Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
disease, typically characterized by symmetrical polyarthritis,
joint destruction and an impaired quality of life (1). The pathogenesis of RA remains to be
elucidated. Numerous factors and inflammatory cells, including
monocytes, B cells, T cells, endothelial cells and fibroblasts
operate in unison to initiate a chronic inflammatory process
(2–5). Increasing evidence has been obtained
regarding the pivotal role of B cells involved in the immune
dysregulation in RA, which is supported by the clinical
improvements in the patients with RA receiving B-cell depleting
therapies such as rituximab, an anti-CD20 antibody (6–9).
However, the absence or loss of B cells was shown to exacerbate
disease symptoms in experimental autoimmune encephalomyelitis,
collagen-induced arthritis (CIA) and colitis, suggesting that B
cells, or specific B-cell sub-sets, are also able to negatively
regulate immune responses (10–13).
Suppressor B-cells were initially observed in 1974
for their suppressive effects on delayed hypersensitivity (14). Further interest in suppressor
B-cells was generated by studies demonstrating a profound
inhibitory function in the inflammatory context, in vitro
and in adoptive transfer experiments (15–20).
Cumulative evidence suggested that a certain B-cell sub-set
predominantly exerted regulatory functions through the production
of the regulatory cytokine interleukin (IL)-10; this B-cell sub-set
was therefore named as B10 cells (16). Additional types of IL-10-producing
or regulatory B10 cells also exist, including transforming growth
factor-β producing B-cells (12,17,21,22).
Dissimilar to the CD1dhiCD5+CD19hi
sub-set in the spleen, which was labeled as B10 cells in mice, the
identification of B10 cells using membrane markers has, thus far,
not been successful in humans (23). The B10 cells were functionally
identified by their ability to express cytoplasmic IL-10 following
an ex vivo stimulation. Recently, the
CD19+CD24hiCD38hiIL-10+
and
CD19+CD24hiCD27+IL-10+
B-cell subsets were investigated in humans, such as B10 cells of
autoimmunity and immune-mediated inflammation, which further
facilitated the investigation of regulatory B10 cells (24–27).
Contradictory to the findings in the animal models, the negative
regulatory function of B10 cells was impaired in the patients with
thrombocytopenia and systemic lupus erythematosus (SLE) (26,28).
In the CIA model, B10 cells demonstrated their novel and effective
suppressive effects as well as their potential therapeutic value in
the in vivo treatment of severe RA, with resistance to
current therapies (12). However,
the frequency of B10 cells in the patients with RA and the
demonstration of their similar inhibitory effects to those found in
animal models require further study.
In the present study, the frequency of B10 cells in
the peripheral blood (PB) and their correlation with clinical
characteristics, laboratory characteristics and inflammatory
cytokines in a total of 97 patients with RA with different disease
activity and status were investigated. In addition, the frequency
of B10 cells in the paired synovial fluid (SF) from 13 patients was
evaluated. Finally, the IL-10 production and suppression capacities
of the B10 cells on T-cells in the patients of different disease
status were investigated in order to examine their potential
clinical application.
Patients and methods
Patients and healthy controls
Informed consent was obtained from 97 patients (aged
≥18 years) using the revised American College of Rheumatology
criteria from 1987 (29). The
patients with RA with complicated infections were not enrolled in
the present study. A total of 24 healthy individuals were recruited
following informed consent as a control group and these patients
were age and gender matched with the patients with RA. The Ethics
Committee at Xijing Hospital (Xi'an, China) granted ethical
approval of the present study. Demographic and clinical parameters
were also collected at the same time as the blood and synovial
samples. These included age, gender, disease duration/duration of
flare, use of drugs, tender joint count (28 joints), swollen joint
count (28 joints), erythrocyte sedimentation rate, level of
C-reactive protein (CRP), patient global visual analogue scale
(VAS; 0–10 cm), disease activity score in 28 joints (DAS28)-CRP
scores, rheumatoid factor, anti-cyclic citrullinated peptide 2
antibodies, anti-mutated citrullinated vimentin antibody, and
immunoglobulins, immunoglobulin (Ig)G, IgM, and IgM.
A total of 22 patients reached a DAS value of
<2.6 for at least 6 months and were in clinical DAS28 remission.
Out of the 75 patients with active disease, 51 individuals received
no treatment or were only treated with non-steroidal
anti-inflammatory drugs (NSAIDs), while 24 patients experienced a
disease relapse. A flare was defined as any increase in the disease
activity (DAS28≥2.6) and required a change in therapy (30).
