Introduction
Bladder cancer (BC) is a common malignancy of the
urinary tract (1,2). Bone metastasis occurs in ~40% of
patients with BC (3,4). Zoledronic acid (ZA) is effective in
patients with bone metastases from bladder cancer (5–7). In
addition to its bone-protective effects, ZA can prevent tumor
progression (8). Due to its
inhibition of the prenylation of small GTP-binding proteins, such
as Ras, ZA inactivates Ras signaling and inhibits Ras-dependent
cell proliferation (9–11).
P73 was identified to be a member of the p53 family
and is frequently overexpressed in human tumors (12–14).
Although p53 is firmly established to be a tumor suppressor, the
role of p73 in human tumorigenesis is not well understood (15,16).
The p73 gene contains two promoters and thus encodes the
transcriptional domain-containing (TAp73) and the amino deleted
(ΔNp73) isoforms (17). TAp73
isoforms contain an amino-terminal transactivation domain and thus
can activate the promoters of p53-target genes and induce apoptosis
(18,19). ΔNp73 isoforms, which lack the
transactivation domain of TAp73 protein and retain the DNA-binding
and oligomerization domains, act as dominant-negative inhibitors
for p53 family members by forming inactive hetero-oligomers or
competing for p53-DNA-binding (20).
During rapid cell growth, adequate levels of
intracellular nicotinamide adenine dinucleotide phosphate (NADPH),
generated predominantly through the pentose phosphate pathway
(PPP), are critical for cell survival. NADPH is required for DNA,
protein and lipid biosynthesis, and is also required to generate
sufficient material to support cancer cell proliferation (21–23).
The tumor suppressor p53 was reported to bind to
glucose-6-phosphate dehydrogenase (G6PD), the rate-limiting enzyme
of the PPP, and inhibit its activity. Thus, p53 can suppress the
glucose consumption and NADPH production of cells (24). Conversely, TAp73 was reported to
activate the expression of G6PD and thus promote the PPP flux and
NADPH production (25). The
present study investigated the effects of ZA on the PPP flux and
the proliferation of tumor cells.
Materials and methods
Reagents
ZA and cycloheximide (CHX) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The mouse monoclonal anti-human
Ras (05-1072; 1:1,000 dilution) and rabbit monoclonal anti-human
phospho-extracellular signal-regulated kinase (ERK) 1/2
(Thr202/Tyr204 and Thr185/Tyr187; 05-797R; 1:1,000 dilution) were
obtained from Millipore (Bedford, MA, USA). The rabbit polyclonal
anti-human G6PD (8866; 1:1,000 dilution) and β-actin (4967; 1:3,000
dilution) antibodies were obtained from Cell Signaling Technology
Inc. (Beverly, MA, USA). Rabbit polyclonal anti-human p73
(ab137797; 1/800 dilution) was from Abcam (Cambridge, MA, USA). FPT
inhibitor II was purchased from Santa Cruz Biotechnology Inc.
(Dallas, TX, USA). The real-time PCR Master Mix kit (SYBR green PCR
master mix) was purchased from Takara Bio Inc. (Dalian, China). To
evaluate Ras activity [Ras-guanosine-5′-triphosphate (GTP) levels],
affinity precipitation of active Ras was performed using a Ras
activation assay according to the manufacturer's instructions
(Millipore).
Cell culture
T24 human bladder cancer cells and 293T cells were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). Cells were cultured in RPMI-1640
medium (Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum, penicillin (100 U/ml) and
streptomycin (100 µg/ml) (all from Invitrogen Life
Technologies) in a humidified atmosphere of 5% CO2 at
37°C.
mRNA extract and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
To examine the expression of G6PD and TAp73 mRNA in
T24 cells, RT-qPCR was performed. Total RNA was extracted from the
cell lines using the RNAiso reagent (Takara, Otsu, Japan). The
first-strand cDNA was generated using TransScript First-Strand cDNA
Synthesis SuperMix kit (Transgen, Beijing, China). Amplification
and data acquisition were run on a real-time PCR system (ABI Prism
7500; Applied Biosystems, Foster City, CA, USA) for SYBR green PCR
master mix. The quantity of the cDNA sample was 1 µl. RT-PCR
was performed by 3 min incubation at 95°C and 40 amplification
cycles (95°C for 10 sec; 56°C for 15 sec; and 72°C for 35 sec).
