Introduction
Although chondocytes account for only 1–5% of the
entire cartilage tissue volume, their degeneration contributes to
the metabolic and structural changes observed in osteoarthritis
(OA) (1–6).
Apoptosis is the genetically regulated form of cell
death, which occurs when the cell is exposed to physiological,
pathogenic or cytotoxic stimuli, and enables the organism to
maintain its homeostasis (7). The
susceptibility of cells to apoptosis is regulated by complex
molecular signaling systems; proteins encoded by the B-cell
CLL/lymphoma 2 (BCL2) gene family are major regulatory
components of the apoptotic pathway (8–10).
The pro-survival family members, including BCL2, are
integral mitochondrial membrane proteins, which can inhibit
apoptosis. The proapoptotic BCL2-associated X protein
(BAX) products, localize to the cytoskeleton in healthy
cells, however, following a death signal, they interact
predominantly by heterodimerizing with, and inhibiting, the
antiapoptotic proteins, thus initiating apoptosis (7,11,12).
The expression ratio of BCL2 to BAX appears to be an
important determinant of cell susceptibility to apoptosis (9,13)
Cartilage tissue homeostasis is mediated by the
resident chondrocytes, and cellular loss leads to the
characteristic features of OA tissue, including decrease of
cartilage extracellular matrix (ECM) and abnormal tissue remodeling
(4,14,15).
Due to the chronic nature of OA and the fact that chondrocytes
account for little of the total cartilage volume, several studies
have reported contradictory results regarding the relative presence
of apoptotic cells; thus, the definite contribution of apoptotic
cell death in the pathogenesis of the disease is difficult to
determine (4,8,16,17).
However, the assumption that cell death is a central feature in
cartilage degradation has been examined in various studies, the
majority of which report that apoptotic chondrocyte death occurs
more frequently in OA than in healthy cartilage (17–23).
Furthermore, the cartilage in human OA exhibits nuclear and
cytoplasmic features consistent with apoptotic cell death, and the
presence of apoptosis in OA cartilage degeneration has been well
demonstrated in in vitro and in vivo models (16,24).
There is increasing evidence that the BCL2
gene family-apoptotic pathway may be important in the regulation of
chondrocyte apoptosis and the aforementioned observed features of
OA cartilage degeneration (16,25,26).
The mRNA and protein levels of BAX and BCL2 are
detectable in chondrocytes of osteoarthritic and normal cartilage.
Furthermore their protein levels in OA are reported to
differentiate compared with those of the normal cartilage, with
differences in their expression patterns between lesional and
non-lesional areas of the same osteoarthritic cartilage (16,25,26).
The above-mentioned observations suggest that the
relative expression levels of BAX and BCL2 may be a
regulator of chondrocyte apoptosis; alterations in the classical
apoptotic BCL2/BAX expression ratio may contribute to
the process of cartilage degeneration and may be involved in the
pathogenesis of OA. In order to investigate any potential
association between the expression profiles of these classic
apoptosis-associated genes and the biochemical pathways of OA, the
present study quantitatively analyzed the mRNA levels of BAX
and BCL2 in normal and osteoarthritic human articular
cartilage tissue.
Materials and methods
Cartilage tissue samples
The present study was performed in accordance with
the ethical standards set out at the Declaration of Helsinki and
was approved by the institutional review board of Attikon
University Hospital (Athens, Greece). Patients' written informed
consent were obtained prior to the start of the study. Cartilage
tissue samples were isolated from 78 patients undergoing orthopedic
surgical intervention, which were divided in two groups. The first
group was termed the OA group and consisted of 50 specimens
isolated from the visibly evident lesions located on the femoral
and the tibial articular surface of the knee joint from patients
undergoing total arthroplasty for OA. Images of the lesions were
captured for documentation and the cartilage specimens were
isolated from the lateral compartment of the osteoarthritic knee.
The samples were obtained intraoperatively with the use of a
sterile scalpel, irrigated with 10cc normal saline, snap frozen and
stored at −80°C until subsequent analysis. The second group was the
control group, consisting of 28 tissue specimens that were isolated
from non-weight bearing areas of the lateral femoral condyle
articular surface during arthroscopic and reconstructive procedures
for causes other than OA (n=16), or from visibly healthy weight
bearing areas of the lateral tibial or femoral articular cartilage
during above knee amputations or joint salvage procedures in
patients with non-osteoarthritic knees (n=12). In order to
investigate the expression of apoptosis-associated genes in
different stages of OA, the radiographic criteria of the
Kellgren-Lawrence grading scale (27) were used. According to this
classification system, 27 OA samples belonged to patients with
stage III osteoarthritis and 23 OA samples belonged to patients
with stage IV disease (Table
I).
 | Table ICharacteristics of the normal and
osteoarthritic tissue samples. |
Table I
Characteristics of the normal and
osteoarthritic tissue samples.
| Characteristic | Cartilage tissue
sample, n (%)
|
|---|
| Normal (n=28) | Osteoarthritis
(n=50) |
|---|
| Age (median, mean ±
standard error of the mean) | 38.5, 40.9±3.4 | 73.0,
72.7±0.87 |
| Gender (n, %) |
| Female | 14 (50.0) | 42 (84.0) |
| Male | 14 (50.0) | 8 (16.0) |
| Stage of
osteoarthritis |
| III | | 27 (54.0) |
| IV | | 23 (46.0) |
RNA extraction and cDNA synthesis
The osteoarthritic and normal cartilage tissue
specimens (~100 mg of each sample) were frozen in liquid nitrogen
and pulverized until fine powder was obtained. Total RNA was
extracted from the 78 tissue samples using TRIzol
reagent® (Molecular Research Center, Inc., Cincinnati,
OH, USA), according to the manufacturer's instructions. The
resulting RNA pellet was dissolved in a maximum of 5 µl RNA
Storage solution (Applied Biosystems/Ambion, Austin, TX, USA) and
stored in aliquots at −80°C until use. The integrity of the RNA was
confirmed in randomly selected samples via agarose gel
electrophoresis. Following RNA extraction, the total quantity of
RNA from each tissue sample was utilized for first-strand cDNA
synthesis. The reaction mix also contained 1 µg
oligo(dT)18 reverse transcription primer, 50 mM Tris-HCl
(pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol,
0.5 mM each dNTP, 100 units M-MuLV reverse transcriptase RNase
H™(Finnzymes Oy, Espoo, Finland), 20 units RNase
inhibitor (HT biotechnology LTD, Cambridge, England) and
diethylpyrocarbonate-treated (DEPC) water to a total volume of 20
µl. The reverse transcription reaction was incubated at 37°C
for 60 min and was terminated by incubation at 70°C for 15 min.