B-cell surface and cytoplasmic expression
analysis
Mononuclear cells in PB (PBMCs) and SF (SFMCs) were
isolated by density gradient centrifugation using Ficoll-Hypaque
(1.077 g/ml; Sigma-Aldrich, St. Louis, MO, USA) and were
centrifuged at 400 × g for 30 min at room temperature. PBMCs and
SFMC were re-suspended for 48 h prior to stimulation
(2×106 cells/ml) in a RPMI-1640 medium containing 10%
fetal calf serum (Invitrogen Life Technologies, Carlsbad, CA, USA),
4 mM L-glutamine (Invitrogen Life Technologies), 10 µg/ml
lipopolysaccharide (LPS; Sigma-Aldrich) and 1 µg/ml CD40L
(R&D Systems, Minneapolis, MN, USA). Phorbol myristate acetate
(PMA; 50 ng/Ml, Sigma-Aldrich), ionomycin (1 µg/ml;
Sigma-Aldrich) and GolgiPlug (Brefeldin A; BD Biosciences, San
Jose, CA, USA) were added 5 h prior the end of the culture.
Following harvesting, B-cell phenotypic analysis was performed
using fluorescent-conjugated mouse anti-human monoclonal antibodies
against human CD19-APC (cat. no. 555415), CD24-PerCP-Cy5.5 (cat.
no. 561647), and CD38-FITC (cat. no. 555459) for 30 min on ice in
the dark. The antibodies were purchased from BD Biosciences. The
cytoplasmic IL-10 expression was analyzed according to the
manufacturer's instructions, using a Cytofix/Cytoperm kit (BD
Biosciences) and staining with anti-human IL-10 or an
isotype-matched control. Subsequently, the cells were analyzed
using flow cytometry (FACSort; BD Biosciences).
Serum cytokine concentration assays
Quantitative levels of cytokines IL-1β, IL-6, IL-21,
IL-8, IL-17A and interferon (IFN)-γ were measured in the serum of
the patients, following the manufacturer's instructions (BD
Biosciences). In brief, six bead populations with distinct
fluorescence intensities coated with capture antibodies specific
for IL-1β, IL-6, IL-21, IL-8, IL-17A, and IFN-γ were mixed and
added to each assay tube at room temperature followed by incubation
for 3 h. Cells were then washed with wash buffer and re-suspended
in a wash buffer prior to detection using flow cytometry. The data
were analyzed using the BD CBA analysis software (FCAP Array v3.0;
BD Biosciences). The concentration of each cytokine in the plasma
was determined in reference to a standard curve.
Cell sorting and functional assays
A total of five patients with active disease and
three healthy controls provided formal consent for the attainment
of an additional 200–300 ml PB, which was necessary for cell
sorting and functional assays. Table
I details the characteristics of the five patients and the
three healthy controls. PBMCs were isolated as described above. The
CD19+CD24hiCD38hi cells were
separated using a fluorescence-activated cell sorting Moflo sorter
(Beckman Coulter, Inc, Brea, CA, USA) following surface staining
using fluorescently-conjugated antibodies against CD24, CD19 and
CD38. CD3+ T cells were later enriched using MicroBeads
(Miltenyi Biotech, Bergisch Gladbach, Germany) according to the
manufacturer's instructions. The secretion of IL-10 by the
CD19+CD24hiCD38hi cells was
stimulated with LPS and CD40L for 48 h. Flow cytometric analysis
was used to detect the cells as described above.
 | Table ICharacteristics of patients with
rheumatoid arthritis and healthy controls based on functional
assays. |
Table I
Characteristics of patients with
rheumatoid arthritis and healthy controls based on functional
assays.
| Subject | Age (years) | Gender | Therapy | IL-10+ B
cells (%) |
|---|
| Patient with active
disease | | | | |
| 1 | 18 | F | No | 0.55 |
| 2 | 58 | F | No | 0.69 |
| 3 | 29 | F | No | 4.26 |
| 4 | 46 | M | NSAID | 4.58 |
| 5 | 37 | F | No | 7.13 |
| Healthy control
subject | | | | |
| 1 | 23 | M | | 1.59 |
| 2 | 51 | F | | 0.98 |
| 3 | 46 | F | | 2.11 |
CD19+CD24hiCD38hi
cells were then added to the CD3+ T-cell culture in
96-well round-bottom plates at a ratio of 1:2 (1×105
cells: 2×105 cells) and stimulated with CD3 monoclonal
antibody (mAb; 0.5 µg/ml) for 48 h. Cultured CD3+
T cells stimulated with CD3 mAb in the absence of
CD19+CD24hiCD38hi cells served as
a control. The proliferation of CD3+ T cells was
observed using carboxyfluorescein succinimidyl ester (CFSE;
Molecular Probes, Eugene, OR, USA) staining, which was quantified
by flow cytometric analysis. ELISA was used to detect the cytokine
tumor necrosis factor (TNF)-α in the supernatant using an IFN-γ
high sensitivity ELISA (cat. no. BMS228HS; eBioscience, San Diego,
CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS 10.0
statistical software (SPSS, Inc., Chicago, IL, USA). For all the
comparisons described in the present study Mann-Whitney U-test or
t-test were used. P<0.05 was considered to indicate a
statistically significant difference. Correlations were
investigated using Spearman's rank correlation coefficient.