β-actin served as a control. The primer sequences used were as
follows: Forward: 5′-GTA CCA CTG GCA TCG TGA TGG ACT-3′ and reverse
5′-CCG CTC ATT GCC AAT GGT GAT-3′ for β-actin; forward: 5′-TGC CCC
CGA CCG TCT AC-3′ and reverse: 5′-ATG CGG TTC CAG CCT ATC TG-3′ for
G6PD; and forward: 5′-GCA CCT ACT TTG ACC TCC CC-3′ and reverse:
5′-GCA CTG CTG AGC AAA TTG AAC-3′ for TAp73.
Metabolism assays
Glucose consumption was measured in the cell lysates
with Glucose Uptake Colorimetric Assay kit from (BioVision, San
Francisco, CA, USA). The production of NADPH was measured in the
cell lysates with NAD+/NADH Quantification Colorimetric
kit (BioVision, San Francisco, CA, USA). Following the
manufacturer's instructions for these measures.
Vector and cell transfection
pCMV6-TAp73 (NM_005427) was obtained from OriGene
(Rockville, MD, USA). For the transfection of the T24 cells, pCMV6
empty vector (OriGene, Rockville, MD, USA) and pCMV6-TAp73 vector
were trans-fected into cells using Lipofectamine™ 2000 (Invitrogen
Life Technologies). After 6 h, the medium was refreshed and
cultured for 48 h. Then the cells were treated with ZA (200
µM).
Viral production and infection
Expression plasmids for small hairpin (sh)RNAs of
TAp73 were made in pLKO.1-puro plasmids (Sigma-Aldrich). The
targeted sequences were: 5′-GGA TTC CAG CAT GGA CGT CTT-3′ (sh1)
and 5′-CCA AGG GTT ACA GAG CAT TTA-3′ (sh2). A negative control
vector containing scrambled shRNA was also obtained from
Sigma-Aldrich. The plasmids were prepared with a plasmid maxi kit
and transfected in 293T cells (Invitrogen Life Technologies) with
the Lipofectamine™ 2000 to produce lentiviral particles. Then, the
T24 cells were infected with the lentiviral particles for 24 h.
Cells were then selected with 2 mg/ml puromycin (Sigma-Aldrich) for
72 h.
Western blot analysis
Cells were lysed in a radioim-munoprecipitation
assay buffer containing and complete Protease/Phosphatase Inhibitor
Cocktail (Cell Signaling Technology, Inc.). The lysate was
centrifuged at 11,500 × g for 15 min at 4°C and the supernatant was
collected. Protein concentration was determined using a Bradford
protein assay kit (Bio-Rad, Hercules, CA, USA). The proteins were
separated by 10% SDS-PAGE gel (RSBM, Taiyuan, China) and then
transferred onto a polyvinylidene difluoride membrane (Millipore).
The membranes were blocked with 10% milk in Tris-buffered saline
with Tween-20 (TBST) for 1 h and then were incubated with
antibodies overnight at 4°C. After washing with TBST three times,
the membranes were probed with horseradish peroxidase-conjugated
secondary antibodies in TBST for 1 h at room temperature, then
washed with PBST three times. The immunobinding signals were
detected by a chemiluminescence kit (Millipore).
Cell proliferation analysis
Cell proliferation was examined by using a Cell
Counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan). Cells were plated
and treated with ZA (200 µM) in 96-well plates at 2,000
cells per well and cultured in growth medium for 20 h. After 20 h,
CCK-8 (10 µl) was added to each well containing 100
µl RPMI-1640 medium. Then the plate was incubated for 2 h at
37°C. Absorbance was measured at 450 nm using a microplate reader
(SpectraMax M5 Microplate Reader; BioTek, Winooski, VT, USA).
