Introduction
The overall 5-year survival rate among patients with
pancreatic cancer is <5%, and it is the fourth leading cause of
cancer-related mortality, which affects males and females (1,2). In
recent years, there have been important advances in the
understanding of the molecular mechanisms underlying the
pathogenesis of pancreatic cancer. However, little progress has
been made in terms of prevention or treatment, in particular for
those individuals with advanced-stage disease (3,4).
A previous study by this groups reported that
triptolide (TL), the primary extract of the Chinese herb
Tripterygium wilfordii hook, induces apoptosis in pancreatic
cancer cell lines in vitro (5). TL also exhibits antitumor effects in
numerous types of tumor, including breast, prostate and lung
cancer, and sensitizes tumor cells to death induction by a variety
of agents, such as Apo2/Trail (6)
and tumor necrosis factor-α (7).
In the present study, the mechanisms underlying TL-induced cell
death and suppression of tumor growth were investigated in a mouse
pancreatic cancer xenograft model.
Pancreatic cancer is characterized by
hypovasculature, which is due to the fast proliferation of cancer
cells and results in a poor blood supply and tumor hypoxia
(8). However, malignant cells may
undergo genetic or adaptive changes that allow them to survive
during oxygen and nutrition deprivation. Hypoxia-inducible factor-1
(HIF-1) is an important regulator under these conditions. HIF-1 is
composed of two subunits HIF-1α and HIF-1β. HIF-1α is a unique
oxygen-regulated component, which determines HIF-1 activity, such
as induction of the expression of a number genes related to tumor
angiogenesis, cell proliferation and metabolism (9,10).
In order to investigate the role of HIF-1α in
TL-induced cell death, HIF-1α gene transcription and protein level
in pancreatic cancer cell lines were measured following TL
treatment in vitro and in vivo. By microarray
analysis of gene expression, TL target genes were searched and the
effects of TL on gene expression were confirmed by quantitative
polymerase chain reaction (qPCR). The current results suggest that
TL possesses strong antitumor effects via suppression of HIF-1α and
other important target genes, including the key oncogene c-Myc in
pancreatic cancer cells.
Materials and methods
Cell culture and materials
The PANC1, ASPC1 and SW1990 human pancreatic cancer
cell lines were purchased from the American Type Culture Collection
(Rockville, MD, USA). PANC1 and SW1990 were cultured in Dulbecco's
modified Eagle's medium (Invitrogen Life Technologies, Carlsbad,
CA, USA) and ASPC1 cells were grown in RPMI 1640 media (Invitrogen
Life Technologies). Media were supplemented with 10% fetal bovine
serum (Invitrogen Life Technologies) and cells were grown as
monolayers in a humidified atmosphere at 37°C. Crystalline TL
(PG490, purity 99.5%) was obtained from Shanghai DND
Pharm-Technology Co., Inc. (Shanghai, China). The present study was
approved by the ethics committee of Nantong University (Nantong,
China).
Cell proliferation analysis
Cells (1×104) in 200 µl medium
were seeded into each well of 96-well cell culture plates.
Following overnight incubation, cells were treated with 10, 20 and
50 ng/ml TL. A cell proliferation assay was performed at 6, 12, 24
and 48 h following incubation, using a Cell Counting kit-8
(Dojindo, Kumamoto, Japan). The cell numbers were evaluated with
the absorbance at 450 nm, which was measured on an MR7000 plate
reader (Dynatech Laboratories, Chantilly, VA, USA). Control cells
were treated with dimethyl sulfoxide (DMSO) only.
Xenograft tumor model and treatments
Athymic nude mice (BALB/c nu/nu, 5-week old females;
n=32) were purchased from Shanghai Laboratory Animal Center of
Chinese Academy of Science (Shanghai, China). SW1990 cells
(107) in 100 µl phosphate-buffered saline were
injected into the backs of BALB/c nude mice. TL was dissolved in
60% ethanol, 30% DMSO and 10% phosphate buffer (pH 6.0) at a
concentration of 1 mg/ml. TL was injected intraperitoneally (IP)
into the mice on a daily basis once visible tumors reached ~100
mm3. The following formula for calculating tumor volume
was used: Length × width × (length+width/2) × 0.526. Three weeks
post-injection, the animals were sacrificed with CO2 and
the tumors were carefully dissected. The tumors were measured using
calipers and the following formula was used to calculate tumor
volume: Tumor volume = length × width × (length + width / 2) ×
0.526.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated with TRIzol (Invitrogen Life
Technologies) and 1 µg RNA was used for reverse
transcription with a SuperScript® VILO™ cDNA Synthesis
kit (Invitrogen Life Technologies). SYBR Green Master mix (Clontech
Laboratories, Inc., Mountain View, CA, USA) was used for the
RT-qPCR reaction. RT-qPCR was performed using a cycler (Roche Mol,
IND) and SYBR green dye. For data analysis, a method designated as
2−ΔΔCT was used to calculate fold changes. The following
primers (Sangon Biotech Co., Ltd., Shanghai, China) were used:
Forward: 5′-ATTGCCGACAGGATGCAGA-3′ and reverse:
5′-GAGTACTTGCGCTCAGGAGGA-3′ for β-actin; forward:
5′-TCAAAAACAGAGACGAAGGACA-3′ and reverse:
5′-GATTCAAAGTGGCAGACAGGTT-3′ for HIF-1α; forward:
5′GGGCCTCCGAAACCATGAACTT-3′ and reverse: 5′-TCGCATCAGGGGCACACAG-3′
for VEGF; forward: 5-′CAAACCTCCTCACAGCCCACT-3′ and reverse:
5′-TGACACTGTCCAACTTGACCC-3′ for c-Myc; forward:
5′-CACCTTTGATGGGTCCCTGTT-3 and reverse: 5′-CTGGCATACCTGTTGCTCACT-3′
for Ets2; and forward: 5′-ACCACAGTCCATGCCATCAC-3′ and reverse:
5′-TCCACCACCCTGTTGCTGT-3′ for GAPDH. The cycling conditions were as
follows: 95°C for 2 min for initial denatureation, 40 cycles of
95°C for 15 sec and 60°C for 1 min.