Quantitative (q) polymerase chain
reaction (PCR)
Gene specific primers were designed and synthesized
by VBC-Biotech GmbH (Vienna, Austria) for the amplification of the
mRNA transcripts of the BCL2 and BAX target genes, as
well as for beta-2-microglobulin (B2M), which was used as a
reference gene. The previously published genomic sequences were
consulted for the design of the primers (GenBank™ accession nos.
NG_009361.1, NG_012191.1 and NG_012920.1 for BCL2,
BAX and B2M, respectively). B2M was selected
for normalization purposes due its reported biological stability in
human osteoarthritic articular cartilage (28). The BCL2 specific primers
were: forward 5′-TCGCCCTGTGGATGACTGA-3′ and reverse
5′-CAGAGACAGCCAGGAGAAATCA-3′, producing a 134 base pair (bp) PCR
amplicon, the BAX specific primers were: forward
5′-TGGCAGCTGACATGTTTTCTGAC-3′ and reverse
5′-TCACCCAACCACCCTGGTCTT-3′, generating an amplicon of 195 bp and
the B2M primers were: forward 5′-ACTGAATTCACCCCCACTGA-3′ and
reverse 5′-AAGCAAGCAAGCAGAATTTGGA-3′, resulting in a product of 167
bp.
Reverse transcription-qPCR analysis was performed in
96-well plates on an ABI Prism 7500 Thermal Cycler (Applied
Biosystems). The reaction mixture contained 0.2 µl cDNA,
50–75 nM primers, 5 µL Kapa SYBR® Fast Universal
2X qPCR Master mix (Kapa Biosystems, Inc., Woburn, MA, USA), 0.2
µL 50X Rox Low passive reference dye (Kapa Biosystems) and
DEPC water in a total volume of 10 µl. The reactions were
performed in duplicate under the following conditions: 95°C for 3
min as a polymerase activation step, 40 cycles of 95°C for 15 sec
for denaturation, and 60°C for 1 min for primer annealing,
extension and fluorescence detection. Each run included a negative
control (no cDNA), as well as a common calibrator sample, which
consisted of cDNA reverse transcribed from RNA isolated from the
PC-3 human prostate cancer cell line (American Type Culture
Collection, Manassas, VA, USA) that was found to be steadily
expressed in all genes under investigation, and was therefore used
for normalization between the runs. Dissociation curves (60–95°C,
with a heating rate of 0.1°C/sec) and fluorescence data (every
0.3°C) were produced following the amplification, in order to
corroborate the presence of the predominant reaction products,
through their unique melting temperatures (Tm), and the absence of
any non-specific products and/or primer-dimers. The Tm of the
specific PCR amplicons were, 83.9, 85.7 and 81.3°C for BCL2,
BAX and B2M, respectively. Randomly selected PCR
products were also electrophoresed on 3% w/v agarose gels in order
to confirm the presence of a unique amplicon.
Calculations were performed using Sequence Detection
system, version 1.2.3 computer software (Applied Biosystems). Gene
expression analysis was performed using the comparative
Ct (2−ΔΔCt) method (29). Briefly, the PCR products were
detected by measuring the emitted fluorescence (Rn) at
the end of each reaction cycle and average CT values
were calculated for subsequent expression analysis. The threshold
cycle (Ct) corresponds to the number of cycles required
to detect a fluorescence signal above the baseline. The relative
quantification units (RQ units=2−ΔΔCt), representative
of the normalized expression of the target genes, were calculated
for each sample. ΔΔCt is the difference between the
ΔCt value of a cartilage tissue sample and the
ΔCT for the calibrator sample, whereas ΔCt is
the difference between the Ct value of the target gene
(BCL2 or BAX) and the Ct of the endogenous
reference gene (B2M).