Statistical analysis
Data were analyzed using Student's t-test. Graphpad
6.01 Prism software (GraphPad, Inc., La Jolla, CA, USA) was used
for statistical analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
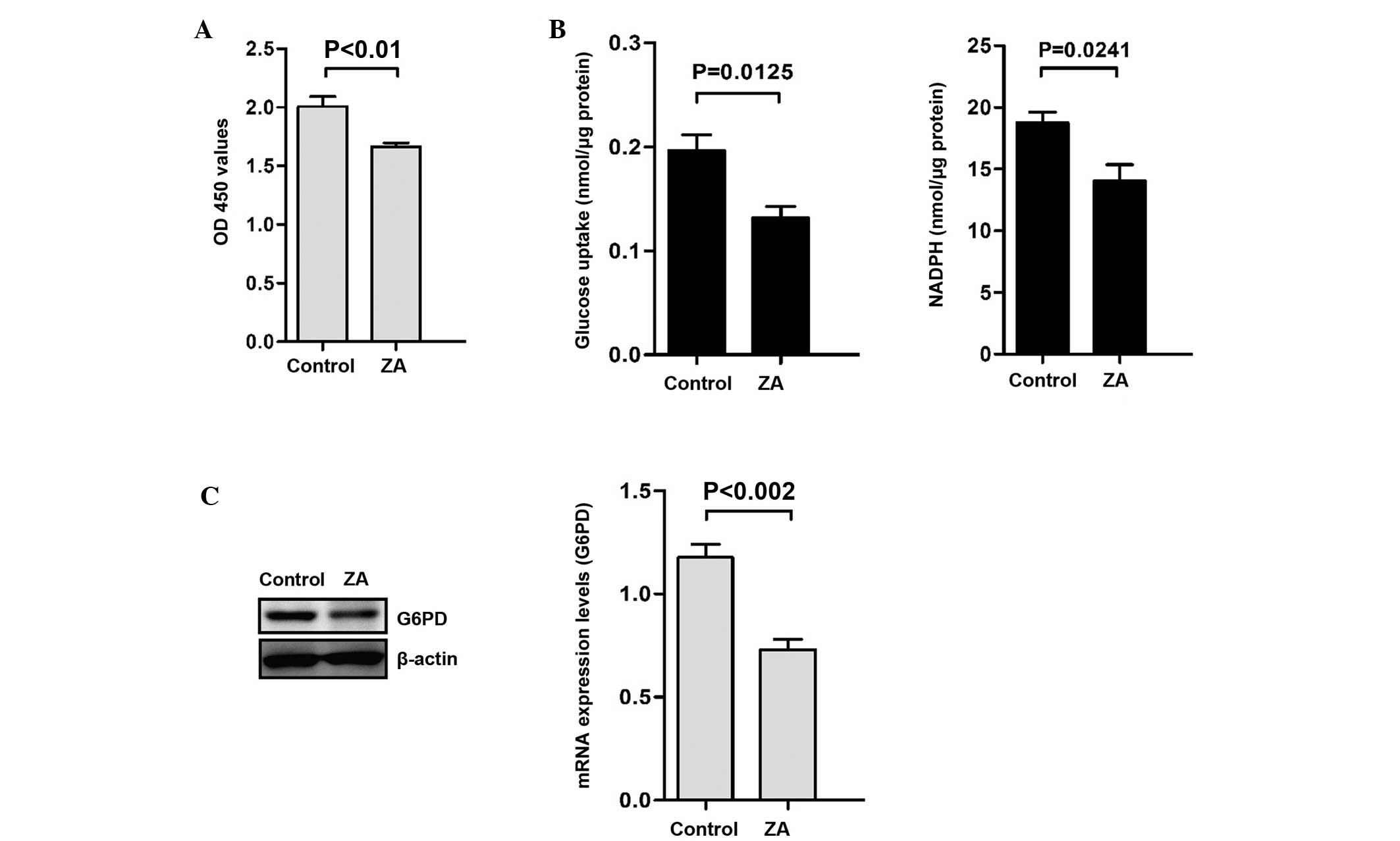
ZA inhibits the proliferation of bladder
cancer cells and attenuates the PPP
To confirm the effects of ZA on the proliferation of
bladder cancer cells, T24 human bladder cancer cell lines were
treated with ZA (200 µM) for 20 h. As shown in Fig. 1A, ZA significantly reduced the
proliferative activity of T24 cells. The PPP and NADPH produced in
the PPP are required for rapid cancer cell growth. Thus, it was
examined whether ZA affects the PPP flux in bladder cancer cell
lines. It was demonstrated that glucose consumption and NADPH were
inhibited by treatment with ZA in T24 cells (Fig. 1B). Furthermore, G6PD, the
rate-limiting enzyme of the PPP, was found to be downregulated at
the mRNA and protein level (Fig.
1C).
ZA decreases the stability of TAP73
TAp73 was reported to activate the expression of
G6PD and thus promote the PPP flux and NADPH production (25). As shown in Fig. 2A, it was demonstrated that the
levels of TAp73 decreased following treatment with ZA in T24 cells
(Fig. 2A). The expression of TAp73
mRNA was then examined by RT-qPCR. The data show that there were no
significant changes in TAp73 mRNA levels following treatment with
ZA (Fig. 2B). It was hypothesized
that the regulation of TAp73 levels by ZA may be the result of
protein stability regulation. The TAp73 stability in T24 cells was
then determined by treatment with CHX. As shown in Fig. 2C, following pre-treatment with ZA,
the stability of TAp73 decreased.