Results
Heterogeneity in the frequency of B10
cells with a CD19+CD24hiCD38hi
phenotype in patients with RA
The median frequency of the IL-10-competent
CD19+ B cells from the patients with RA with active
disease was significantly increased following a 48-h stimulation
with LPS plus CD40L and subsequent 5-h re-stimulation with PMA +
ionomycin (Fig. 1A) as compared
with that in the healthy controls [median (% range), 3.38
(0.26–9.74) vs. 1.78 (0.86–4.11); P<0.0001] and patients with RA
in remission [median (% range), 3.38 (0.26–9.74) vs. 1.39
(0.69–4.04); P<0.0001]. Although IL-10-competent
CD19+ CD21hi CD38hi B-cells were
enhanced in patients with RA with active disease, the increases
were not as significant in patients with low disease status [median
(% range), 3.84 (1.99–5.12)], moderate disease status [median (%
range), 3.19 (1.44–9.44)] and high disease status [median (%
range), 3.53 (0.26–8.28] (Fig.
1B). However, regardless of the disease status, the frequency
of IL-10+ B cells was higher in the SF as compared with
that in the paired PB samples (Fig.
1C). An additional analysis was conducted on patients that
received no treatment or were treated only with NSAIDs, and
patients with RA with a disease relapse and an active disease
status (Fig. 1D). Individuals that
received no treatment/NSAIDs exhibited a lower level of
IL-10+ B cells [median (% range), 3.21 (0.26–9.74)] as
compared with that in patients experiencing a disease relapse
[median (% range), 4.01 (1.88–8.28)]; however, the difference was
not statistically significant (P=0.10). Of note, 13 individuals
(presented under the dashed line in Fig. 1B and D), which received no
treatment/NSAIDs and who had a high disease status, exhibited a
significantly lower IL-10+ B-cell frequency as compared
with that in the other 38 patients who received no treatment/NSAIDs
[median (% range), 0.55 (0.26–1.00] vs. 3.8 (1.44–9.74);
P<0.0001]. Similarly, the 24 patients with a disease relapse
exhibited a higher IL-10+ B-cell frequency compared with
those with <1% IL-10+ B cells among the patients with
no treatment/NSAIDs [median (% range), 4.01 (1.88–8.28) vs. 0.55
(0.26–1.00); P<0.0001] (Fig.
1E). In addition, the individuals with ≤1% IL-10+ B
cells exhibited a longer symptom duration, a greater number of
tender and swollen joints, and a higher patient global visual
analogue scale and DAS28-CRP, respectively (Table II).
 | Figure 1Frequency and phenotype of the
regulatory B10 cells in the patients with RA. (A) The frequency of
IL-10+ B cells was significantly increased in the
patients with active disease (P<0.0001) as compared with that in
the healthy control group (P<0.0001). However, patients in
remission exhibited a similar frequency of IL-10+ B
cells to that in the healthy controls (P=0.191). (B) Patients with
a low, moderate and high disease status exhibited no significant
differences in the frequency of IL-10+ B cells. However,
13 patients with a high active disease status exhibited a low
frequency of IL-10+ B cells (≤1%) (under dashed line).
(C) The frequency of IL-10+ B cells in synovial fluid
was increased as compared with that in the paired peripheral blood
samples. (D) Among the patients with active disease status,
patients with no treatment/NSAID exhibited a non-significant
frequency of IL-10+ B cells as compared with those in
patients with a disease relapse. 13 patients with no
treatment/NSAID (under dashed line) exhibited a low frequency of
IL-10+ B cells (≤1%). (E) In 13 patients with high
disease status who received no treatment/NSAID, the number of
IL-10+ B cells was decreased as compared with that in
another 38 patients with RA who received no treatment/NSAID
(P<0.0001) and 24 patients with a disease relapse (P<0.0001).
Horizontal lines represent the median, while highest and lowest
data points represent the range. *P<0.001. (F)
Representative frequencies of IL-10-producing cells. The frequency
of IL-10-producing cells in total CD19+ B cells was
4.11%. Using the indicated gates, IL-10 was found to be
predominantly produced by the CD24hiCD38hi
B-cell sub-population of CD19+ cells. RA, rheumatoid
arthritis; NSAID, non-steroidal anti-inflammatory drugs; IL,
interleukin; SF, synovial fluid; PB, peripheral blood; SSC, side
scatter. |
 | Table IIDemographic and clinical
characteristics of patients with RA with active disease. |
Table II
Demographic and clinical
characteristics of patients with RA with active disease.