In order to confirm that amplification was performed
with equal efficiencies for the target and the reference genes,
thus allowing relative quantification according to the
2−ΔΔCT formula, validation experiments were performed
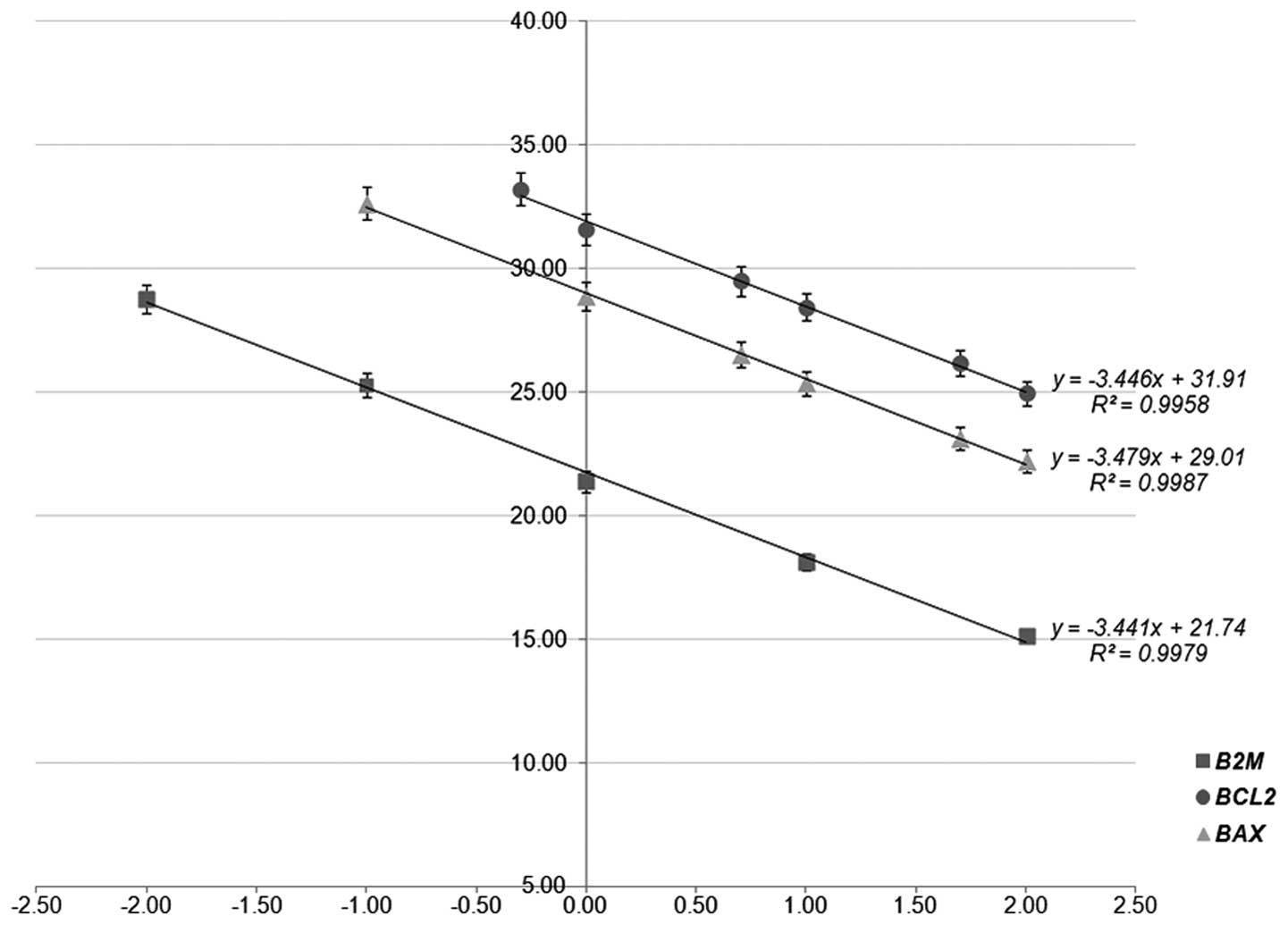
using different quantities of input cDNA (Fig. 1). The cDNA dilution series,
covering several orders of magnitude, were used for BCL2,
BAX and B2M amplification, and the resulting
Ct values were plotted against Log10 [cDNA quantity].
The reaction efficiencies (E%) were estimated using the following
formula: E% = [−1 + 10(−1/slope)] × 100.
Statistical analysis
Non-parametric statistical analyses were performed
as the distribution of variables between the groups was not
Gaussian. For calculation of the BCL2/BAX ratio, the
RQ units of BCL2 were divided by those of BAX for
each sample. The Mann-Whitney U was used to analyze the
differences in the normalized expression levels of BCL2,
BAX and BCL2/BAX between the groups of
individuals. Spearman's correlation coefficient (rs) was
used in order to examine associations between the continuous
variables in the investigation. Receiver operating characteristic
(ROC) curves were generated for the expression levels of
BCL2/BAX by plotting sensitivity against
(1-specificity). The calculations for the Area Under the Curve
(AUC) were based on Hanley and McNeil's method (30). To calculate the mRNA expression
frequencies of BCL2 and BAX in normal and OA tissue
samples, the following cutoff points were used: the 50th
percentile (median) for the expression of BCL2 and the
65th percentile for the expression of BAX;
differences were assessed using Fisher's exact test. Binary
logistic regression models were used in order to estimate the odds
ratio (OR) for the presence of OA. All statistical analyses were
performed using SPSS 17.0 software (SPSS Inc., Chicago Il,
USA).
Results
Quantitative assessment of the mRNA
expression levels of BAX and BCL2
A highly sensitive and specific qPCR assay was
developed and evaluated for quantification of the mRNA levels of
BAX and BCL2. The specific amplification of the
expected products, according to primer design, was evidenced by a
peak in the melting curve analysis and the detection of a single
distinctive band in agarose gel electrophoresis for randomly
selected cartilage tissue specimens (data not shown). Validation
experiments were also performed in order to calculate the qPCR
reaction efficiency for each amplicon, using a wide range of inital
cDNA template quantities. The amplification efficiencies of
BCL2, BAX and B2M, calculated from the slopes
of the calibration curves deriving from the validation experiments
(slopeBCL2=−3.446, r2= 0.996;
slopeBAX=−3.479, r2= 0.999 and
slopeB2M= −3.441, r2= 0.998,
respectively) were 95.1, 93.8 and 95.3%, respectively (Fig. 1). These data confirmed that the PCR
amplicons were produced with similar efficiencies, which
consequently enabled the use of the ΔΔCt method for
calculating the RQ expression units of the BAX and
BCL2 mRNA transcripts.
Distribution of the mRNA levels of BAX
and BCL2 mRNA and the BCL2/BAX expression ratio in OA compared with
normal cartilage
The comparative study of the mRNA levels of
BAX and BCL2 between the normal and OA tissue
cartilage samples enabled identification of important differential
expression patterns. The mRNA levels of BAX presented an
increasing trend in patients with primary OA (median=0.739 RQ
units) compared with the normal individuals (median=0.244 RQ units;
Table II), however this was not
statistically significant (P=0.099). When the individuals were
dichotomized according to the BAX mRNA expression status,
the expression frequency of BAX was significantly higher
(P=0.026) in the OA group compared with the normal group (44.0, vs
17.9%, respectively; Table III).