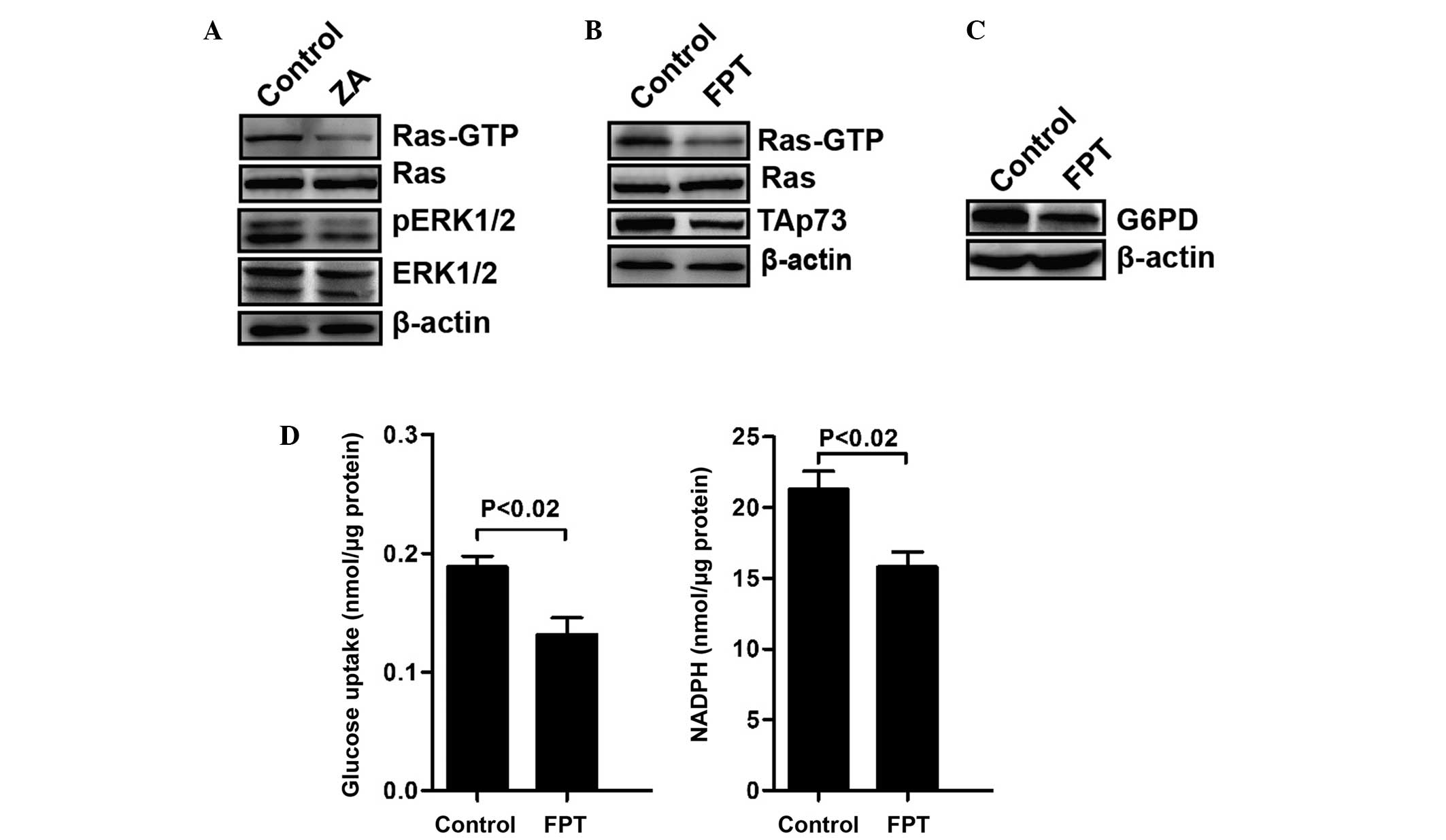
Stability of TAp73 regulated by ZA may
depend on the inhibition of the activity of Ras
Activated Ras was reported to stabilize TAp73
inducing its accumulation (26).
The activity of Ras in T24 cells treated with ZA was then examined.
