Introduction
Breast cancer has become a leading cause of
mortality among females in China, with 1.1 million novel cases
annually (1). Decades of studies
have revealed the molecular profiling of breast cancer using
expression arrays (2–4), which may aid in identifying the
underlying pathogenic mechanisms and thus determining effective
novel therapeutic strategies for the treatment of breast
cancer.
MicroRNAs (miRNAs), a class of small endogenous RNA
molecules, modulate gene expression through negatively regulating
the stability or translational efficiency of specific mRNAs
(5,6). In addition, studies have reported the
dysregulation of several miRNAs in breast cancer, which may
contribute to tumor initiation and progression (7,8). One
study reported that the downregulation of miR-515-5p via the
estrogen receptor, promoted breast cancer cell proliferation
through modulating sphingosine kinase 1 (9), while miR-221/222 targeted adiponectin
receptor 1 in order to promote epithelial-to-mesenchymal transition
in breast cancer cells (10).
The function of miR-802, located on chromosome 21,
in cancer biology remains to be elucidated. The present study aimed
to investigate the role of miR-802 as a positive regulator of the
proliferation of breast cancer cells, as well as elucidate the
mechanism underlying its action in human cancers.
Materials and methods
Tissue samples
A total of 20 pairs of tumor tissues and adjacent
normal tissues were obtained from patients who underwent surgery at
the Department of Breast Cancer at Hubei Cancer Hospital (Wuhan,
China). Written informed consent was obtained from each patient and
the study was approved by the Department of Breast Cancer at Hubei
Cancer Hospital institutional review board, and the Ethics
Committee of Hubei Cancer Hospital.
Cell culture
Breast cancer cell lines (MCF-7, MDA-MB-453,
MDA-MB-468 and ZR-75-1) and normal breast epithelial cells
(HBL-100) were obtained from the Chinese Academy of Sciences Cell
Bank (Shanghai, China). Cells were maintained in Dulbecco's
modified Eagle's medium (Invitrogen Life Technologies, Carlsbad,
CA) supplemented with 10% fetal bovine serum (Invitrogen Life
Technologies). Cultures were maintained at 37°C in a humidified
incubator containing 5% CO2.
Quantitative polymerase chain reaction
(qPCR)
Total RNA from tissues or cells was harvested using
an miRNA Isolation kit (Ambion, Austin, TX, USA). Expression of
mature miRNAs was assayed using a Taqman MicroRNA assay (Applied
Biosystems, Foster City, CA, USA) specific for human miR-802. qPCR
was performed using a 7900 Real-time PCR system (Applied
Biosystems). All samples were normalized to the internal control,
U6 small nuclear RNA. All RNA samples were examined as to their
concentration and purity. RNA purity was measured using the
NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific,
Waltham, MA, USA). Based on the absorbance ratio at 260/280 nm
(mean ± standard deviation =1.86±0.02), all RNA samples were pure
and protein free. The reaction conditions were as follows: Initial
holding period at 95°C for 5 min, then 40 cycles of 95°C for 5 sec
and 60°C for 30 sec. The primer sequences used were as follows:
p27, forward 5′-ATGAGCCGCAAACTGGGT C-3′ and reverse
5′-AGAGCCGAACTCCACAAT CTC-3′; cyclin A, forward
5′-CGCTGGCGGTACTGAAGTC-3′ and reverse 5′-GAGGAACGGTGACATGCTCAT-3′;
cyclin B1 forward 5′-AATAAGGCGAAGATCAACATGGC-3′ and reverse
5′-TTTGTTACCAATGTCCCCAAGAG-3′. Relative quantitation analysis of
gene expression data was performed according to the
2−ΔΔCt method.
Cell proliferation assay
Transfected cells were plated onto 12-well plates at
a density of 1×104 per well and cultured for 1–3 days.
Cell counts were estimated by trypsinizing (Invitrogen Life
Technologies) the cells and performing analysis using a Coulter
counter (Beckman Coulter, Fullerton, CA, USA). For
bromodeoxyuridine (BrdU; Beyotime Institute of Biotechnology,
Shanghai, China) incorporation assays, a cell proliferation
enzyme-linked immunosorbent assay (Beyotime Institute of
Biotechnology) was used to analyze the incorporation of BrdU during
DNA synthesis according to the manufacturer's instructions.