The mRNA levels of BCL2 remained predominantly unchanged
(P=0.904) in the OA group (median=57.2 RQ units) compared with the
normal group (median=41.8 RQ units; Table II). The expression frequencies of
BCL2 were also similar (P=0.479) between the OA and normal
groups (54.0, vs 42.9%, respectively; Table III).
 | Table IIDistribution of mRNA expression
levels of BAX and BCL2, and BCL2/BAX
ratio in osteoarthritic and normal tissues. |
Table II
Distribution of mRNA expression
levels of BAX and BCL2, and BCL2/BAX
ratio in osteoarthritic and normal tissues.
| Variable | Mean ± SEM | Range | Percentile
|
|---|
| 10 | 25 | 50 (median) | 75 | 90 |
|---|
| Osteoarthritis
(n=50) |
| BAX
expressiona | 1.64±0.31 | 0.00426–7.68 | 0.00426 | 0.0444 | 0.739 | 2.58 | 5.59 |
| BCL2
expressiona | 118±31 | 0.342–1,171 | 0.342 | 1.04 | 57.2 | 132 | 229 |
|
BCL2/BAX ratiob | 208±54 | 0.0454–1,598 | 0.840 | 18.8 | 61.2 | 308 | 467 |
| Normal (n=28) |
| BAX
expressiona | 0.772±0.258 | 0.00426–6.39 | 0.00426 | 0.0166 | 0.244 | 0.870 | 2.26 |
| BCL2
expressiona | 86.2±26.1 | 0.342–664 | 0.947 | 12.3 | 41.8 | 93.9 | 210 |
|
BCL2/BAX ratiob | 418±117 | 8.38–2,020 | 33.5 | 68.4 | 169 | 558 | 1,508 |
 | Table IIIFrequencies of the mRNA expression
levels of BAX and BCL2 between normal and OA tissue
samples |
Table III
Frequencies of the mRNA expression
levels of BAX and BCL2 between normal and OA tissue
samples
| Variable | Individuals
(n) | Individuals, n (%)
| P-valuea |
|---|
| Normal | OA |
|---|
| BAX | | | | 0.026 |
| Negative | 51 | 23 (82.1) | 28 (56.0) |
| Positive | 27 | 5 (17.9) | 22 (44.0) |
| BCL2 | | | | 0.479 |
| Negative | 39 | 16 (57.1) | 23 (46.0) |
| Positive | 39 | 12 (42.9) | 27 (54.0) |
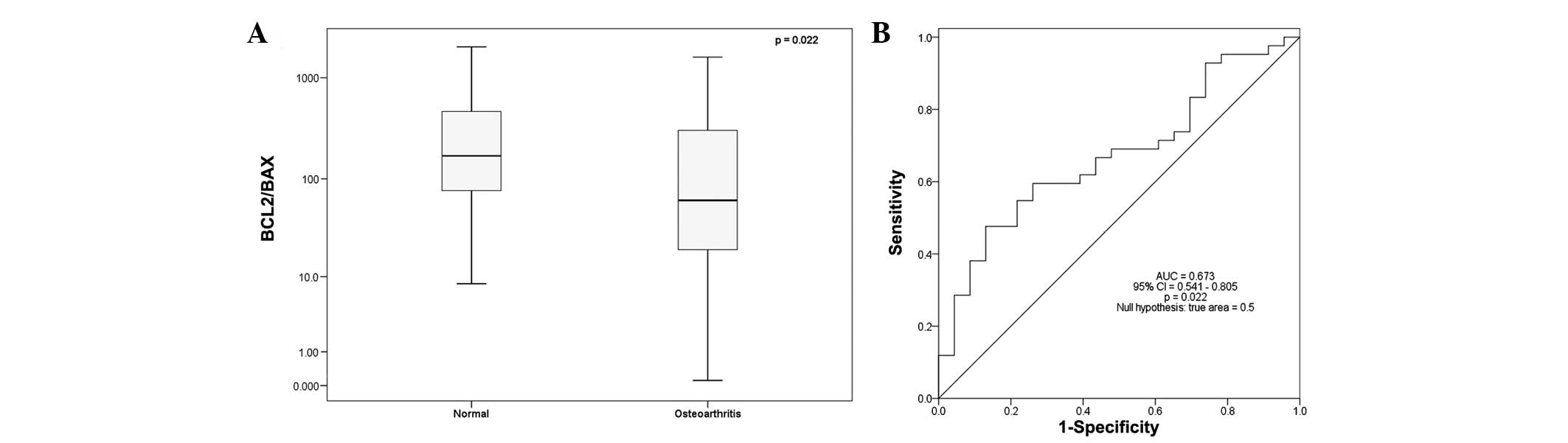
However, the BCL2/BAX expression ratio
was significantly decreased (2.76-fold) in the OA group compared
with the normal group (P=0.022), with a median
BCL2/BAX expression ratio of 169 in the normal group
and 61.2 in the OA group (Table
II; Fig. 2A). Binary logistic
regression analysis revealed that individuals with higher
BCL2/BAX expression ratios were significantly less
likely to suffer from OA (OR=0.400, 95% CI= 0.181–0.884; P= 0.024),
as shown in Table IV.