Western blotting
Western blotting was performed as previously
described (11). Specific
antibodies for HIF-1α and c-Myc were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Following washing with rinse
buffer, the blot membranes were incubated with 1:3,000 diluted
horseradish peroxidase-conjugated secondary antibody (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and samples were then
developed using enhanced chemiluminescence reagents (Amersham,
Little Chalfont, UK).
Immunohistology
Tumor samples were isolated and immediately fixed in
10% pH-neutral phosphate-buffered formalin. The fixed tissues were
then embedded in paraffin and kept until required. Paraffin
sections (4 µm) were cut, deparaffinized and hydrated.
Antigens were retrieved in 10 mM sodium citrate buffer (pH 6.0) and
preheated to 95°C for 10 min. Immunohistochemical staining of the
protein was performed using the streptavidin-peroxidase method with
antibodies specific to HIF-1α (rabbit polyclonal, cat. no. 3716;
1:1,000 dilution) and anti-VEGF mouse monoclonal antibody (cat. no.
M727; 1:1,000; Clone VG1; Dakopatts, Copenhagen, Denmark). The
stained sections were examined and scored using a microscope
(Olympus IX51; Olympus, Hamburg, Germany). In order to measure
microvessel density (MVD), paraffin-embedded and formalin-fixed
sections were stained with anti-CD31 mouse monoclonal antibody
(cat. no. 14-0318-93; 1:40; Dakopatts). The
immunohistochemically-stained microvessels were counted in five
areas with the highest vascular density per section of tumor, as
described previously (12).
Microarray of gene expression
SW1990 cells treated with or without TL for 4 h were
harvested and total RNA was isolated. cDNA probes were synthesized
and labeled with Cy5-dCTP or Cy3-dCTP (Amersham Biosciences,
Piscataway, NJ, USA) for TL-treated cells or control cells,
respectively. A BiostarH-40s microarray (Biostar Genechip Inc.,
Shanghai, China) containing 4,097 human genes was used and the
service was provide by Shanghai United Gene Group Ltd. (Shanghai,
China). Data were collected using a ScanArray4000 scanner
(Perkin-Elmer, Boston, MA, USA). GenePixPro3.0 (Axon Instruments,
Union City, CA, USA) was used to analyze the differences in gene
expression between TL-treated cells and control cells.
Statistical analysis
All data are expressed as the mean ± standard
deviation. The significance for the difference between groups was
assessed by one-way analysis of variance using SPSS software
version 11.5 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
TL reduces HIF-1α expression in
pancreatic cancer cells
The growth of the PANC1, ASPC1 and SW1990 pancreatic
cancer cell lines following TL treatment was measured and compared
with growth in these cells lines without TL. TL was found to induce
cell death in all three cell lines (data not shown), with SW1990
cells being the most sensitive to TL (Fig. 1A). At a concentration of 20 ng/ml,
TL led to a significant increase in the death of SW1990 cells after
24 h compared with the control cells (Fig. 1B). It has previously been reported
that HIF-1α is highly expressed in tumor tissues of patients with
pancreatic cancer (13,14). The present study tested the
hypothesis that TL suppresses HIF-1α expression in these cell
lines, thereby leading to cell death. Using RT-qPCR, a substantial
quantity of HIF-1α transcription in all three cell lines was
detected (Fig. 1C). Western
blotting was performed in order to determine changes in the
expression of HIF-1α in SW1990 cells following treatment with TL.
The results showed that HIF-1α expression was suppressed at 24 h
following TL treatment, and that this effect occurred in a
dose-dependent manner as shown in Fig.