Absorbance was measured at 450 nm using the Spectra Max 190 ELISA
reader (Molecular Devices, Sunnyvale, CA, USA).
Plasmid construction and
transfection
For the miR-802 expression plasmid, human miR-802
precursor was cloned by PCR and inserted into the XbaI and XhoI
sites of pSilencer (Ambion). The negative control plasmid consists
of a scrambled sequence (Ambion). MCF-7 cells were transfected with
the miR-802 precursor or the negative control using Lipofectamine
2000® (Invitrogen Life Technologies) according to the
manufacturer's instructions.
Cell cycle analysis
Cells were resuspended in 70% ethanol and fixed at
room temperature for 30 min. Cells were then washed three times
with phosphate-buffered saline (PBS; Beyotime Institute of
Biotechnology) and resuspended in propidium iodide (PI) stain
solution, consisting of 50 µg/ml PI (Beyotime Institute of
Biotechnology) and 100 µg/ml ribonuclease A (Sangon Biotech,
Shanghai, China) in PBS. The cells were incubated in PI stain
solution for 30 min and were then analyzed by flow cytometry using
a FACScalibur (BD Biosciences, Oxford, UK). The flow cytometry data
were analyzed using FlowJo (Tree Star, Inc., Ashland, OR, USA).
Western blot analysis
Cells were harvested and lysed using ice-cold lysis
buffer (50 mM Tris-HCl, pH 6.8; 802 mM 2-ME, 2% w/v SDS, and 10%
glycerol). Following centrifugation at 20,000 × g for 10 min at
4°C, proteins in the supernatants were quantified and separated
using 10% SDS-PAGE, then transferred to nitrocellulose membranes
(GE Healthcare, Little Chalfont, UK). Following blocking with 10%
non-fat milk in PBS, membranes were immunoblotted with the
following primary antibodies: Anti-p27 (ab32034; rabbit monoclonal;
1:2,000), cyclin A (ab2097; rabbit polyclonal; 1:2,000), cyclin B1
(ab72; mouse monoclonal; 1:1,000) and Forkhead box protein M1
(FoxM1) (ab83097; rabbit polyclonal; 1:1,000) antibodies purchased
from Abcam (Cambridge, MA, USA). Protein levels were normalized to
that of GAPDH (sc-365062; mouse monoclonal, 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). The blots were then
incubated with horseradish peroxidase-linked secondary antibodies
(Cell Signaling Technologies, Inc., Danvers, MA, USA). Antibody
signals were detected using a SuperSignal West Pico
Chemiluminescent Substrate kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA) according to manufacturer's instructions.
Luciferase reporter assay
The potential targets of miR-802 were analyzed by
TargetScan (www.targetscan.org) and miRWalk
(www.umm.uni-heidelberg.de/apps/zmf/mirwalk)
softwares. Wild-type and mutant 3′-untranslated region (UTR)
fragments of the FoxM1 gene were cloned into pMir-Report (Ambion),
yielding pMir-Report-FoxM1. Mutations were introduced into
potential miR-802 binding sites using a QuikChange site-directed
mutagenesis kit (Stratagene Inc., La Jolla, CA, USA). For
luciferase assays, cells were seeded in 24-well plates and
transfection efficiency was normalized by cotransfecting the Simian
virus 40 (SV40) plasmid. Values were determined using the
Dual-Luciferase Reporter Assay system (Promega Corporation,
Madison, WI, USA).
Tumor growth assay
Male BALB/c nude mice (age, five weeks) were
purchased from the Experimental Animal Center of the Third Military
Medical University (Chongqing, China). A total of 8×105
MCF-7 cells stably expressing miR-802 or negative control were
injected subcutaneously into the skin under the front legs of the
mice. Mice were observed over four weeks for tumor formation. At
four weeks, the mice were sacrificed via cervical dislocation,
tumors were recovered and the wet weights of each tumor were
determined. Experiments were performed using six mice per group
(two groups; MCF-7 cells with stable overexpression of miR-802
precursors and negative controls).
Statistical analysis
Values are presented as the mean ± standard error of
the mean (n>3). Differences between groups were analyzed using
Student's t-test using GraphPad Prism software, version 6.0.1
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05, P<0.01
and P<0.001 were considered to indicate statistically
significant differences.