GTP-bound Ras (Ras-GTP), a marker of Ras activation, as well as the
quantity of ERK1/2 phosphorylation were decreased in T24 cells
following treatment with ZA for 20 h (Fig. 3A). FPT inhibitor II, exhibited
inhibitory effects of Ras farnesylation and activity, was
substituted for ZA to treat T24 cells. As shown in Fig. 3B, FPT inhibitor II significantly
reduced the levels of Ras-GTP and TAp73. In addition, FPT inhibitor
II also inhibited the expression of G6PD (Fig. 3C), glucose consumption and NADPH
production (Fig. 3D). These
results support the role of ZA in the regulation of the stability
of TAp73 via inhibiting the activity of Ras.
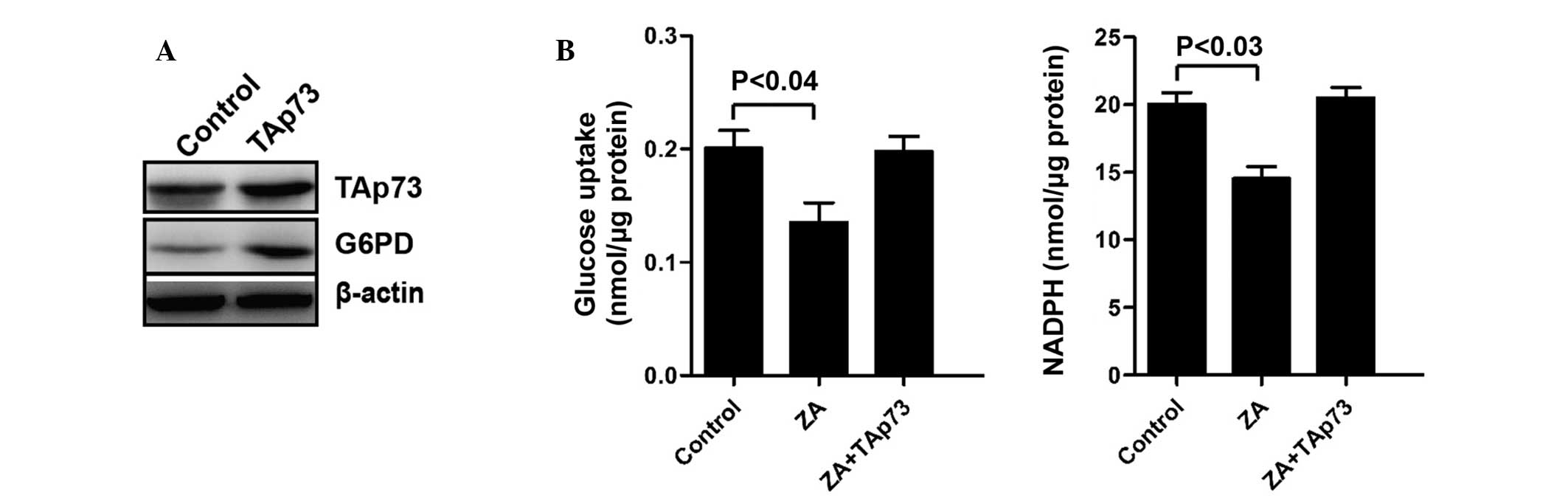
ZA regulates PPP through the regulation
of TAp73
In order to further investigate the function of ZA
in the regulation of the PPP. Following transfection with TAp73 for
48 h (Fig. 4A), the inhibitory
effect on the PPP by ZA, as well as the glucose consumption and
NADPH production was attenuated by TAp73 overexpression (Fig. 4B). Furthermore, the expression of
G6PD was also upregulated (Fig.
4A). These results further support that ZA inhibits the
expression of G6PD and then attenuates the PPP through the
downregulation of TAp73.