1D. These data suggest that HIF-1α is highly expressed in
pancreatic cancer cells lines and that downregulation of HIF-1α may
be responsible for cell death in pancreatic cell lines.
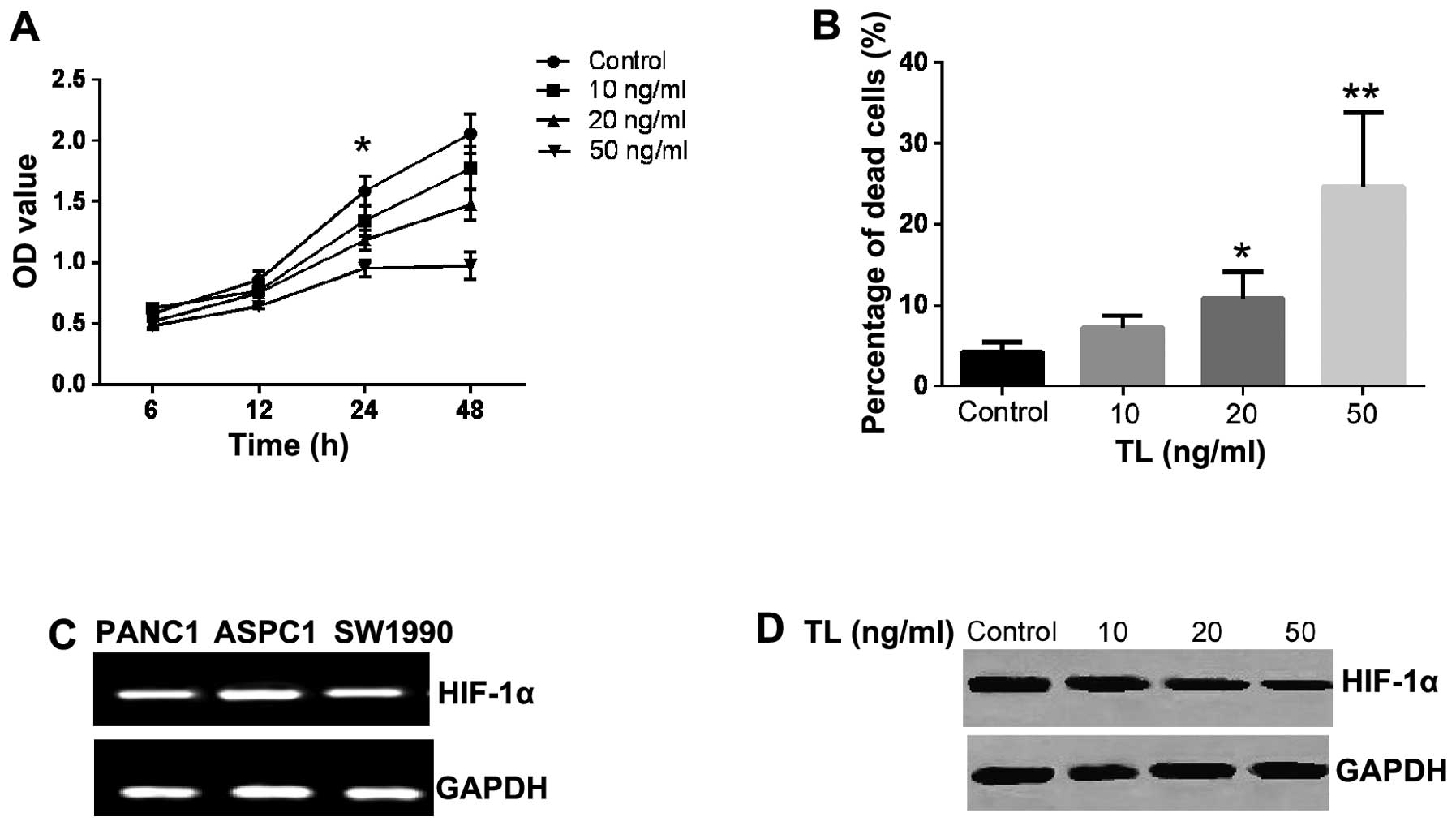 | Figure 1TL inhibits growth of SW1990 cells and
expression of HIF-1α. (A) Growth of SW1990 cells was measured after
6, 12, 24 and 48 h of TL treatment at concentrations of 10, 20 and
50 ng. Significant suppression of cell growth was initially
observed after 24 h of treatment with 20 ng/ml TL.