Results
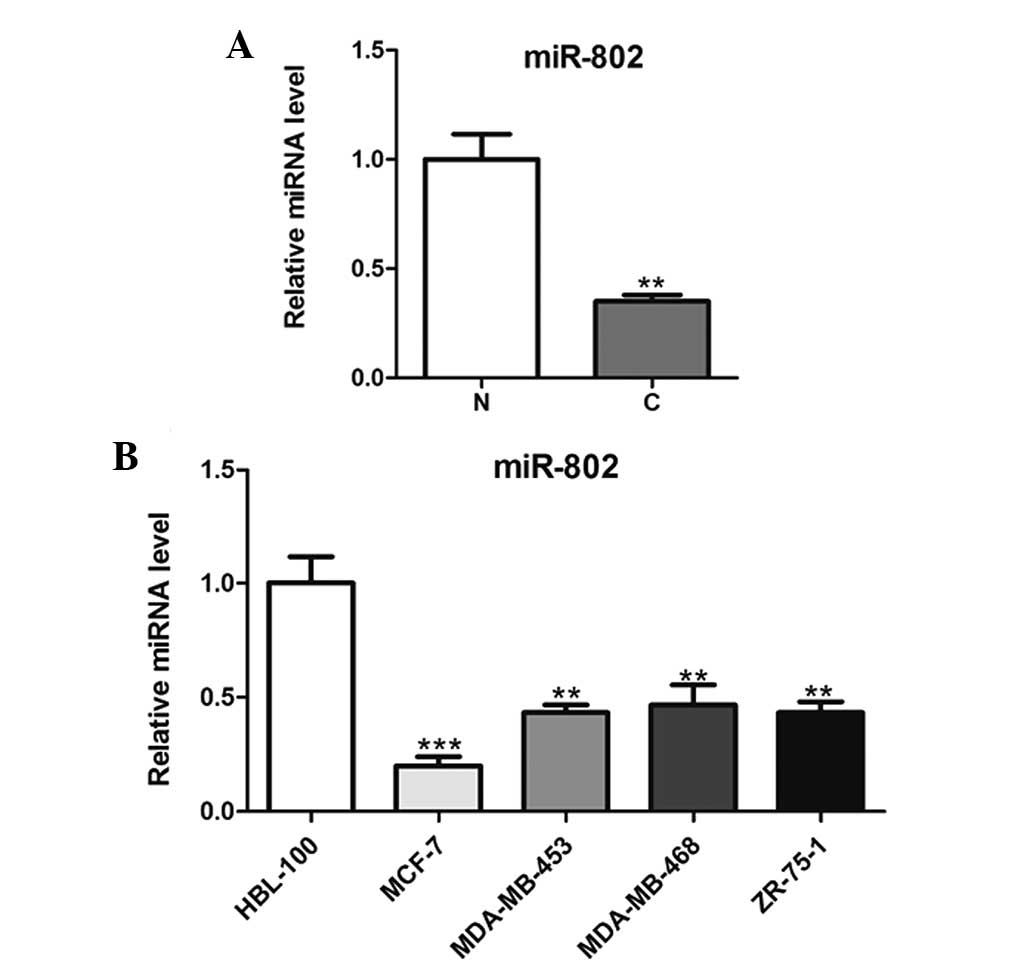
miR-802 is downregulated in breast cancer
tissues and cells
In order to explore the role of miR-802 in breast
cancer carcinogenesis, microRNAs were extracted from malignant and
normal breast tissue biopsies and then analyzed using qPCR. As
shown in Fig. 1A, miR-802
expression was significantly downregulated in cancer tissues
compared with that of the adjacent non-cancerous tissues. In
addition, miR-802 expression was significantly decreased in the
breast cancer cell lines compared with that of the normal breast
epithelial cells (Fig. 1B).