Additionally, ROC curve analysis revealed the important
BCL2/BAX ratio value in effectively discriminating
the normal from OA samples (AUC=0.673, 95% CI=0.541–0.805, P=0.022;
Fig. 2B).
 | Table IVBinary logistic regression analysis
for the occurrence of osteoarthritis. |
Table IV
Binary logistic regression analysis
for the occurrence of osteoarthritis.
| Covariant | Crude odds
ratio | 95% CI | P-value |
|---|
|
Log10BAX | 1.39 | 0.891–2.16 | 0.147 |
|
Log10BCL2 | 0.828 | 0.517–1.33 | 0.432 |
|
Log10BCL2/BAX | 0.400 | 0.181–0.884 | 0.024 |
| Age | 1.29 | 1.13–1.48 | <0.001 |
mRNA expression levels of BAX, BCL2 and
the BCL2/BAX ratio in radiographical stages of OA
The expression levels of the apoptosis-associated
genes investigated in the present study were also examined at
different stages of OA, according to the radiographic criteria of
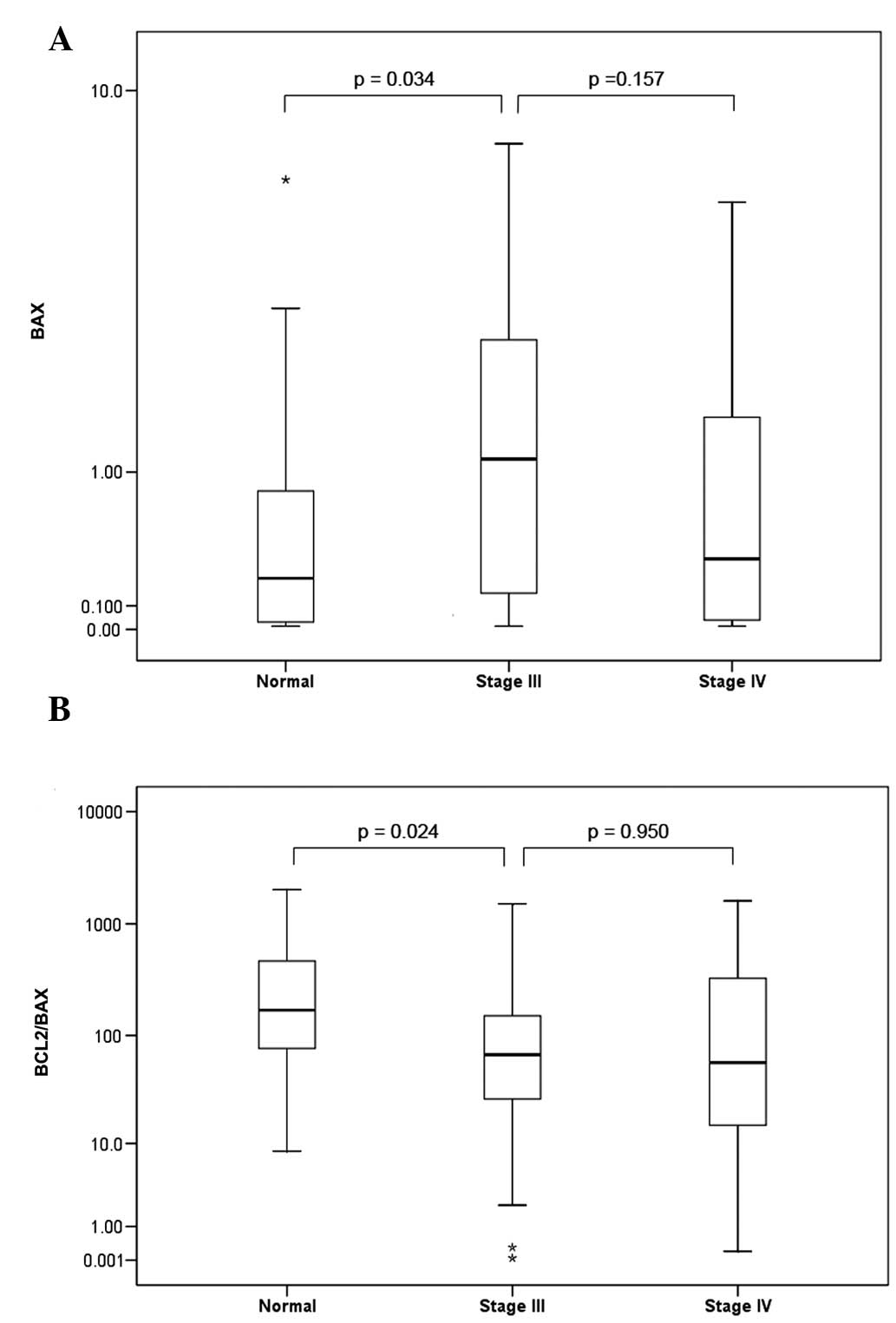
the Kellgren-Lawrence grading scale (27). No statistically significant change
was found between stage III and stage IV OA in the mRNA expression
levels of BAX (P= 0.157) or BCL2 (P= 0.395), or the
BCL2/BAX expression ratio (P=0.950).
Notably, a significant 4.6-fold overexpression of
median BAX mRNA levels was observed between the normal group
and the stage III OA group (P=0.034), with the stage III OA group
having a median expression of 1.12 BAX RQ units, compared
with 0.244 in the normal group (Fig.
3A).
In addition, the BCL2/BAX ratio was
markedly decreased (2.5-fold) between the normal group and the
stage III OA group (Fig. 3B). The
normal group had a median BCL2/BAX expression of 169,
which was significantly (P=0.024) lower compared with that in the
stage III OA group (66.8; Fig.
3B). By contrast, the mRNA levels of BCL2 did not
differentiate substantially (P=0.419) between the stage III OA and
normal group.
Associations between the mRNA expression
levels of BAX, BCL2, BCL2/BAX and patient characteristics
A significant positive correlation was observed
between the mRNA levels of BAX and BCL2 in the normal
group (rs=0.728; P<0.001); and in the OA group,
although to a lesser degree (rs= 0.532; P<0.001).
High mRNA levels of BAX were also correlated with increased
age, which was statistically significant only for the set of tissue
samples derived from normal individuals (rs=0.502;
P=0.007). Similarly, a marked negative correlation was found
between the BCL2/BAX expression ratio and increased
age (rs=−0.580; P=0.004) within the normal group, but
not in the OA group. Elevated mRNA levels of BCL2 were
weakly correlated with increased age within the OA group
(rs= 0.396; P= 0.004), but not among the normal samples.