| Variable | RA with no
treatment/NSAID
| RA with flare | Significance |
|---|
| IL-10+B
cells % ≤1% | IL-10+B
cells % >1% |
|---|
| Number (F/M) | 13 (12/1) | 38 (32/6) | 24 (20/4) | |
| Age (years) | 54 (18–64) | 43 (21–75) | 54 (18–70) | Ns |
| Symptom
durationa (years) | 15 (6–40) | 2 (0.16–10)b | 0.56
(0.08–4)c | P<0.001b,c |
| Tender joints | 23 (12–28) | 11.5 (4–28)b | 10 (3–28)c | P<0.001b,c |
| Swollen joints | 13 (4–22) | 4 (0–24)b | 3 (1–21)c | P<0.001b,c |
| VAS global
(cm) | 9 (6–10) | 5 (1–10) | 5 (2–10) | P<0.001b,c |
| CRP (mg/dl) | 3.8 (1.2–10.8) | 2.6 (0.8–19.5) | 3.6
(0.25–16.9) | Ns |
| ESR (mm/h) | 81 (35–140) | 61 (19–110) | 63 (15–108) | Ns |
| DAS28-CRP | 7.36
(5.3–8.16) | 5.50
(3.05–8.05)b | 5.24
(3.20–8.45)c | P<0.001b,c |
| RF (IU/ml) | 373 (0–7,630) | 278 (0–3,680) | 220 (0–3,190) | Ns |
| Anti-CCP2
(RU/ml) | 140 (0–1,291) | 218.5
(0–1,750) | 193.5
(30–1,800) | Ns |
| Anti-MCV
(RU/ml) | 106 (0–1,300) | 117 (0–1,340) | 87.5 (0–1,200) | Ns |
| IgG (mg/dl) | 1480
(719–3,040) | 500
(737–2,460) | 1535
(925–1,980) | Ns |
| IgM (mg/dl) | 143 (69–275) | 180 (75.6–343) | 192 (75–262) | Ns |
| IgA (mg/dl) | 140 (94–291) | 127.5 (75–535) | 123 (57–394) | Ns |
IL-10+ B cells were isolated by flow
cytometry for further functional assays, in which the phenotype of
the IL-10+ B cells was identified. As Fig. 1F demonstrates, the
CD19+CD24hiCD38hi cells
predominantly produced IL-10 following the treatment described
above. Thus, regulatory B10 cells predominantly represent a small
sub-set of cells within the
CD19+CD24hiCD38hi B-cell
sub-population, which was consistent with the findings of previous
studies (26,27).
B10 cell frequency is negatively
correlated with symptom duration, but not with pro-inflammatory
cytokines in patients with active RA
The correlation of clinical characteristics of
patients with active RA with the frequency of IL-10+ B
cells was further analyzed in order to investigate the effect of
clinical characteristics on the frequency of IL-10-producing B
cells. As shown in Table III,
IL-10+ B cells exhibited a negative association with the
symptom duration (r=−0.44, P<0.0001) and the number of swollen
joints (r=−0.24, P=0.036), while no association with other clinical
characteristics was identified.
 | Table IIICorrelation of clinical
characteristics of patients with active rheumatoid arthritis as
well as correlation of serum cytokines with the frequency of
IL-10+ B cells. |
Table III
Correlation of clinical
characteristics of patients with active rheumatoid arthritis as
well as correlation of serum cytokines with the frequency of
IL-10+ B cells.
| Variable | Spearman | P-value
(two-tailed) |
|---|
| Clinical
characteristic | | |
| Age | 0.072 | 0.54 |
| Symptom
duration | −0.44 | <0.0001a |
| Tender joints | −0.19 | 0.09 |
| Swollen
joints | −0.24 | 0.036a |
| VAS global | −0.14 | 0.22 |
| CRP | 0.08 | 0.47 |
| ESR | −0.18 | 0.12 |
| DAS28-CRP | −0.15 | 0.31 |
| RF | −0.09 | 0.42 |
| Anti-CCP2 | 0.07 | 0.57 |
| Anti-MCV | 0.18 | 0.13 |
| IgG | 0.13 | 0.28 |
| IgM | −0.10 | 0.39 |
| IgA | −0.11 | 0.34 |
| Cytokine | | |
| IL-1β | −0.28 | 0.35 |
| IL-6 | −0.36 | 0.23 |
| IL-21 | −0.33 | 0.28 |
| IL-8 | −0.18 | 0.55 |
| IL-17A | −0.35 | 0.26 |
| IFN-γ | −0.20 | 0.51 |
Pro-inflammatory mediators, including IL-1β, IL-6,
IL-21, IL-8, IL-17A, and IFN-γ, are involved in the pathology of RA
(4,31). The association of these cytokines
with the regulatory B10 cells was further assessed in the present
study. Although IL-1β, IL-6, IL-21, IL-8, IL-17A and IFN-γ levels
were significantly increased in the serum of patients with RA with
active disease as compared with those in the healthy controls,
neither of these cytokines demonstrated any correlation with the
B10 cells (Table III).