*P<0.05, compared with control. (B) TL induced cell
death in SW1990 cells. *P<0.05 and
**P<0.01, compared with control. (C) Reverse
transcription-polymerase chain reaction results indicated that
HIF-1α is expressed in the pancreatic cancer cell lines, PANC1,
ASPC1 and SW1900. (D) HIF-1α protein in SW1990 cells was detected
using western blotting and its expression levels was reduced by TL
in a dose-dependent manner. TL, triptolide; HIF-1α,
hypoxia-inducible factor-1α; OD, optical density. |
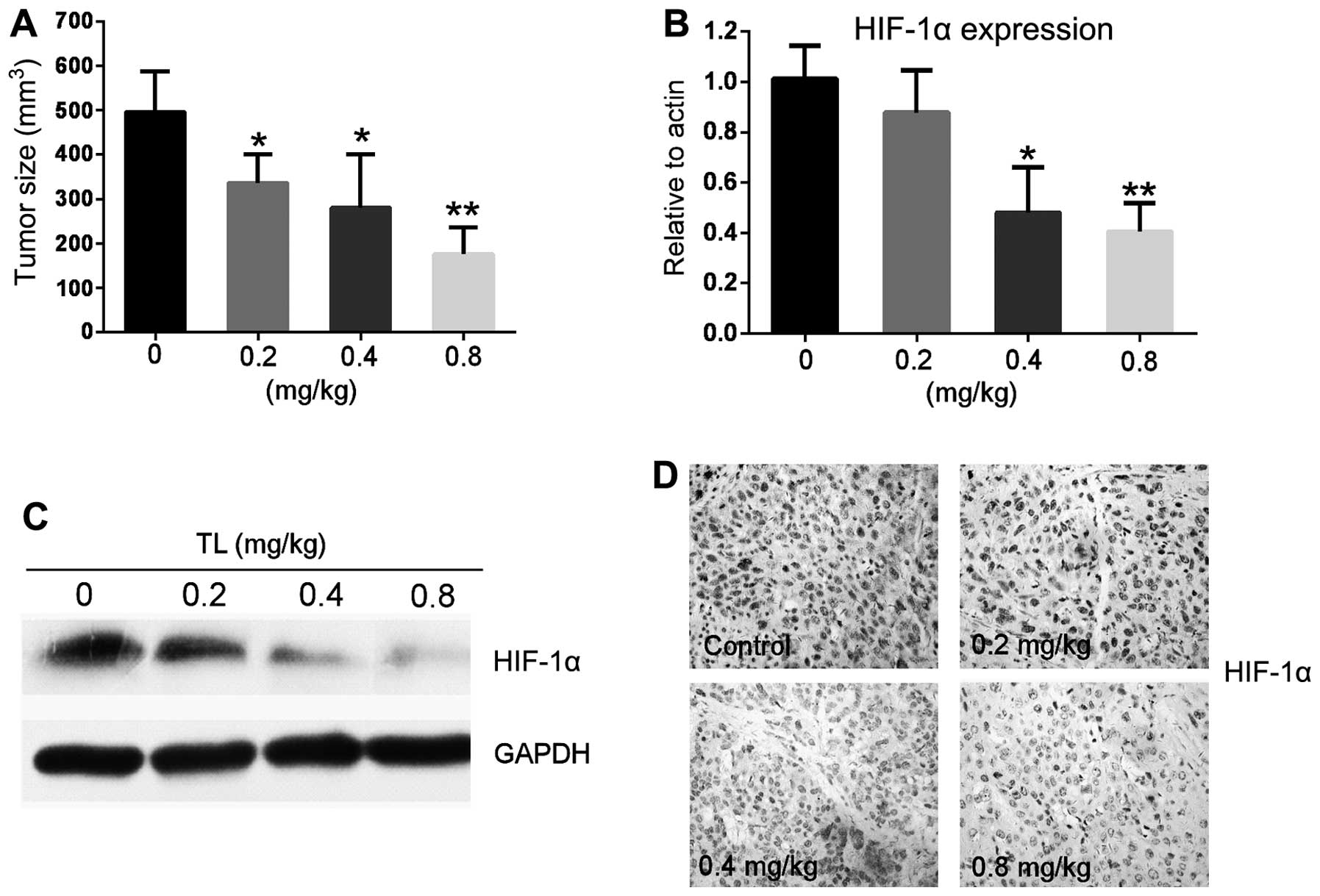
HIF-1α expression in tumor tissues is
downregulated following TL treatment
In order to test whether TL suppresses HIF-1α
expression in vivo, a xenograft model of pancreatic cancer
was established using SW1990 cells. In the present study, the
transplanted tumor was usually visible five days following
injection of tumor cells, and grew to ~100 mm3 by day
10. From day 11, TL at various doses was injected IP, daily for
three weeks. The results confirmed that treatment with TL
significantly suppressed tumor growth, as evaluated by tumor size
at the end of treatment (Fig. 2A).
Tumor tissues were collected and HIF-1α expression levels were
measured using RT-qPCR. The results demonstrated that HIF-1α
transcriptional levels in tumor tissues were significantly lower in
mice that had been treated with TL, compared with those in control
mice (Fig. 2B). HIF-1α protein
levels in these tumors were also measured using western blotting.