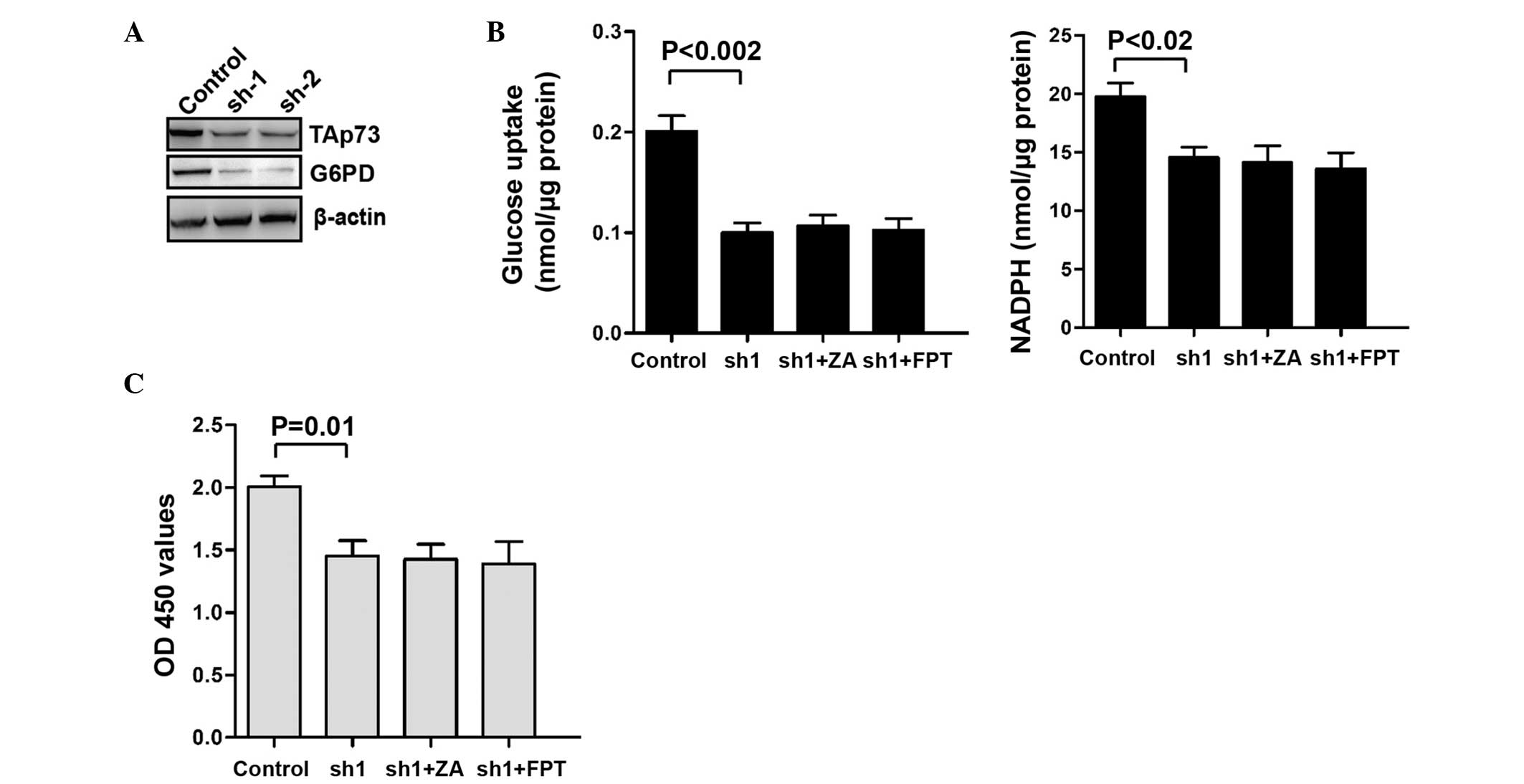
Knockdown of TAp73 inhibits the PPP of
T24 cells independent of Ras activity or ZA treatment
shRNA-mediated TAp73 knockdown was then performed in
T24 cells and the PPP flux was examined. It was demonstrated that
the expression of G6PD (Fig. 5A)
and the PPP flux (Fig. 5B) were
inhibited following knockdown of TAp73 in T24 cells. Moreover, FPT
inhibitor II and ZA did not affect the PPP flux after the knockdown
of TAp73 (Fig. 5B). Similarly, the
proliferation of T24 cells was not significantly changed by the FPT
inhibitor II or ZA after the knockdown of TAp73 (Fig. 5C). These results confirm the role
of TAp73 in the inhibition of the PPP flux by ZA.
Discussion
In the present study, it was demonstrated that ZA
inhibited the proliferation of bladder cancer cells and the PPP.
Moreover, ZA was found to decrease the stability of TAp73, which
activates the expression of G6PD (the rate-limiting enzyme of PPP).
Decreased levels of Ras-GTP and p-ERK1/2 were also found to be
associated with the treatment with ZA. Furthermore, the inhibition
of Ras activity by PT Inhibitor II significantly reduced the levels
of TAp73, G6PD, glucose consumption and NADPH production.
ZA is effective in patients with bone metastases
from bladder cancer. In addition to its bone-protective effects, ZA
can prevent tumor progression (27). ZA was reported to inhibit the
prenylation of small GTP-binding proteins, such as Ras. Thus, ZA
was hypothesized to inactivate Ras signaling and inhibit
Ras-dependent cell proliferation (9,28).