Gender was not associated in any case with the mRNA levels of
BAX or BCL2 or the BCL2/BAX expression
ratio (P>0.05, Mann-Whitney U test).
Discussion
The hypothesis that chondrocyte cell death is a
central feature of the pathogenesis of OA has been examined
previously (1,2,22,23).
Numerous studies have reported either direct or indirect evidence
of increased apoptosis associated with human osteoarthritis
(16,24,31–34).
In vitro and in vivo studies have implicated the
BCL2 apoptotic gene family in the regulation of chondrocyte
apoptosis and cartilage degradation (16,22,23,25,26).
The majority of studies examining the expression
status of the classical BCL2 and BAX apoptotic genes
in human OA have demonstrated altered gene expression patterns at
the protein level (16,25,26).
Due to the limited material often available from cartilage human
specimens and the difficulties in isolating total RNA from these
samples, there has not been, to our best knowledge, a study that
quantitatively analyzes the mRNA expression of apoptosis-associated
genes. The present study is the first, to the best of our
knowledge, to investigate the differential gene expression of
BCL2 and BAX in human OA and normal articular
cartilage at the mRNA level, via a hypersensitive and specific qPCR
method.
The results of the qPCR demonstrated that the ratio
of BCL2/BAX mRNA expression levels was significantly
decreased (2.76-fold) in the OA tissues compared with the normal
cartilage tissues (P=0.022). It is well established that a decrease
in the BCL2/BAX expression ratio is associated with
the induction of apoptosis in several human tissues, whereas an
increase in this apoptotic ratio is associated with a poor
prognosis in several types of cancer, and can render tumor cells
resistant to the induction of apoptosis by drug therapy (9,13,35,36).
Furthermore a study by Chen et al suggested that the
BCL2/BAX mRNA ratio is important in governing the
susceptibility of human chondrocytes to apoptosis (37).
The gene expression levels of BCL2 and
BAX in normal and osteoarthritic tissue samples were also
determined in the present study; the mRNA levels of BAX in
the OA tissues was increased, but without significance, compared
with the healthy tissues. However, the expression frequency of
BAX was significantly elevated in the OA group (P=0.026).
This trend was also observed by Hu et al in a small sample
size of nine OA and six normal cartilage samples, who reported the
overexpression of BAX mRNA in the OA samples, compared with
the normal samples (25). In terms
of the gene expression of BCL2, no significant difference
was observed between the two groups. These results differ from
those of Kim et al, who reported significantly higher
protein levels of BCL2 levels in normal cartilage, obtained
from autopsy, compared with OA cartilage, obtained from patients
with knee OA (16). These
differences may be attributed to the use of autopsy specimens as
healthy controls, since differences in gene expression patterns may
be affected by issues of biomolecule stability or alterations in
chondrocyte phenotype following death (38).
The importance of the BCL2-BAX
apoptotic pathway in cartilage degradation was further supported by
differences in gene expression levels observed between the normal
tissues and different stages of OA, and positive correlations
between the mRNA levels of BAX and BCL2 in both the
normal and OA groups. Since the OA samples in the present study
were obtained from patients during knee arthroplasty, the tissue
samples represented only late stages of the disease (KL grading
scale III or IV). However, although no statistically significant
differences were found between the stage III and stage IV OA
samples, a significant (P=0.034) increase in the mRNA levels of
BAX and a notable decrease in BCL2/BAX ratio
were observed between the normal samples and stage III samples
(P=0.024). These observations suggest that a balance between the
gene expression levels of BCL2 and BAX is required to
maintain tissue homeostasis and, when this balance is disturbed,
the cell response to apoptotic stimuli may drive the progression of
OA.
A correlation between the expression of classical
apoptotic genes and patient age was also observed. A positive
correlation between the mRNA levels of BAX and increasing
age, and a negative correlation between the BCL2/BAX
ratio and increasing age were observed. Several studies have
implicated the expression levels of BCL2 and BAX in
the molecular basis of age-related changes in several human tissues
(39–43). In a comparative study of a mouse OA
model Mistry et al reported that the protein levels of
BCL2 and BAX, analyzed by immunohistochemistry,
decreased with age (44). In the
present study, the groups were not age-matched, since the
prevalence of OA in young individuals is low, and obtaining healthy
knee cartilage specimens from aged individuals is extremely
difficult (38). The association
between the qualitative and/or quantitative differences in the
extracellular matrix of the articular cartilage and aging may
contribute to the reduction in the maintenance and repair potential
of old cartilage and the increased incidence of degenerative
cartilage disease (4,45–47).
However, whether alterations in the differential expression
patterns of BCL2 and BAX contribute to the
vulnerability of aging cartilage, or vice versa, during the
development of OA remain to be elucidated. Further investigation is
necessary to identify whether the alterations in the relative gene
expression levels of the BCL2/BAX apoptotic pathway
reflect the response of chondrocytes to the increased biomechanical
load, decrease of the cartilage repair potential due to the normal
aging process or the severity of OA.
Although the role of apoptosis and of the
BCL2/BAX gene apoptotic pathway in the development of
OA remains to be fully elucidated, delineation of the apoptotic
mechanisms occurring in the articular cartilage tissue may offer
potentially useful therapeutic targets for OA. Chen et al
reported that selenium partly inhibits the apoptotic cell death
induced by T-2 mycotoxin in human chondrocytes by decreasing the
BCL2/BAX ratio (37). Feng et al reported that
chondrocytes overexpressing BCL2 are resistant to apoptosis
induced by serum withdrawal and retinoic acid treatment (48), while Mukherjee et al
reported that staurosporine-mediated chondrocyte death coincided
with increased mRNA expression levels of BAX:BCL-X,
and that pretreatment of cultures with nimesulide or ibuprofen,
protects chondrocytes against cell death (49). Furthermore, Amling et al
demonstrated that PTHrP stimulates the protein expression of
BCL2 in chondrocytes in vivo and in vitro
(50). Further investigations may
reveal whether the pharmacological inhibition of cell death in
chondrocyes is clinically valuable for OA (4).