As shown in Fig. 2C, the level of
the HIF-1α protein was significantly reduced following TL
treatment. In accordance with this, immunohistological staining
demonstrated that HIF-1α expression was reduced in tumors from mice
treated with TL, compared with that in the control group (Fig. 2D). No obvious side effects were
observed in the mice treated with 0.2–0.4 mg/kg TL for 3 weeks,
however certain side effects, including skin irritation, edema and
bleeding of skin capillary vessels, were observed in a number of
the mice treated with >0.5 mg/kg TL.
TL suppresses vascular endothelial growth
factor (VEGF) expression and microvessel density in tumor
tissues
As TL was shown to suppress HIF-1α expression, it
was hypothesized that the expression of VEGF, which is a HIF-1α
target gene, may also be reduced following TL treatment. The
expression of VEGF in tumor tissues from mice, with or without TL
treatment, was therefore measured using immunohistological
staining. As hypothesized, the results showed that the expression
of VEGF was reduced in tumor tissues from mice treated with TL,
compared with that in the control group (Fig. 3A, upper panel). In addition, TL
reduced the MVD in tumor tissue, as shown in Fig. 3A (lower panel). The inhibitory
effect of TL on the expression of VEGF in the SW1990 cell line was
also significant when measured using RT-qPCR (Fig. 3B). These data indicate that TL
suppresses the expression of HIF-1α and inhibits its downstream
function, such as the expression of VEGF and the regeneration of
microvessels in tumor tissues.
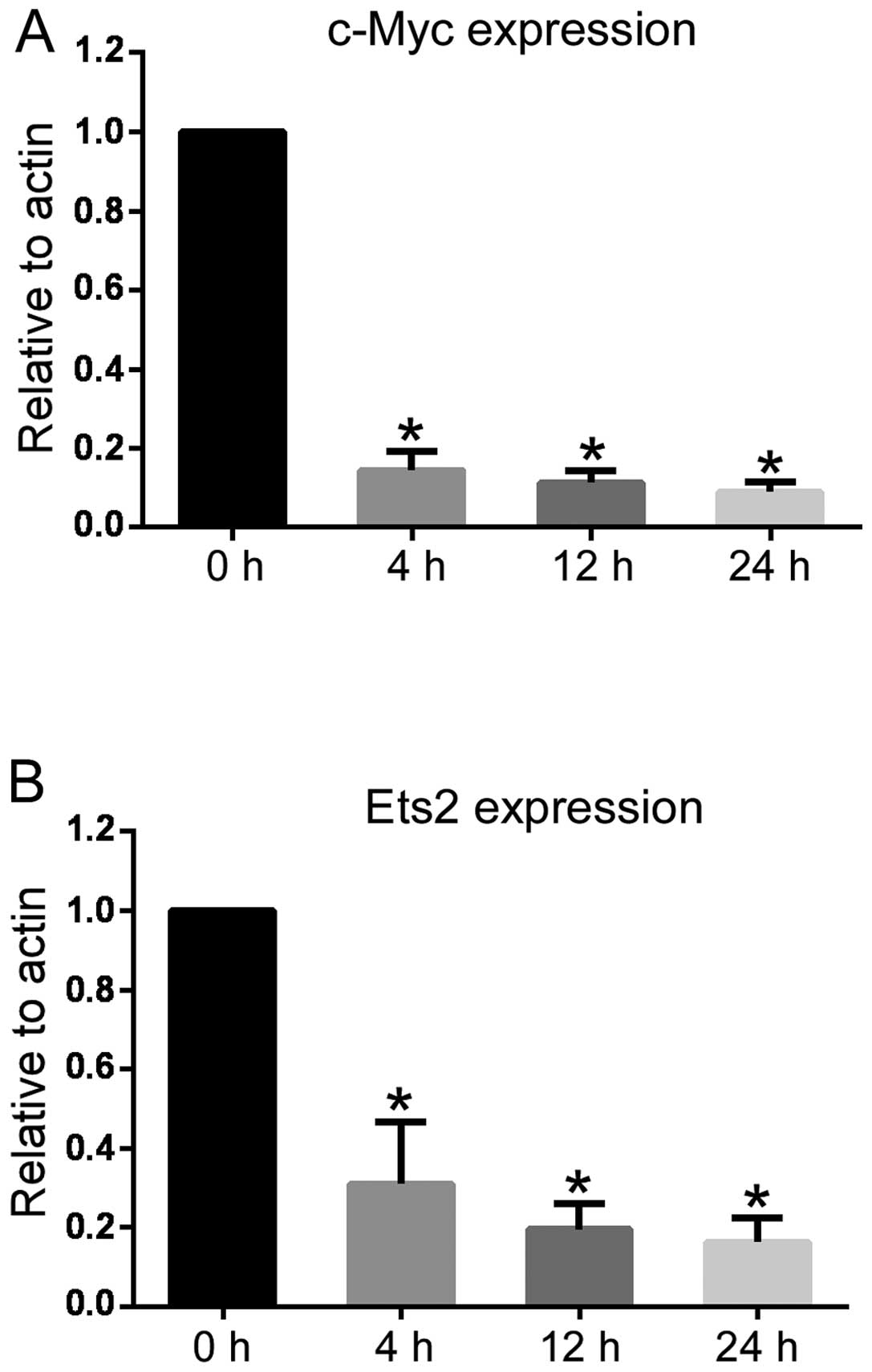
TL treatment alters the expression levels
of multiple cancer-related genes and signal transduction
In order to gain insight into the mechanisms
underlying TL-mediated antitumor effects in pancreatic cancer, the
gene expression profile in pancreatic cancer cells was examined
following TL treatment. SW1990 cells were treated with 40 ng/ml TL
for 4 h, and the expression level of 4,097 genes was measured. The
results showed that the expression of 11 genes was downregulated in
TL-treated cells compared with untreated cells, as shown in
Table I. The most significant
change was observed in the c-Myc gene, which was reduced to 10–20%
of the level of that in the control group in TL-treated cells.