Previous studies have shown that ZA inhibited the activity of Ras
and hence inhibit the expression of hypoxia-inducible factor 1-α
(HIF1A) (29). NADPH is required
for DNA, protein and lipid biosynthesis during the rapid cell
growth of cancer cells. The PPP is the predominant source of NADPH
and it is often enhanced during cell cycle progression of cancer
cells (23,30).
In this study, the PPP flux was found to be
inhibited by ZA. In addition, the expression of G6PD, the
rate-limiting enzyme of the PPP, was also found to be inhibited by
ZA. Activated Ras was reported to stabilize TAp73 protein, ZA was
shown to decrease the levels of Ras-GTP and p-ERK1/2 with treatment
with ZA. FPT inhibitor II is the inhibitor of Ras farnesylation and
its activity. Similar to ZA, FPT inhibitor II significantly reduced
the levels of Ras-GTP, G6PD, TAp73 and the PPP flux. It was also
demonstrated that knockdown of TAp73 resulted in an inhibition of
the PPP flux. Moreover, ZA could not regulate the PPP flux in TAp73
knockdown cells. These results implied that ZA inhibits the PPP
flux and the expression of G6PD via blocking Ras signaling and
attenuating the stability of TAp73 in bladder cancer cells. The
findings of the present study revealed a novel mechanism for ZA to
regulate the PPP. Besides the bone-protective effects, ZA
restrained the PPP flux of cancer cells and inhibited their
proliferation. The mechanistic target of ZA was TAp73, and thus,
TAp73-overexpressing tumors may show enhanced sensitivity to ZA.
Due to its targeting of TAp73, ZA may potentially be utilized in
cancer therapies.
References
|
1
|
Parkin DM: The global burden of urinary
bladder cancer. Scand J Urol Nephrol. (Suppl): 12–20. 2008.
View Article : Google Scholar
|
|
2
|
Noon AP, Albertsen PC, Thomas F, Rosario
DJ and Catto JW: Competing mortality in patients diagnosed with
bladder cancer: Evidence of undertreatment in the elderly and
female patients. Br J Cancer. 108:1534–1540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zarogoulidis K, Boutsikou E, Zarogoulidis
P, Eleftheriadou E, Kontakiotis T, Lithoxopoulou H, Tzanakakis G,
Kanakis I and Karamanos NK: The impact of zoledronic acid therapy
in survival of lung cancer patients with bone metastasis. Int J
Cancer. 125:1705–1709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirata H, Hinoda Y, Ueno K, Shahryari V,
Tabatabai ZL and Dahiya R: MicroRNA-1826 targets VEGFC,
beta-catenin (CTNNB1) and MEK1 (MAP2K1) in human bladder cancer.
Carcinogenesis. 33:41–48. 2012. View Article : Google Scholar :
|
|
5
|
Alcaraz A, González-López R, Morote J, de
la Piedra C, Meseguer C, Esteban E, Climent M, González-Gragera B,
Alvarez-Ossorio JL, Chirivella I, et al: Biochemical markers of
bone turnover and clinical outcome in patients with renal cell and
bladder carcinoma with bone metastases following treatment with
zoledronic acid: The TUGAMO study. Br J Cancer. 109:121–130. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zaghloul MS, Boutrus R, El-Hossieny H,
Kader YA, El-Attar I and Nazmy M: A prospective, randomized,
placebo-controlled trial of zoledronic acid in bony metastatic
bladder cancer. Int J Clin Oncol. 15:382–389. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saad F and Eastham JA: Zoledronic acid use
in patients with bone metastases from renal cell carcinoma or
bladder cancer. Semin Oncol. 37(Suppl 1): S38–S44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gouin F, Ory B, Rédini F and Heymann D:
Zoledronic acid slows down rat primary chondrosarcoma development,
recurrent tumor progression after intralesional curretage and
increases overall survival. Int J Cancer. 119:980–984. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goffinet M, Thoulouzan M, Pradines A,
Lajoie-Mazenc I, Weinbaum C, Faye JC and Séronie-Vivien S:
Zoledronic acid treatment impairs protein geranyl-geranylation for
biological effects in prostatic cells. BMC Cancer. 6:602006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koto K, Murata H, Kimura S, Sawai Y, Horie
N, Matsui T, Ryu K, Ashihara E, Maekawa T, Kubo T, et al:
Zoledronic acid significantly enhances radiation-induced apoptosis
against human fibrosarcoma cells by inhibiting radioadaptive
signaling. Int J Oncol. 42:525–534. 2013.
|
|
11
|
Rowinsky EK, Windle JJ and Von Hoff DD:
Ras protein farne-syltransferase: A strategic target for anticancer
therapeutic development. J Clin Oncol. 17:3631–3652.