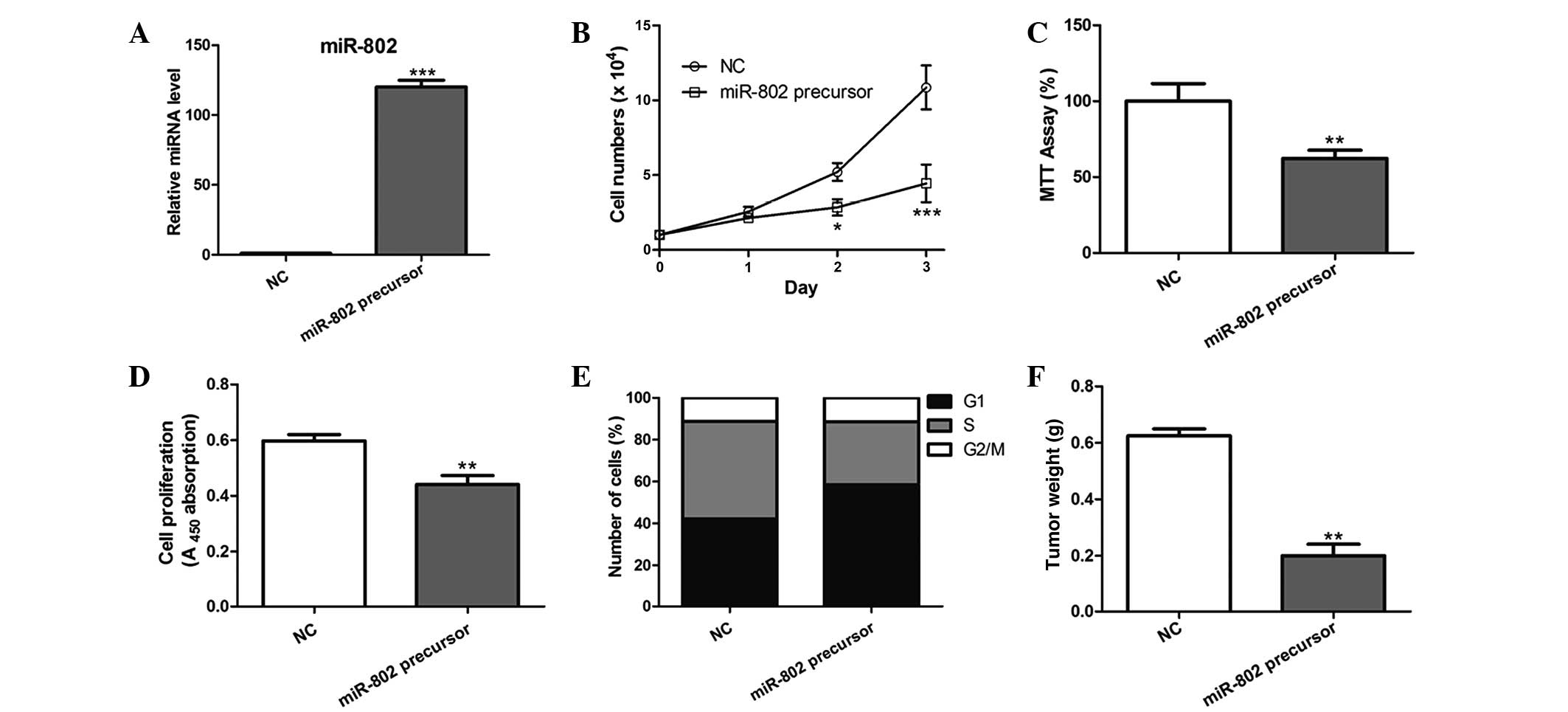
miR-802 overexpression inhibits breast
cancer cell proliferation in vitro and in vivo
In order to determine the effect of miR-802 on
breast cancer cell growth, MCF-7 cells overexpressing miR-802
precursor were constructed, which led to a significant increase in
miR-802 expression (Fig. 2A). As a
result, miR-802 overexpression significantly inhibited the
proliferative ability of MCF-7 cells post-transfection compared
with that of the negative control (Fig. 2B–D). Furthermore, cell cycle
analysis revealed that a significantly higher percentage of cells
overexpressing miR-802 were in G1/G0 phase and a decreased
percentage of these cells were in S phase, compared with that of
the negative control-transfected cells (Fig. 2E).
MCF-7 cells with stable overexpression of miR-802
were then evaluated for tumorigenic potential in vivo. Cells
were injected subcutaneously into the skin under the front legs of
nude mice and tumor growth was closely monitored for four weeks. As
a result, the tumor size and weight was markedly reduced in
miR-802-overexpressing tumors compared with that of the control
tumors (Fig. 2F), suggesting that
miR-802 suppressed breast cancer growth in vivo.
miR-802 targets the FoxM1 3′UTR and
downregulates its expression
In order to understand the underlying mechanisms of
miR-802-induced growth inhibition in MCF-7 cells, potential targets
of miR-802 were searched using TargetScan and miRWalk software. The
results showed that FoxM1, an oncogene in human cancer, harbored a
potential miR-802 binding site (Fig.