Function of B10 cell heterogeneity in
patients with RA
CD19+CD24hiCD38hi
cells were isolated from five patients with RA with active disease
(the frequency of IL-10+ B cells was ≤1% in two patients
and 1% in three patients) and three healthy controls (Table I). Following 48-h treatment with
LPS plus CD40 L, the
CD19+CD24hiCD38hi cells isolated
from patients in whom the IL-10+ B-cell frequency was
≤1% secreted significantly lower levels of IL-10 compared with
those from the healthy controls (6.24±2.31 vs. 13.64±3.77;
P<0.001) and patients with >1% IL-10+ B cells
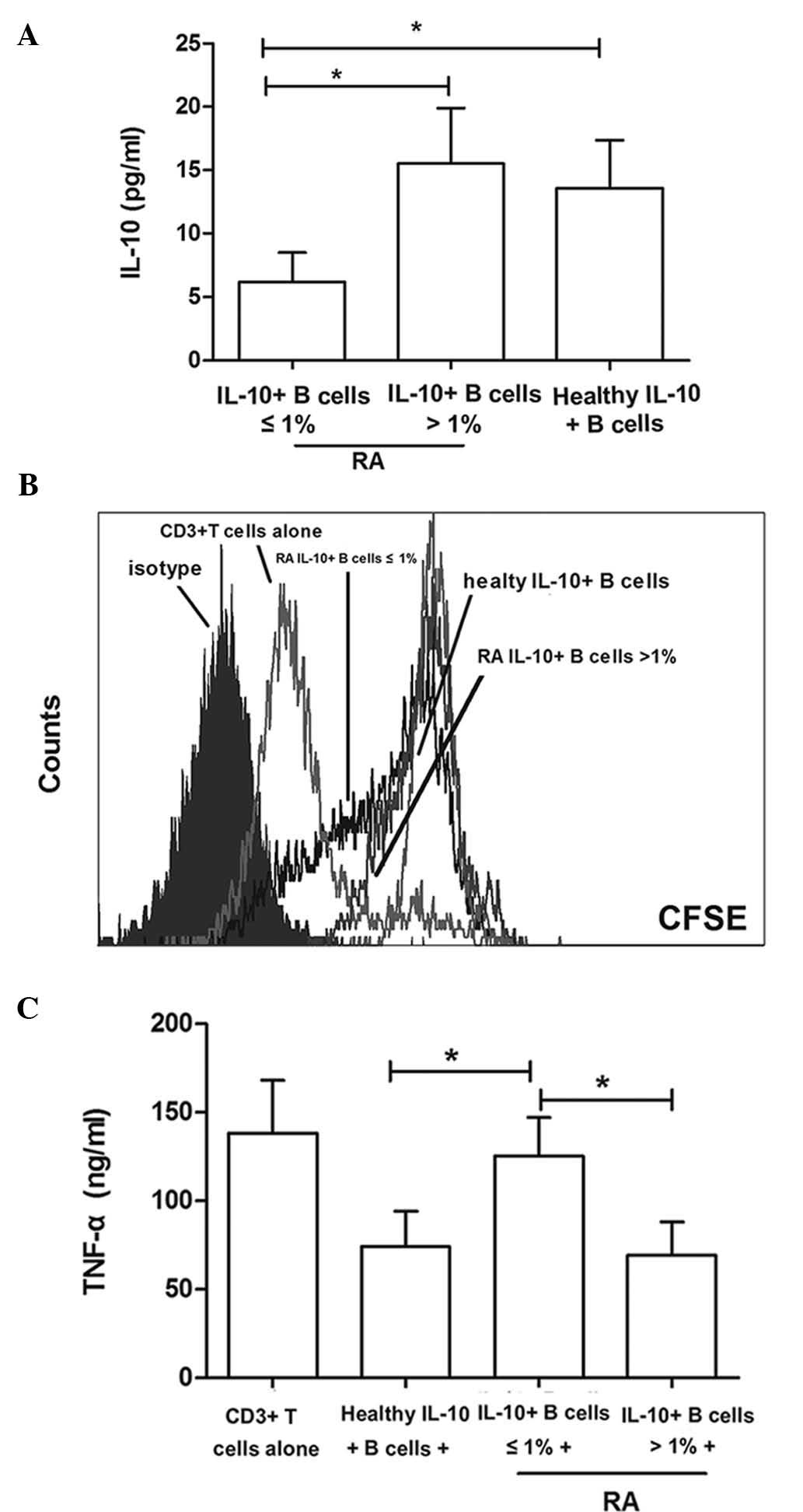
(6.24±2.31 vs. 15.57±4.40; P<0.001) (Fig. 2A).
CFSE labeling of the respective cells was used to
assess the effect of B10 cells on the proliferation of
CD3+ T cells. This dye was retained in the cytoplasm and
was diluted with each cell division. As shown in Fig. 2B, the proliferation of
CD3+ T cells was significantly inhibited in the presence
of CD19+CD24hiCD38hi cells
isolated from healthy controls and patients with >1%
IL-10+ B cells as compared with CD3+ T cells
stimulated with CD3 mAb only. By contrast, the growth inhibitory
function of CD19+CD24hiCD38hi
cells from patients with ≤1% IL-10+B cells was observed
to be decreased (Fig. 2B).