Other genes that were downregulated include the oncogene Ets2, and
certain transcription factors involved in tumorigenesis, such as
SOX9 (15), dickkopf WT signaling
pathway inhibitor 1 (16) and
hairy and enhancer of split-1 (17). The marked change in the expression
of the c-Myc gene is noteworthy, as c-Myc is a well-known oncogene,
and its upregulation has been reported in numerous types of tumor
(18–20). RT-qPCR was used to confirm data
obtained from the microarray experiments. As shown in Fig. 4A and B, the expression of c-Myc and
Ets2 was reduced following 40 ng/ml TL treatment for 4–24 h.
 | Table IDownregulated genes in SW1990 cells
following triptolide treatment. |
Table I
Downregulated genes in SW1990 cells
following triptolide treatment.
| Gene ID | Exp/Control | Gene name | Abbreviation |
|---|
| NM_002467 | 0.185 | V-myc
myelocytomatosis viral oncogene homolog (avian) | c-MYC |
| NM_004655 | 0.285 | Axin 2 | AXIN2 |
| NM_000346 | 0.370 | SRY (sex determining
region Y)-box 9 | SOX9 |
| NM_012242 | 0.390 | Dickkopf WNT
signaling pathway inhibitor 1 | DKK1 |
| NM_018976 | 0.391 | Solute carrier
family 38, member 2 | SLC38A2 |
| NM_005019 | 0.437 | Phosphodiesterase
1A, calmodulin-dependent | PDE1A |
| NM_005239 | 0.446 | V-ets
erythroblastosis virus E26 oncogene homolog 2 (avian) | ETS2 |
| NM_170695 | 0.453 | TGFB-induced factor
(TALE family homeobox) | TGIF |
| NM_002167 | 0.477 | Inhibitor of DNA
binding 3, dominant negative helix-loop-helix protein | ID3 |
| NM_005524 | 0.481 | Hairy and enhancer
of split 1, (Drosophila) | HES1 |
| NM_019058 | 0.500 |
DNA-damage-inducible transcript 4 | DDIT4 |
Discussion
TL treatment leads to cell death in a number of
types of tumor cell line in vitro. In addition, it has
potent antitumor effects in numerous animal models of cancer, and
has potential for use as a chemotherapeutic agent (21–24).
TL alters the expression of certain genes in different experimental
settings and a significant effort has been made to investigate the
common mechanism among them (25).
A previous study indicated that TL inhibits NF-κB activity and the
expression of its downstream genes (26), which are involved in inflammation
and tumorigenesis. However, other studies have indicated that the
spectrum of genes inhibited by TL may be considerably broader than
that of NF-κB target genes. Recent reports have demonstrated that
TL exerts global transcriptional inhibitory activity by inducing
proteasome-dependent degradation of the largest subunit of RNA
polymerase II in cancer cells (27–29).
As a transcription inhibitor, the use of TL in patients should be
carefully evaluated as potential side effects may occur as a result
of non-specific inhibition of global transcription. We propose that
two issues should be thoroughly investigated prior to its
development as a chemotherapeutic agent.
Firstly, the side effects of TL require assessment
in order to evaluate the tolerance of this drug throughout the
course of treatment. In the present study, the xenograft mouse
model of pancreatic cancer was shown to be tolerant to 0.2–0.4
mg/kg TL, and no obvious side effects were observed during the
three weeks of treatment they received, compared with the control
group. However, certain side effects, including skin irritation,
edema and bleeding of skin capillary vessels, were observed in the
present study in a number of the mice treated with >0.5 mg/kg
TL. Although these side effects were not life-threatening, it is
likely that the combined use of low dose of TL with other
therapeutic approaches may, in addition to delivering more potent
antitumor effects, also minimize the side effects of TL that are
experienced at higher doses.