3A). In order to verify whether FoxM1 is a direct target of
miR-802, a luciferase reporter vector was constructed, which
contained the putative miR-802 binding sites within the FoxM1
3′-UTR. As shown in Fig. 3B,
miR-802 overexpression significantly repressed luciferase activity
when the reporter construct contained the FoxM1 3′UTR in MCF-7
cells (Fig. 3B). However, mutation
of the miR-802 binding site from the FoxM1 3′-UTR abolished the
effect of miR-802, suggesting that miR-802 directly inhibited FoxM1
expression through targeting its 3′-UTR (Fig. 3B).
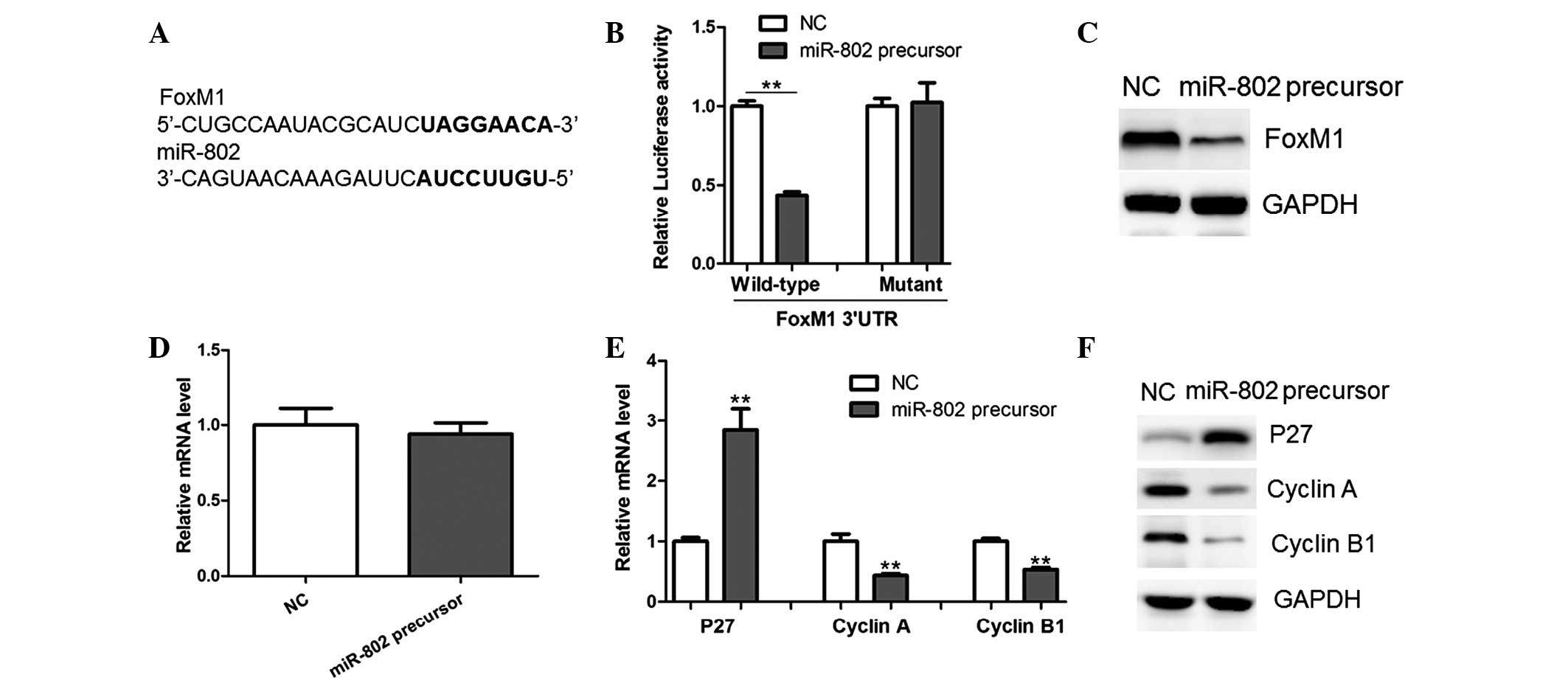 | Figure 3miR-802 represses FoxM1 expression
through targeting its 3′UTR. (A) Prediction of miR-802 binding
sites in the 3′UTRs of human FoxM1 gene as determined using
TargetScan and miRWalk software (bold, potential binding site). (B)
Luciferase reporter activity in MCF-7 breast cancer cells. Cells
were transfected with 100 ng wild-type or mutant 3′-UTR-reporter
constructs together with 25 nM miR-802 precursor or NC. (C) Protein
and (D) mRNA levels of FoxM1 were determined by western blot
analysis and qPCR, respectively, in MCF-7 cells transfected with
miR-802 precursor or NC. (E) mRNA and (F) protein levels of p27,
Cyclin A and Cyclin B1 were determined by western blot analysis and
qPCR, respectively, in MCF-7 cells transfected with miR-802
precursor or NC. miR-802, microRNA-802; FoxM1, Forkhead box protein
M1; UTR, untranslated region; NC, negative control; qPCR,
quantitative polymerase chain reaction. |
A shown in Fig. 3C,
western blot analysis revealed that the miR-802 precursor
significantly decreased the protein expression of FoxM1, while its
mRNA levels remained unchanged (Fig.