Furthermore, the effect of
CD19+CD24hiCD38hi cells on the
TNF-α production by CD3+ T cells was investigated.
CD19+CD24hiCD38hi cells isolated
from patients with ≤1% IL-10+ B cells did not exert any
inhibitory effects on the TNF-α production by CD3+ T
cells in comparison to CD3+ T cells stimulated by CD3
mAb (125.37±22.14 vs. 138.19±30.74; P= 0.437). However,
CD19+CD24hiCD38hi isolated from
healthy controls (125.37±22.14 vs. 74.33±20.08; P=0.015) and
patients with >1% IL-10+ B cells (125.37±22.14 vs.
69.50±19.31; P=0.012) exerted significant inhibitory effects on the
TNF-α production by CD3+ T cells compared with those of
patients with ≤1% B10 cells, respectively (Fig. 2C).
Discussion
In the present study, the heterogeneity in the
frequency of IL-10-competent CD19+ B cells in patients
with RA with different disease status was demonstrated, which was
in parallel with their inhibitory effect on CD3+ T
cells. The median frequency of B10 cells was significantly higher
in RA patients with an active disease as compared with that in the
healthy controls and patients with RA in remission.
Recent studies on B10 cells in animal models
demonstrated that IL-10-competent B-cells exerted profoundly
negative regulatory functions on innate and adaptive immunity, and
revealed their therapeutic potential in the treatment of autoimmune
diseases (15). However, studies
of IL-10-competent B-cells in humans yielded diverse results, which
were difficult to unify into a coherent model. In 2009, Blair et
al (26) identified a specific
sub-set of human Breg cells with a phenotype of
CD19+CD24hiCD38hi, which were
previously associated with immature transitional B-cells, but were
able to produce IL-10 in response to stimulation in vitro.
In this study, CD19+CD24hiCD38hi
cells from patients with SLE secreted low levels of IL-10 and
exerted defective suppressive effects on CD4+ T cells as
opposed to those from healthy controls. Sequentially, a deficit of
this B10-cell suppressive ability was also found in patients with
immune thrombocytopenia (28). A
recent study revealed that patients with anti-neutrophil
cytoplasmic antibody (ANCA)-associated vasculitis had diminished
levels of IL-10-producing B-cells (32). All of these results indicated that
B10-cell dysfunction may be implicated in a number of diseases in
humans.
RA is an auto-inflammatory disease that is
characterized by complex pathogenic inflammation that differs from
SLE and ANCA-associated vasculitis. Although several studies
(12,17,21)
on CIA demonstrated the negative regulatory effects and therapeutic
potential of B10 cells in RA treatment, the characteristics of B10
cells in patients with RA have not been well-investigated. In 2011,
Iwata et al (25) reported
that IL-10-producing B-cells increased in the PB of patients with
RA. However, the authors did not correlate the characteristics of
B10 cells in patients with RA with different disease stages as only
19 patients were recruited in the study. It was therefore critical
to further assess the characteristics of B10 cells in patients with
RA prior to their use in the clinical practice. Therefore, the
present study recruited a total of 97 patients with RA with a range
of disease statuses. The results revealed that the frequency of B10
cells significantly increased in the patients with RA with an
active disease status compared with that of patients with RA in
remission or in healthy controls. This suggested that the
inflammatory factors increased the frequency of B10. This was
confirmed by the observed higher frequency of B10 cells in the SF
as compared with that in the PB of the patients with RA. The
frequency of B10 cells at low, moderate and high disease severity
status revealed no significant differences, which suggested that
the disease status did not affect the frequency of B10 cells. B10
cells from patients with RA with an active disease status were also
investigated and it was found that the frequency of B10 cells
exhibited no difference between an untreated sub-group and a
sub-group undergoing a disease relapse. This indicated that the
frequency of B10 cells was not affected by previous therapy. It is
important to note that a small group of patients with active RA
with a high disease status that received no treatment were found to
exhibit a significantly decreased frequency of IL-10+ B
cells (≤1%). When compared with patients with >1%
IL-10+ B cells in the untreated group and the patients
with a disease relapse, the individuals in the ≤1%
IL-10+ B-cell sub-group exhibited a significantly longer
symptom duration, a greater number of tender and swollen joints and
a higher global VAS and DAS28-CRP score for the demographic and
clinical characteristics. In order to further investigate the
factors that affected the frequency of B10 cells in the patients
with RA, the correlation of the B10-cell frequency in the blood
with the clinical characteristics was assessed. It was identified
that the B-cell frequency exhibited a negative correlation with
symptom duration and the number of swollen joints, which suggested
that disease duration and inflammatory factors may be critical in
decreasing the B10-cell frequency. As the pro-inflammatory
cytokines, including IL-1β, IL-6, IL-21, IL-8, IL-17A, and IFN-γ,
are considered to have an important pathogenic role in RA and were
produced at increased levels in the patients with RA with an active
disease status compared with those in the other groups, a
correlation analysis of these cytokines with the B10-cell frequency
was also performed. However, no significant correlation was
identified between these inflammatory factors and B10-cell
frequency. This indicated that multiple factors in the regulation
of B10-cell production and other unknown inflammatory cytokines may
have affected the B10-cell frequency in patients with RA.
