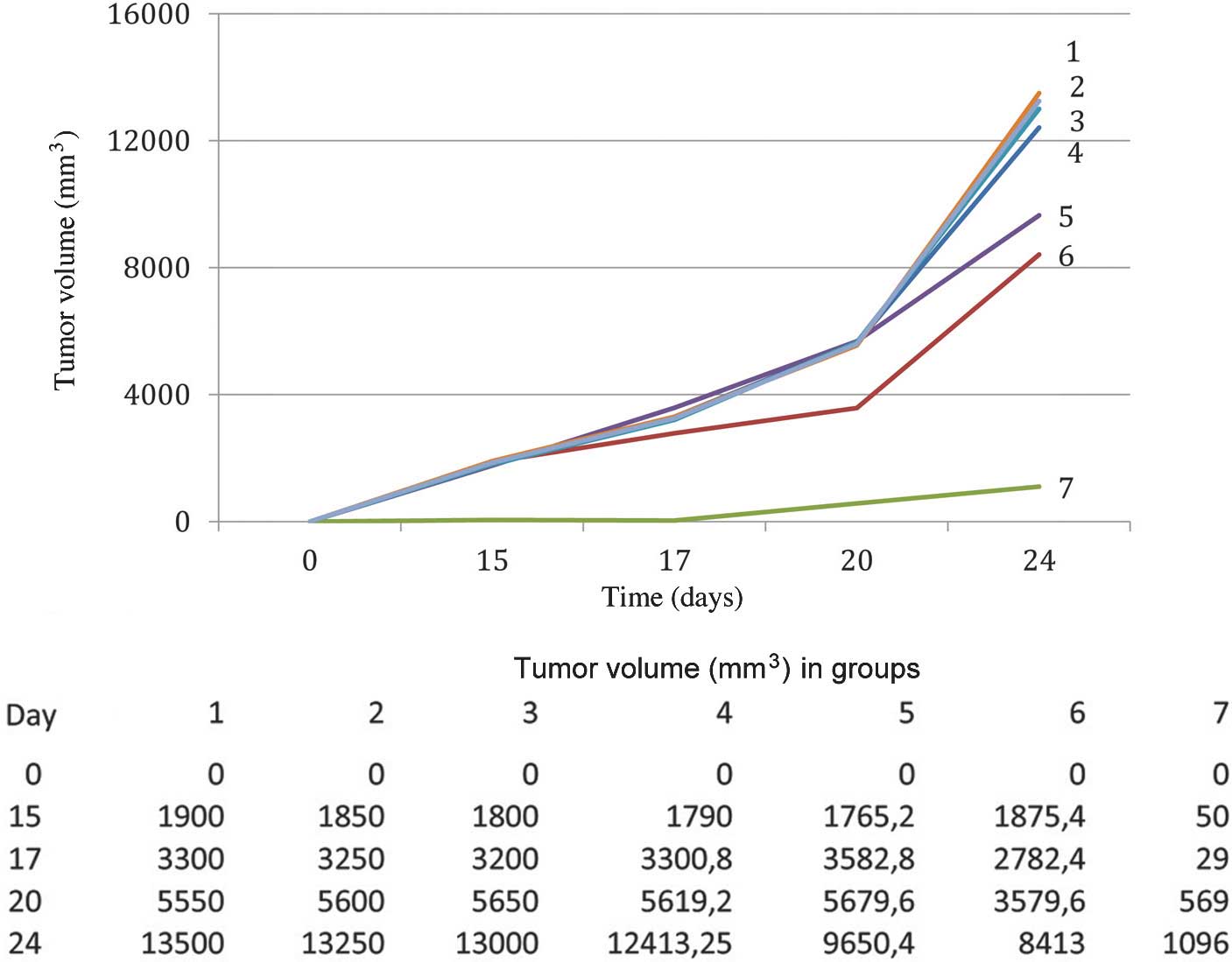
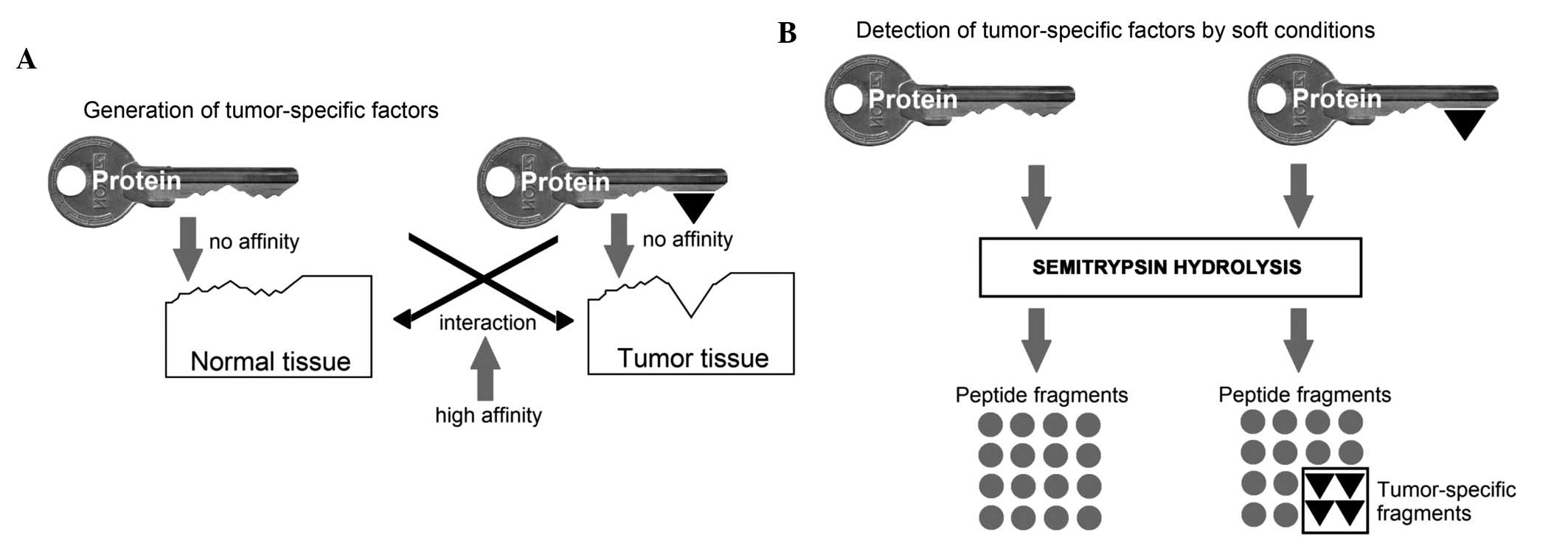
Kormosh NG, Davidova TV, Kopyltsov VN, Serebryakova MV, Kabieva AO, Voyushin KE, Sitdikova SM, Amandzholov BS, Kiselevskii MV, Donenko FV, Donenko FV, et al: Conformational changes in inter‑α‑trypsin inhibitor heavy chain 4 activate its tumor‑specific activity in mice with B16 melanoma. Mol Med Rep 12: 4483-4493, 2015.
APA
Kormosh, N.G., Davidova, T.V., Kopyltsov, V.N., Serebryakova, M.V., Kabieva, A.O., Voyushin, K.E. ... Donenko, F.V. (2015). Conformational changes in inter‑α‑trypsin inhibitor heavy chain 4 activate its tumor‑specific activity in mice with B16 melanoma. Molecular Medicine Reports, 12, 4483-4493. https://doi.org/10.3892/mmr.2015.3961
MLA
Kormosh, N. G., Davidova, T. V., Kopyltsov, V. N., Serebryakova, M. V., Kabieva, A. O., Voyushin, K. E., Sitdikova, S. M., Amandzholov, B. S., Kiselevskii, M. V., Donenko, F. V."Conformational changes in inter‑α‑trypsin inhibitor heavy chain 4 activate its tumor‑specific activity in mice with B16 melanoma". Molecular Medicine Reports 12.3 (2015): 4483-4493.
Chicago
Kormosh, N. G., Davidova, T. V., Kopyltsov, V. N., Serebryakova, M. V., Kabieva, A. O., Voyushin, K. E., Sitdikova, S. M., Amandzholov, B. S., Kiselevskii, M. V., Donenko, F. V."Conformational changes in inter‑α‑trypsin inhibitor heavy chain 4 activate its tumor‑specific activity in mice with B16 melanoma". Molecular Medicine Reports 12, no. 3 (2015): 4483-4493. https://doi.org/10.3892/mmr.2015.3961