Secondly, overexpression of oncogenes is known to
initiate tumorigenesis, and to drive growth or metastasis of tumor
cells. However, different oncogenes are involved in different tumor
types. For example, the present study showed that c-Myc and HIF-1α
are constitutively overexpressed in pancreatic cancer cell lines
and pancreatic tumor tissues. c-Myc is an oncogene and has been
shown to be overexpressed in numerous types of tumor (30). However, HIF-1α usually is induced
by hypoxia and is not expressed in normoxic conditions, while it is
highly expressed in pancreatic tumors and is associated with vessel
generation in a number of tumor tissues (31). In accordance with recent reports
(13,14), the present study found that TL
reduced HIF-1α expression in pancreatic cancer cell lines and in a
tumor mouse model, suggesting that HIF-1α is involved in the
development of pancreatic cancer. Furthermore, the current results
indicate that the constitutive expression of HIF-1α may be
associated with a high level of c-Myc in pancreatic cancer cells,
as suggested in a recent study on colon cancer (32). We hypothesize that overexpression
of c-Myc and HIF-1α increase the sensitivity of pancreatic tumor
cells to TL compared with that of normal cells, thereby resulting
in a more targeted effect of this treatment. Therefore, the pattern
of expression of key oncogenes in certain types of tumor, may
affect the sensitivity of cells to TL and thus the efficacy of this
drug.
The duration of TL treatment may also be important,
as c-Myc gene expression is suppressed at an early stage in
pancreatic cancer cell lines, which indicated an approach by which
to minimize side effects. That is, through short-term treatment
rather than continued use of the drug. c-Myc overexpression is
estimated to occur in 70% of human tumors (33). Furthermore, inhibition of c-Myc was
recently demonstrated to be an effective method with which to treat
lung cancer (34). These findings
suggest that further investigation into the use of c-Myc as a
target for tumor therapy is required (18,35,36).
Although the finding that the c-Myc gene is involved in the
tumorigenesis of pancreatic cancer was not a novel one, the present
study did show that c-Myc is a target of TL and its expression is
markedly inhibited by treatment with TL for a duration over which
the expression of the majority of genes is not affected. Further
investigation is required to examine how to utilise this activity
of TL in the treatment of patients with pancreatic cancer who
exhibit high levels of expression of c-Myc.
Acknowledgments
This study was supported by the National Natural
Scientific Grants, P.R. China (grant no. 81072028) and the
Foundation for Key Medical Talents in Jiangsu Province (grant no.
RC2007085).
References
|
1
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castellanos E, Berlin J and Cardin DB:
Current treatment options for pancreatic carcinoma. Curr Oncol Rep.
13:195–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maitra A and Hruban RH: Pancreatic cancer.
Annu Rev Pathol. 3:157–188. 2008. View Article : Google Scholar
|
|
5
|
Zhou GX, Ding XL, Huang JF, Zhang H, Wu
SB, Cheng JP and Wei Q: Apoptosis of human pancreatic cancer cells
induced by Triptolide. World J Gastroentero. 14:1504–1509. 2008.
View Article : Google Scholar
|
|
6
|
Carter BZ, Mak DH, Schober WD, Dietrich
MF, et al: Triptolide sensitizes AML cells to TRAIL-induced
apoptosis via decrease of XIAP and p53-mediated increase of DR5.
Blood. 111:3742–3750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang WT, Kang JJ, Lee KY, et al:
Triptolide and chemotherapy cooperate in tumor cell apoptosis. A
role for the p53 pathway. J Biol Chem. 276:2221–2227. 2001.
View Article : Google Scholar
|
|
8
|
Kulke MH: Systemic therapy for advanced
pancreatic neuroendocrine tumors. Semin Oncol. 40:75–83. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otrock ZK, Hatoum HA, Awada AH, Ishak RS
and Shamseddine AI: Hypoxia-inducible factor in cancer
angiogenesis: structure, regulation and clinical perspectives. Crit
Rev Oncol Hematol. 70:93–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Semenza GL: HIF-1: upstream and downstream
of cancer metabolism. Curr Opin Genet Dev. 20:51–56. 2010.
View Article : Google Scholar :
|
|
11
|
Ding X, Zhu C, Qiang H, Zhou X and Zhou G:
Enhancing antitumor effects in pancreatic cancer cells by combined
use of COX-2 and 5-LOX inhibitors. Biomed Pharmacother. 65:486–490.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
13
|
Hoffmann AC, Mori R, Vallbohmer D, et al:
High expression of HIF1a is a predictor of clinical outcome in
patients with pancreatic ductal adenocarcinomas and correlated to
PDGFA, VEGF, and bFGF. Neoplasia. 10:674–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun HC, Qiu ZJ, Liu J, et al: Expression
of hypoxia-inducible factor-1 alpha and associated proteins in
pancreatic ductal adenocarcinoma and their impact on prognosis. Int
J Oncol. 30:1359–1367. 2007.PubMed/NCBI
|
|
15
|
Tanaka T, Kuroki T, Adachi T, et al:
Evaluation of SOX9 expression in pancreatic ductal adenocarcinoma
and intraductal papillary mucinous neoplasm. Pancreas. 42:488–493.