3D). Therefore, the results suggested that miR-802 negatively
regulated FoxM1 expression at the translational level.
FoxM1 was previously reported to transcriptionally
downregulate the expression of p27, while upregulating Cyclin A and
Cyclin B1, key regulators of cell-cycle progression (11). In concurrence with this previous
study, the present study observed the enhanced expression of p27
and downregulation of Cyclin A and Cyclin B1 in MCF-7 cells
overexpressing miR-802 (Fig. 3E and
F). Therefore, these results further indicated that FoxM1 was
an important target gene of miR-802 in breast cancer cells.
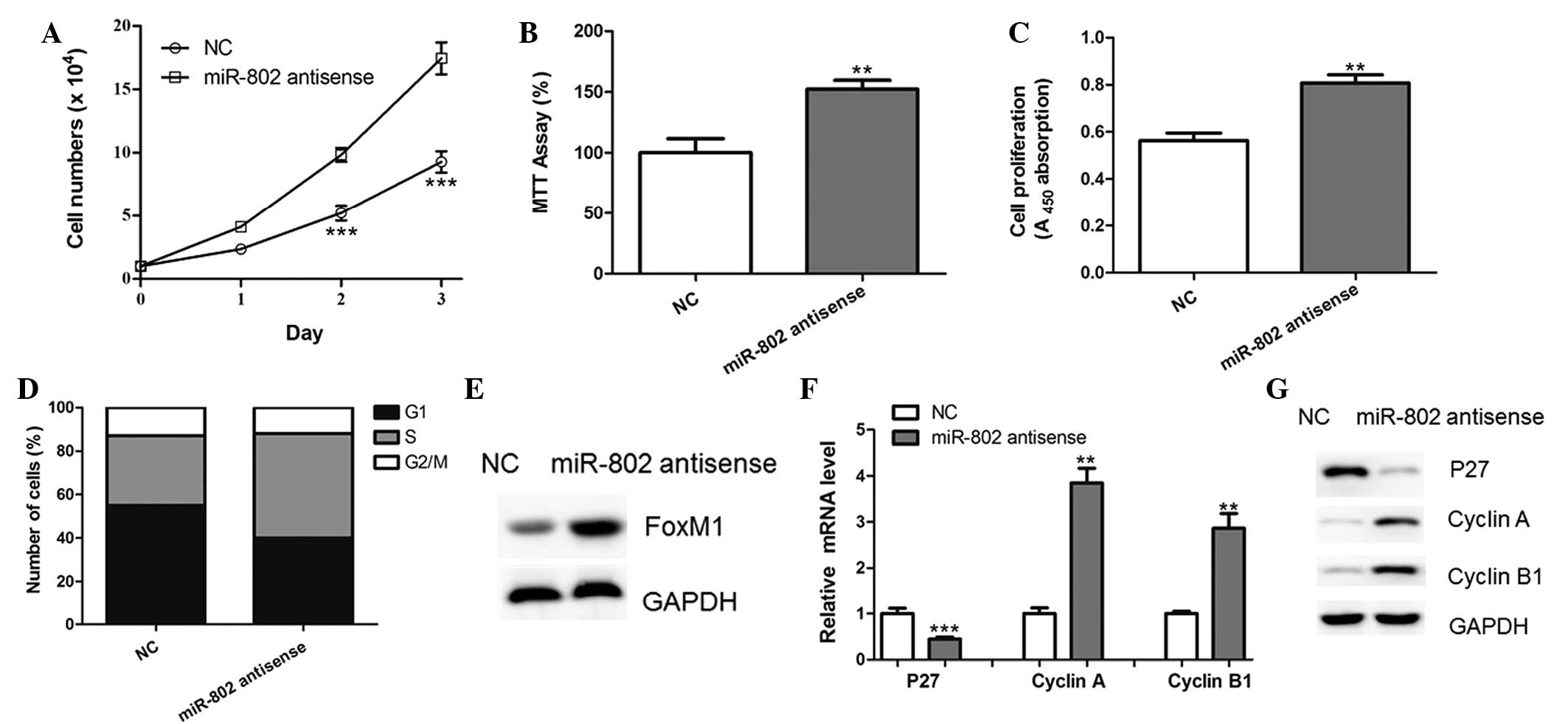
miR-802 antisense promotes the
proliferation of breast cancer cells
MCF-7 cells were transfected with miR-802 antisense
in order to block the functions of endogenous miR-802. As a result,
ectopic expression of the miR-802 antisense led to the increased
proliferative ability of MCF-7 cells, compared with that of the
negative control-transfected cells (Fig. 4A–C). In addition, the inhibition of
miR-802 significantly reduced the percentage of cells in G0/G1
phase and increased the percentage of cells in S phase (Fig. 4D). Furthermore, protein levels of
FoxM1 were upregulated in MCF-7 cells transfected with miR-802
antisense (Fig. 4E). The
downregulation of p27 and upregulation of Cyclin A and Cyclin B1
was also observed following miR-802 antisense transfection
(Fig. 4F and G). These results
therefore supported the conclusion that miR-802 regulated FoxM1
expression in breast cancer cells.
Discussion
miR-802 has been shown to modulate the biological
efficacy of Ang II in the human gastrointestinal tract via
downregulation of angiotensin II type 1 receptor (12). In addition, the role of miR-802 in
the development of obesity-associated impairment of hepatic glucose
metabolism was previously identified (13). In the present study, miR-802
expression and its role in breast cancer were determined and
suggested its potential therapeutic tumor suppressor role in breast
cancer. Cell viability assays and cell cycle analysis demonstrated