Subsequently, the regulatory ability of B10 cells on
the proliferation of CD3+ T cells was assessed. It was
identified that CD19+CD24hiCD38hi
cells predominantly produced IL-10, which was consistent with
previous studies (25–27). Therefore, they have been termed
'B10 cells' in recent years (26).
Similarly, based on the observations regarding B10-cell frequency
detection, the B10 cells from patients with RA with different
disease status revealed heterogenic functions. The B10 cells
isolated from the RA sub-group containing ≤1% IL-10+ B
cells secreted significantly decreased levels of IL-10 and exerted
a significantly diminished suppressive effect on CD3+
T-cell proliferation and TNF-α production compared with those of
healthy controls and the RA sub-group containing
>1%IL-10+ B10 cells. These functional studies on B10
cells further demonstrated the complexity of the mechanisms
underlying B10-cell generation and function in patients with
RA.
In conclusion, the present study demonstrated that
the frequency and function of B10 cells displayed heterogeneity in
patients with RA with a different disease status as well as healthy
controls. Adoptive transfer of B10 cells is considered to be a
potential therapeutic strategy for RA based on the results of
previous studies on animals; however, further study is required
prior to the use of B10 cells in clinical practice.
Acknowledgments
The present study was funded by a grant from the
National Natural Science Foundation of China (grant no. 81001335).
The authors would like to thank Mr. Yanhong Wang and Miss. Chunmei
Fan of the Department of Clinical Immunology, State Key Discipline
of Cell Biology, Xijing Hospital, Fourth Military Medical
University (Xian, China) for their excellent technical
assistance.
References
|
1
|
Drossaers-Bakker KW, de Buck M, van Zeben
D, Zwinderman AH, Breedveld FC and Hazes JM: Long-term course and
outcome of functional capacity in rheumatoid arthritis: The effect
of disease activity and radiologic damage over time. Arthritis
Rheum. 42:1854–1860. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davignon JL, Hayder M, Baron M, Boyer JF,
Constantin A, Apparailly F, Poupot R and Cantagrel A: Targeting
monocytes/macrophages in the treatment of rheumatoid arthritis.
Rheumatology (Oxford). 52:590–598. 2013. View Article : Google Scholar
|
|
3
|
Gol-Ara M, Jadidi-Niaragh F, Sadria R,
Azizi G and Mirshafiey A: The role of different subsets of
regulatory T cells in immunopathogenesis of rheumatoid arthritis.
Arthritis. 2012:8058752012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brennan FM and McInnes IB: Evidence that
cytokines play a role in rheumatoid arthritis. J Clin Invest.
118:3537–3545. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bugatti S, Codullo V, Caporali R and
Montecucco C: B cells in rheumatoid arthritis. Autoimmun Rev.
7:137–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leandro MJ, Cambridge G, Ehrenstein MR and
Edwards JC: Reconstitution of peripheral blood B cells after
depletion with rituximab in patients with rheumatoid arthritis.
Arthritis Rheum. 54:613–620. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mariette X, Rouanet S, Sibilia J, Combe B,
Le Loët X, Tebib J, Jourdan R and Dougados M: Evaluation of
low-dose rituximab for the retreatment of patients with active
rheumatoid arthritis: A non-inferiority randomised controlled
trial. Ann Rheum Dis. 73:1508–1514. 2014. View Article : Google Scholar
|
|
8
|
Reddy V, Croca S, Gerona D, De La Torre I,
Isenberg D, McDonald V, Leandro M and Cambridge G: Serum rituximab
levels and efficiency of B cell depletion: Differences between
patients with rheumatoid arthritis and systemic lupus
erythematosus. Rheumatology (Oxford). 52:951–952. 2013. View Article : Google Scholar
|
|
9
|
Bredemeier M, de Oliveira FK and Rocha CM:
Low-versus high-dose rituximab for rheumatoid arthritis: A
systematic review and meta-analysis. Arthritis Care Res (Hoboken).
66:228–235. 2014. View Article : Google Scholar
|
|
10
|
Goetz M, Atreya R, Ghalibafian M, Galle PR
and Neurath MF: Exacerbation of ulcerative colitis after rituximab
salvage therapy. Inflamm Bowel Dis. 13:1365–1368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolf SD, Dittel BN, Hardardottir F and
Janeway CA Jr: Experimental autoimmune encephalomyelitis induction
in genetically B cell-deficient mice. J Exp Med. 184:2271–2278.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mauri C, Gray D, Mushtaq N and Londei M:
Prevention of arthritis by interleukin 10-producing B cells. J Exp
Med. 197:489–501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Evans JG, Chavez-Rueda KA, Eddaoudi A,
Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR and Mauri C: Novel
suppressive function of transitional 2 B cells in experimental
arthritis. J Immunol. 178:7868–7878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Neta R and Salvin SB: Specific suppression
of delayed hypersensitivity: The possible presence of a suppressor
B cell in the regulation of delayed hypersensitivity. J Immunol.