1999.PubMed/NCBI
|
|
12
|
Wei J, Zaika E and Zaika A: p53 Family:
Role of protein isoforms in human cancer. J Nucleic Acids.
2012:6873592012. View Article : Google Scholar
|
|
13
|
Stiewe T and Putzer BM: Role of p73 in
malignancy: Tumor suppressor or oncogene? Cell Death Differ.
9:237–245. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lunghi P, Costanzo A, Mazzera L, Rizzoli
V, Levrero M and Bonati A: The p53 family protein p73 provides new
insights into cancer chemosensitivity and targeting. Clin Cancer
Res. 15:6495–6502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomkova K, Belkhiri A, El-Rifai W and
Zaika AI: p73 isoforms can induce T-cell factor-dependent
transcription in gastrointestinal cells. Cancer Res. 64:6390–6393.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zaika AI and El-Rifai W: The role of p53
protein family in gastrointestinal malignancies. Cell Death Differ.
13:935–940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Conforti F, Yang AL, Agostini M, Rufini A,
Tucci P, Nicklison-Chirou MV, Grespi F, Velletri T, Knight RA,
Melino G, et al: Relative expression of TAp73 and ΔNp73 isoforms.
Aging (Albany NY). 4:202–205. 2012.
|
|
18
|
Rufini A, Agostini M, Grespi F, Tomasini
R, Sayan BS, Niklison-Chirou MV, Conforti F, Velletri T, Mastino A,
Mak TW, et al: p73 in Cancer. Genes Cancer. 2:491–502. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin D, Cui Z, Kong L, Cheng F, Xu J and
Lan F: p73 participates in WWOX-mediated apoptosis in leukemia
cells. Int J Mol Med. 31:849–854. 2013.PubMed/NCBI
|
|
20
|
Grob TJ, Novak U, Maisse C, Barcaroli D,
Lüthi AU, Pirnia F, Hügli B, Graber HU, De Laurenzi V, Fey MF, et
al: Human delta Np73 regulates a dominant negative feedback loop
for TAp73 and p53. Cell Death Differ. 8:1213–1223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Samudio I, Fiegl M and Andreeff M:
Mitochondrial uncoupling and the Warburg effect: Molecular basis
for the reprogramming of cancer cell metabolism. Cancer Res.
69:2163–2166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jones RG and Thompson CB: Tumor
suppressors and cell metabolism: A recipe for cancer growth. Genes
Dev. 23:537–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang P, Du W, Wang X, Mancuso A, Gao X,
Wu M and Yang X: p53 regulates biosynthesis through direct
inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol.
13:310–316. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du W, Jiang P, Mancuso A, Stonestrom A,
Brewer MD, Minn AJ, Mak TW, Wu M and Yang X: TAp73 enhances the
pentose phosphate pathway and supports cell proliferation. Nat Cell
Biol. 15:991–1000. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fernandez-Garcia B, Vaqué JP,
Herreros-Villanueva M, Marques-Garcia F, Castrillo F,
Fernandez-Medarde A, León J and Marín MC: p73 cooperates with Ras
in the activation of MAP kinase signaling cascade. Cell Death
Differ. 14:254–265. 2007. View Article : Google Scholar
|
|
27
|
Chang J, Wang W, Zhang H, Hu Y and Yin Z:
Bisphosphonates regulate cell proliferation, apoptosis and
pro-osteoclastic expression in MG-63 human osteosarcoma cells.
Oncol Lett. 4:299–304. 2012.PubMed/NCBI
|
|
28
|
Ohtsuka Y, Manabe A, Kawasaki H, Hasegawa
D, Zaike Y, Watanabe S, Tanizawa T, Nakahata T and Tsuji K:
RAS-blocking bisphosphonate zoledronic acid inhibits the abnormal
proliferation and differentiation of juvenile myelomonocytic
leukemia cells in vitro. Blood. 106:3134–3141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riganti C, Castella B, Kopecka J, Campia
I, Coscia M, Pescarmona G, Bosia A, Ghigo D and Massaia M:
Zoledronic acid restores doxorubicin chemosensitivity and
immunogenic cell death in multidrug-resistant human cancer cells.
PLoS One. 8:e609752013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cairns RA, Harris I, McCracken S and Mak
TW: Cancer cell metabolism. Cold Spring Harb Symp Quant Biol.
76:299–311. 2011. View Article : Google Scholar : PubMed/NCBI
|