that selective overexpression of miR-802 inhibited the
proliferative ability of MCF-7 breast cancer cells, while
inhibition of miR-802 promoted cell proliferation. In addition,
FoxM1 was identified as a novel direct target of miR-802 using a
luciferase reporter assay and western blot analysis in MCF-7 cells.
However, larger sample sizes are required in order to further
verify the results of the present study. In addition, further
studies are also required in order to determine whether miR-802 may
be used as a biomarker for the diagnosis and prognosis in breast
cancer. Furthermore, it may be of interest to further investigate
whether miR-802 is dysregulated in other types of human
cancers.
FoxM1 is a member of the Forkhead transcription
factor family, which control cell proliferation and apoptosis
through the regulation of genes associated with cell cycle entry,
including p21 and p27 (14,15).
Previous studies have reported that FoxM1 was upregulated in breast
cancer and its expression was correlated with poor prognosis and
metastasis in breast cancer (16,17).
Therefore, FoxM1 has become a target for therapeutic intervention
in cancer treatment. The combination of oxidative stress and FoxM1
inhibitors has been reported to induce apoptosis in cancer cells as
well as inhibit xenograft tumor growth (18). However, the molecular determinants
for the upregulation of FoxM1 remain to be elucidated. Previous
studies suggested that FoxM1 may be regulated by several miRNAs,
including miR-134, miR-149 and miR-370 (19–21).
This therefore indicated that the dysregulated expression of
certain miRNAs may have a critical role in the aberrant expression
of FoxM1 in human cancers.
In conclusion, the results of the present study
provided the first evidence that overexpression of miR-802
inhibited breast cancer cell growth in vitro and in
vivo through regulating FoxM1 expression. This therefore
indicated the important role of miR-802 in breast cancer
pathogenesis and implicated its potential therapeutic use for the
treatment of breast cancer.