Despite increasing evidence that chondrocyte
apoptosis is associated with the development of OA, the importance
of that link, and whether chondrocyte apoptosis is a cause or a
result of cartilage degeneration, remains to be elucidated
(2,51). The results of the present study
further implicate apoptosis in the pathogenesis of OA, through
molecular mechanisms, which include the aberrant expression of the
BCL2 gene family. Additional investigation of this
association, and the ability to intervene in the process of
apoptosis may reveal novel prognostic biomarkers and potential
targets for early therapeutic interventions in the treatment of
OA.
Acknowledgments
This study was co-financed by the Hellenic
Association of Orthopaedic Surgery & Traumatology and the
University of Athens, Special Account for Research Grant
(Kapodistrias).
References
|
1
|
Chang J, Wang W, Zhang H, Hu Y, Wang M and
Yin Z: The dual role of autophagy in chondrocyte responses in the
pathogenesis of articular cartilage degeneration in osteoarthritis.
Int J Mol Med. 32:1311–1318. 2013.PubMed/NCBI
|
|
2
|
Mutijima E, De Maertelaer V, Deprez M,
Malaise M and Hauzeur JP: The apoptosis of osteoblasts and
osteocytes in femoral head osteonecrosis: its specificity and its
distribution. Clin Rheumatol. 33:1791–1795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vaillancourt F, Fahmi H, Shi Q, et al:
4-Hydroxynonenal induces apoptosis in human osteoarthritic
chondrocytes: the protective role of glutathione-S-transferase.
Arthritis Res Ther. 10:R1072008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grogan SP and D'Lima DD: Joint aging and
chondrocyte cell death. Int J Clin Rheumtol. 5:199–214. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blagojevic M, Jinks C, Jeffery A and
Jordan KP: Risk factors for onset of osteoarthritis of the knee in
older adults: a systematic review and meta-analysis. Osteoarthritis
Cartilage. 18:24–33. 2010. View Article : Google Scholar
|
|
6
|
Felson DT, Lawrence RC, Dieppe PA, et al:
Osteoarthritis: new insights. Part 1: the disease and its risk
factors. Ann Intern Med. 133:635–646. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomadaki H and Scorilas A: BCL2 family of
apoptosis-related genes: functions and clinical implications in
cancer. Crit Rev Clin Lab Sci. 43:1–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnson EO, Charchandi A, Babis GC and
Soucacos PN: Apoptosis in osteoarthritis: morphology, mechanisms,
and potential means for therapeutic intervention. J Surg Orthop
Adv. 17:147–152. 2008.PubMed/NCBI
|
|
9
|
Korsmeyer SJ, Shutter JR, Veis DJ, Merry
DE and Oltvai ZN: Bcl-2/BAX: a rheostat that regulates an
anti-oxidant pathway and cell death. Semin Cancer Biol. 4:327–332.
1993.PubMed/NCBI
|
|
10
|
Reed JC: Double identity for proteins of
the Bcl-2 family. Nature. 387:773–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cory S and Adams JM: The BCL2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hockenbery D, Nuñez G, Milliman C,
Schreiber RD and Korsmeyer SJ: Bcl-2 is an inner mitochondrial
membrane protein that blocks programmed cell death. Nature.
348:334–336. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oltvai ZN and Korsmeyer SJ: Checkpoints of
dueling dimers foil death wishes. Cell. 79:189–192. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iannone F and Lapadula G: The
pathophysiology of osteoarthritis. Aging Clin Exp Res. 15:364–372.
2003. View Article : Google Scholar
|
|
15
|
Martel-Pelletier J, Boileau C, Pelletier
JP and Roughley PJ: Cartilage in normal and osteoarthritis
conditions. Best Pract Res Clin Rheumatol. 22:351–384. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HA, Lee YJ, Seong SC, Choe KW and Song
YW: Apoptotic chondrocyte death in human osteoarthritis. J
Rheumatol. 27:455–462. 2000.PubMed/NCBI
|
|
17
|
Aigner T, Hemmel M, Neureiter D, et al:
Apoptotic cell death is not a widespread phenomenon in normal aging
and osteoarthritis human articular knee cartilage: a study of
proliferation, programmed cell death (apoptosis), and viability of
chondrocytes in normal and osteoarthritic human knee cartilage.
Arthritis Rheum. 44:1304–1312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng AX, Lou SQ, Zhou HW, Wang Y and Ma
DL: Expression of PDCD5, a novel apoptosis related protein, in
human osteoarthritic cartilage. Acta Pharmacol Sin. 25:685–690.
2004.PubMed/NCBI
|
|
19
|
Héraud F, Héraud A and Harmand MF:
Apoptosis in normal and osteoarthritic human articular cartilage.
Ann Rheum Dis. 59:959–965. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Horton WE Jr, Feng L and Adams C:
Chondrocyte apoptosis in development, aging and disease. Matrix
Biol. 17:107–115. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu H, Hou G, Zhang Y, Dai Y and Zhao H:
c-Jun transactivates Puma gene expression to promote
osteoarthritis. Mol Med Rep. 9:1606–1612. 2014.PubMed/NCBI
|
|
22
|
Zamli Z, Adams MA, Tarlton JF and Sharif
M: Increased chondrocyte apoptosis is associated with progression
of osteoarthritis in spontaneous Guinea pig models of the disease.
Int J Mol Sci. 14:17729–17743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thomas CM, Fuller CJ, Whittles CE and
Sharif M: Chondrocyte death by apoptosis is associated with the
initiation and severity of articular cartilage degradation. Int J
Rheum Dis. 14:191–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blanco FJ, Guitian R, Vázquez-Martul E, de
Toro FJ and Galdo F: Osteoarthritis chondrocytes die by apoptosis.