2013. View Article : Google Scholar
|
|
16
|
Gao C, Xie R, Ren C and Yang X: Dickkopf-1
expression is a novel prognostic marker for gastric cancer. J
Biomed Biotechnol. 2012:8045922012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee SH, Hong HS, Liu ZX, Kim RH, Kang MK,
Park NH and Shin KH: TNFα enhances cancer stem cell-like phenotype
via Notch-Hes1 activation in oral squamous cell carcinoma cells.
Biochem Biophys Res Commun. 424:58–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gabay M, Li Y and Felsher DW: MYC
activation is a hallmark of cancer initiation and maintenance. Cold
Spring Harb Perspect Med. 4:a0142412014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stellas D, Szabolcs M, Koul S, et al:
Therapeutic effects of an anti-Myc drug on mouse pancreatic cancer.
J Natl Cancer Inst. 106:dju3202014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou ZL, Yang YX, Ding J, Li YC and Miao
ZH: Triptolide: structural modifications, structure-activity
relationships, bioactivities, clinical development and mechanisms.
Nat Prod Rep. 29:457–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alsaied OA, Sangwan V, Banerjee S, Krosch
TC, Chugh R, Saluja A, Vickers SM and Jensen EH: Sorafenib and
triptolide as combination therapy for hepatocellular carcinoma.
Surgery. 156:270–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carter BZ, Mak DH, Shi Y, Fidler JM, Chen
R, Ling X, Plunkett W and Andreeff M: MRx102, a triptolide
derivative, has potent antileukemic activity in vitro and in a
murine model of AML. Leukemia. 26:443–450. 2012. View Article : Google Scholar
|
|
24
|
Ding X, Zhang B, Pei Q, Pan J, Huang S,
Yang Y, Zhu Z, Lv Y and Zou X: Triptolide induces apoptotic cell
death of human cholangiocarcinoma cells through inhibition of
myeloid cell leukemia-1. BMC Cancer. 14:2712014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Q: Triptolide and its expanding
multiple pharmacological functions. Int Immunopharmacol.
11:377–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yinjun L, Jie J and Yungui W: Triptolide
inhibits transcription factor NF-kappaB and induces apoptosis of
multiple myeloma cells. Leuk Res. 29:99–105. 2005. View Article : Google Scholar
|
|
27
|
Titov DV, Gilman B, He QL, Bhat S, Low WK,
Dang Y, Smeaton M, Demain AL, Miller PS, Kugel JF, et al: XPB, a
subunit of TFIIH, is a target of the natural product triptolide.
Nat Chem Biol. 7:182–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vispé S, DeVries L, Créancier L, Besse J,
Bréand S, Hobson DJ, Svejstrup JQ, Annereau JP, Cussac D, Dumontet
C, et al: Triptolide is an inhibitor of RNA polymerase I and
II-dependent transcription leading predominantly to down-regulation
of short-lived mRNA. Mol Cancer Ther. 8:2780–2790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Lu JJ, He L and Yu Q: Triptolide
(TPL) inhibits global transcription by inducing
proteasome-dependent degradation of RNA polymerase II (Pol II).
PLoS One. 6:e239932011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meyer N and Penn LZ: Reflecting on 25
years with MYC. Nat Rev Cancer. 8:976–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rankin EB and Giaccia AJ: The role of
hypoxia-inducible factors in tumorigenesis. Cell Death Differ.
15:678–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen C, Cai S, Wang G, Cao X, Yang X, Luo
X, Feng Y and Hu J: c-Myc enhances colon cancer cell-mediated
angiogenesis through the regulation of HIF-1α. Biochem Biophys Res
Commun. 430:505–511. 2013. View Article : Google Scholar
|
|
33
|
Gordan JD, Thompson CB and Simon MC: HIF
and c-Myc: sibling rivals for control of cancer cell metabolism and
proliferation. Cancer Cell. 12:108–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Soucek L, Whitfield JR, Sodir NM,
Massó-Vallés D, Serrano E, Karnezis AN, Swigart LB and Evan GI:
Inhibition of Myc family proteins eradicates KRas-driven lung
cancer in mice. Genes Dev. 27:504–513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Soucek L, Whitfield J, Martins CP, Finch
AJ, Murphy DJ, Sodir NM, Karnezis AN, Swigart LB, Nasi S and Evan
GI: Modelling Myc inhibition as a cancer therapy. Nature.
455:679–683. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sodir NM, Swigart LB, Karnezis AN, Hanahan
D, Evan GI and Soucek L: Endogenous Myc maintains the tumor
microenvironment. Genes Dev. 25:907–916. 2011. View Article : Google Scholar : PubMed/NCBI
|