113:1716–1725. 1974.PubMed/NCBI
|
|
15
|
Yang M, Rui K, Wang S and Lu L: Regulatory
B cells in autoimmune diseases. Cell Mol Immunol. 10:122–132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mauri C and Bosma A: Immune regulatory
function of B cells. Annu Rev Immunol. 30:221–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang M, Deng J, Liu Y, Ko KH, Wang X, Jiao
Z, Wang S, Hua Z, Sun L, Srivastava G, et al: IL-10-producing
regulatory B10 cells ameliorate collagen-induced arthritis via
suppressing Th17 cell generation. Am J Pathol. 180:2375–2385. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Braun J and Wei B: Regulatory B
cells in autoimmune diseases and mucosal immune homeostasis.
Autoimmunity. 44:58–68. 2011. View Article : Google Scholar
|
|
19
|
Matsushita T, Yanaba K, Bouaziz JD,
Fujimoto M and Tedder TF: Regulatory B cells inhibit EAE initiation
in mice while other B cells promote disease progression. J Clin
Invest. 118:3420–3430. 2008.PubMed/NCBI
|
|
20
|
Gray M, Miles K, Salter D, Gray D and
Savill J: Apoptotic cells protect mice from autoimmune inflammation
by the induction of regulatory B cells. Proc Natl Acad Sci USA.
104:14080–14085. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lemoine S, Morva A, Youinou P and Jamin C:
Regulatory B cells in autoimmune diseases: how do they work? Ann N
Y Acad Sci. 1173:260–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fillatreau S, Sweenie CH, McGeachy MJ,
Gray D and Anderton SM: B cells regulate autoimmunity by provision
of IL-10. Nat Immunol. 3:944–950. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yanaba K, Bouaziz JD, Haas KM, Poe JC,
Fujimoto M and Tedder TF: A regulatory B cell subset with a unique
CD1dhiCD5+ phenotype controls T cell-dependent inflammatory
responses. Immunity. 28:639–650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zha B, Wang L, Liu X, Liu J, Chen Z, Xu J,
Sheng L, Li Y and Chu Y: Decrease in proportion of CD19+CD24(hi)
CD27+ B cells and impairment of their suppressive function in
Graves' disease. PLoS One. 7:e498352012. View Article : Google Scholar
|
|
25
|
Iwata Y, Matsushita T, Horikawa M, Dilillo
DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM,
Williams AD, et al: Characterization of a rare IL-10-competent
B-cell subset in humans that parallels mouse regulatory B10 cells.
Blood. 117:530–541. 2011. View Article : Google Scholar :
|
|
26
|
Blair PA, Noreña LY, Flores-Borja F,
Rawlings DJ, Isenberg DA, Ehrenstein MR and Mauri C:
CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in
healthy individuals but are functionally impaired in systemic Lupus
Erythematosus patients. Immunity. 32:129–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Newell KA, Asare A, Kirk AD, Gisler TD,
Bourcier K, Suthanthiran M, Burlingham WJ, Marks WH, Sanz I,
Lechler RI, et al: Identification of a B cell signature associated
with renal transplant tolerance in humans. J Clin Invest.
120:1836–1847. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Zhong H, Bao W, Boulad N,
Evangelista J, Haider MA, Bussel J and Yazdanbakhsh K: Defective
regulatory B-cell compartment in patients with immune
thrombocytopenia. Blood. 120:3318–3325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arnett FC1, Edworthy SM, Bloch DA, McShane
DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS,
et al: The American Rheumatism Association 1987 revised criteria
for the classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wells GA, Tugwell P, Kraag GR, Baker PR,
Groh J and Redelmeier DA: Minimum important difference between
patients with rheumatoid arthritis: the patient's perspective. J
Rheumatol. 20:557–560. 1993.PubMed/NCBI
|
|
31
|
Hughes-Austin JM, Deane KD, Derber LA,
Kolfenbach JR, Zerbe GO, Sokolove J, Lahey LJ, Weisman MH, Buckner
JH, Mikuls TR, et al: Multiple cytokines and chemokines are
associated with rheumatoid arthritis-related autoimmunity in
first-degree relatives without rheumatoid arthritis: Studies of the
Aetiology of Rheumatoid Arthritis (SERA). Ann Rheum Dis.
72:901–907. 2013. View Article : Google Scholar :
|
|
32
|
Wilde B, Thewissen M, Damoiseaux J,
Knippenberg S, Hilhorst M, van Paassen P, Witzke O and Cohen
Tervaert JW: Regulatory B cells in ANCA-associated vasculitis. Ann
Rheum Dis. 72:1416–1419. 2013. View Article : Google Scholar : PubMed/NCBI
|