A possible pathway for osteoarthritis pathology. Arthritis Rheum.
41:284–289. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu J, Huang G, Huang S and Yang L: Control
genes of chondrocyte apoptosis in osteoarthritic articular
cartilage. Zhonghua Wai Ke Za Zhi. 38:266–268. 2172000.
|
|
26
|
Wang SJ, Guo X, Chen JH, et al: Mechanism
of chondrocyte apoptosis in Kashin-Beck disease and primary
osteoarthritis: a comparative study. Nan Fang Yi Ke Da Xue Xue Bao.
26:927–930. 2006.PubMed/NCBI
|
|
27
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteoarthrosis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pombo-Suarez M, Calaza M, Gomez-Reino JJ
and Gonzalez A: Reference genes for normalization of gene
expression studies in human osteoarthritic articular cartilage. BMC
Mol Biol. 9:172008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hanley JA and McNeil BJ: The meaning and
use of the area under a receiver operating characteristic (ROC)
curve. Radiology. 143:29–36. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hashimoto S, Ochs RL, Komiya S and Lotz M:
Linkage of chondrocyte apoptosis and cartilage degradation in human
osteoarthritis. Arthritis Rheum. 41:1632–1638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kourí JB, Aguilera JM, Reyes J, Lozoya KA
and González S: Apoptotic chondrocytes from osteoarthrotic human
articular cartilage and abnormal calcification of subchondral bone.
J Rheumatol. 27:1005–1019. 2000.PubMed/NCBI
|
|
33
|
Yatsugi N, Tsukazaki T, Osaki M, Koji T,
Yamashita S and Shindo H: Apoptosis of articular chondrocytes in
rheumatoid arthritis and osteoarthritis: correlation of apoptosis
with degree of cartilage destruction and expression of
apoptosis-related proteins of p53 and c-myc. J Orthop Sci.
5:150–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharif M, Whitehouse A, Sharman P, Perry M
and Adams M: Increased apoptosis in human osteoarthritic cartilage
corresponds to reduced cell density and expression of caspase-3.
Arthritis Rheum. 50:507–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tallman MS, Gilliland DG and Rowe JM: Drug
therapy for acute myeloid leukemia. Blood. 106:1154–1163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Köhler T, Schill C, Deininger MW, et al:
High Bad and BAX mRNA expression correlate with negative outcome in
acute myeloid leukemia (AMl). Leukemia. 16:22–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen JH, Cao JL, Chu YL, Wang ZL, Yang ZT
and Wang HL: T-2 toxin-induced apoptosis involving Fas, p53,
Bcl-xL, Bcl-2, BAX and caspase-3 signaling pathways in human
chondrocytes. J Zhejiang Univ Sci B. 9:455–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Horton WE Jr, Yagi R, Laverty D and Weiner
S: Overview of studies comparing human normal cartilage with
minimal and advanced osteoarthritic cartilage. Clin Exp Rheumatol.
23:103–112. 2005.PubMed/NCBI
|
|
39
|
Lee EK, Jung KJ, Choi J, et al: Molecular
basis for age-related changes in ileum: involvement of
BAX/caspase-dependent mitochondrial apoptotic signaling. Exp
Gerontol. 45:970–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Phaneuf S and Leeuwenburgh C: Cytochrome c
release from mitochondria in the aging heart: a possible mechanism
for apoptosis with age. Am J Physiol Regul Integr Comp Physiol.
282:R423–R430. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gupta S: Molecular and biochemical
pathways of apoptosis in lymphocytes from aged humans. Vaccine.
18:1596–1601. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Das P, Chopra M, Sun Y, Kerns DG,
Vastardis S and Sharma AC: Age-dependent differential expression of
apoptosis markers in the gingival tissue. Arch Oral Biol.
54:329–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Aggarwal S and Gupta S: Increased
apoptosis of T cell subsets in aging humans: altered expression of
Fas (CD95), Fas ligand, Bcl-2, and BAX. J Immunol. 160:1627–1637.
1998.PubMed/NCBI
|
|
44
|
Mistry D, Oue Y, Chambers MG, Kayser MV
and Mason RM: Chondrocyte death during murine osteoarthritis.
Osteoarthritis Cartilage. 12:131–141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Temple MM, Bae WC, Chen MQ, et al: Age-
and site-associated biomechanical weakening of human articular
cartilage of the femoral condyle. Osteoarthritis Cartilage.
15:1042–1052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barbero A, Grogan S, Schäfer D, Heberer M,
Mainil-Varlet P and Martin I: Age related changes in human
articular chondrocyte yield, proliferation and post-expansion
chondrogenic capacity. Osteoarthritis Cartilage. 12:476–484. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang C, Li SW, Helminen HJ, Khillan JS,
Bao Y and Prockop DJ: Apoptosis of chondrocytes in transgenic mice
lacking collagen II. Exp Cell Res. 235:370–373. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Feng L, Precht P, Balakir R and Horton WE
Jr: Evidence of a direct role for Bcl-2 in the regulation of
articular chondrocyte apoptosis under the conditions of serum
withdrawal and retinoic acid treatment. J Cell Biochem. 71:302–309.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mukherjee P, Rachita C, Aisen PS and
Pasinetti GM: Non-steroidal anti-inflammatory drugs protect against
chondrocyte apoptotic death. Clin Exp Rheumatol. 19(Suppl 22):
S7–S11. 2001.PubMed/NCBI
|
|
50
|
Amling M, Neff L, Tanaka S, et al: Bcl-2
lies downstream of parathyroid hormone-related peptide in a
signaling pathway that regulates chondrocyte maturation during
skeletal development. J Cell Biol. 136:205–213. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zamli Z and Sharif M: Chondrocyte
apoptosis: a cause or consequence of osteoarthritis? Int J Rheum
Dis. 14:159–166. 2011. View Article : Google Scholar : PubMed/NCBI
|