References
|
1
|
Cao W, Wang X and Li JC: Hereditary breast
cancer in the Han Chinese population. J Epidemiol. 23:75–84. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Assadi M, Lamerz J, Jarutat T, et al:
Multiple protein analysis of formalin-fixed and paraffin-embedded
tissue samples with reverse phase protein arrays. Mol Cell
Proteomics. 12:2615–2622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asghar U, Witkiewicz AK, Turner NC and
Knudsen ES: The history and future of targeting cyclin-dependent
kinases in cancer therapy. Nat Rev Drug Discov. 14:130–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Atalay C: New concepts in axillary
management of breast cancer. World J Clin Oncol. 5:895–900. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Kouwenhove M, Kedde M and Agami R:
MicroRNA regulation by RNA-binding proteins and its implications
for cancer. Nat Rev Cancer. 11:644–656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh R and Mo YY: Role of microRNAs in
breast cancer. Cancer Biol Ther. 14:201–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Redova M, Sana J and Slaby O: Circulating
miRNAs as new blood-based biomarkers for solid cancers. Future
Oncol. 9:387–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pinho FG, Frampton AE, Nunes J, et al:
Downregulation of microRNA-515-5p by the estrogen receptor
modulates sphingosine kinase 1 and breast cancer cell
proliferation. Cancer Res. 73:5936–5948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hwang MS, Yu N, Stinson SY, et al:
miR-221/222 targets adiponectin receptor 1 to promote the
epithelial-to-mesenchymal transition in breast cancer. PloS One.
8:e665022013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang IC, Chen YJ, Hughes D, et al:
Forkhead box M1 regulates the transcriptional network of genes
essential for mitotic progression and genes encoding the SCF
(Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 25:10875–10894. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sansom SE, Nuovo GJ, Martin MM, Kotha SR,
Parinandi NL and Elton TS: miR-802 regulates human angiotensin II
type 1 receptor expression in intestinal epithelial C2BBe1 cells.
Am J Physiol Gastrointest Liver Physiol. 299:G632–G642. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kornfeld JW, Baitzel C, Konner AC, et al:
Obesity-induced overexpression of miR-802 impairs glucose
metabolism through silencing of Hnf1b. Nature. 494:111–115. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sengupta A, Kalinichenko VV and Yutzey KE:
FoxO1 and FoxM1 transcription factors have antagonistic functions
in neonatal cardiomyocyte cell-cycle withdrawal and IGF1 gene
regulation. Circ Res. 112:267–277. 2013. View Article : Google Scholar :
|
|
15
|
Qu K, Xu X, Liu C, et al: Negative
regulation of transcription factor FoxM1 by p53 enhances
oxaliplatin-induced senescence in hepatocellular carcinoma. Cancer
Lett. 331:105–114. 2013. View Article : Google Scholar
|
|
16
|
Sanders DA, Ross-Innes CS, Beraldi D,
Carroll JS and Balasubramanian S: Genome-wide mapping of FOXM1
binding reveals co-binding with estrogen receptor alpha in breast
cancer cells. Genome Biol. 14:R62013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang C, Chen H, Tan G, et al: FOXM1
promotes the epithelial to mesenchymal transition by stimulating
the transcription of Slug in human breast cancer. Cancer Lett.
340:104–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Halasi M, Pandit B, Wang M, Nogueira V,
Hay N and Gartel AL: Combination of oxidative stress and FOXM1
inhibitors induces apoptosis in cancer cells and inhibits xenograft
tumor growth. Am J Pathol. 183:257–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Wang Y, Luo J, et al: miR-134
inhibits epithelial to mesenchymal transition by targeting FOXM1 in
non-small cell lung cancer cells. FEBS Lett. 586:3761–3765. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ke Y, Zhao W, Xiong J and Cao R: miR-149
Inhibits non-small-cell lung cancer cells EMT by targeting FOXM1.
Biochem Res Int. 2013:5067312013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng Y, Wang L, Zeng J, et al: FoxM1 is
overexpressed in Helicobacter pylori-induced gastric carcinogenesis
and is negatively regulated by miR-370. Mol Cancer Res. 11:834–844.
2013. View Article : Google Scholar : PubMed/NCBI
|