Introduction
Hypertension, a leading risk factor for
cardiovascular diseases (1),
occurs in ~50% of aged individuals (2). It has been reported that long-term
hypertension is associated with cardiac hypertrophy (3). High blood pressure is associated with
the aberrant expression of genes, including angiotensinogen (AGT),
which has an important role in the pathogenesis of hypertension
(4–6). AGT is secreted by the liver and
sequentially cleaved by renin and angiotensin I-converting enzyme
(7,8). This results in production of an
active hormone, angiotensin II, which induces hypertension
(9). AGT has been suggested as an
important determinant of blood pressure and cardiac hypertrophy
(10). The roles of AGT in the
pathogenesis of hypertension have been investigated by molecular
genetic approaches, including transgenic animals (11), anti-sense oligodeoxynucleotides
(12), linkage analysis of the AGT
gene on chromosome 1q42-43 and essential hypertension (13).
Spontaneously hypertensive rats (SHR), have been
widely used as an animal model of human primary hypertension
(14,15). The mean arterial blood pressure of
SHR is significantly increased from 16–20 weeks of age, which is
associated with a series of changes in their pathogenesis and
pathophysiology (16–18). The pathological changes in SHR are
similar to those in humans with primary hypertension (19,20).
Therefore, SHR are an ideal animal model for studies on the
etiology of human primary hypertension and may serve as a screening
model for novel anti-hypertensive drugs (21).
Overexpression of type 1 angiotensin II receptors
(AT1R) in renal proximal tubules has been detected in young SHR
(22). To explore its role in
hypertension, RNA interference (RNAi) techniques have often been
used to investigate the functional consequences of AT1R silencing.
However, most AT1R-knockdown studies are performed in vitro,
while only a few studied have used in vivo animal models
(23,24). In the present study, knockdown of
AT1R (25) and
angio-tensin-converting enzyme (ACE) (26) were investigated in SHR, as AT1R and
ACE are associated with the function of angiotensin II (AngII) in
hypertension. Of note, AGT gene expression in SHR has rarely been
studied (27).
RNAi has been widely used to selectively inhibit
gene expression due to its target specificity. The specific
inhibitory effect of small interfering (si)RNA on its target gene
is determined by the formation of RNA-induced silencing complexes
(28). As RNAi is a useful tool
for gene functional studies, it may also potentially be used in
gene therapy. However, at present, gene therapy remains challenging
due to lack of efficient and safe delivery systems to enable the
silencing of a specific gene in the target tissue (29,30).
To address this issue, numerous novel gene carriers have been
developed, among which cationic polymers are showing promise as a
safer strategy for gene delivery, owing to its several advantages,
including lower immunogenicity, the ease of chemical modification
and the ability to transfer larger plasmid DNA molecules (31,32).
Currently, the cationic polymer polyethylenimine (PEI) and its
biscarbamate-crosslinked derivatives (PEI-Et) (33) and galactose-polyethylene
glycol-PEI-Et (GPE) (34,35), have been successfully used for
non-viral transfection with markedly low cytotoxicity, potent
transfection efficiency and high hepatocyte-targeting properties.
GPE, a hepatocyte-targeting gene carrier, has the ability to
condense plasmid DNA (pDNA) into nanoparticles, providing lower
cytotoxicity, high transfection efficiency and preferential
accumulation in the liver.
In the present study, it was hypothesized that
specific RNAi with the AGT gene may have effects on long-term
hypertension and may improve hypertensive cardiac hypertrophy.
Therefore, effects of AGT-specific small hairpin (sh)RNA coupled to
GPE carrier molecules were evaluated in SHRs.
Materials and methods
Reagents
The GPE carrier was a gift from Professor Jin Tuo of
the School of Pharmacy, Jiao Tong University (Shanghai, China). PEI
was purchased from Sigma-Aldrich (St. Louis, MO, USA). shRNA
plasmids (UUGAUAUCCG) for the negative control (NT) and AGT gene
were products of Genechem Biotechnology Co. (Shanghai, China).
Plasmid purity was assessed by electrophoresis, and the
concentration of pDNA was determined by spectrophotometry (DU-800;
Beckman Coulter, Inc., Krefeld, Germany) with absorption at 260/280
nm. The mouse AGT immunoglobulin G (IgG) antibody (cat. no. 77;
1:1,000; overnight incubation at 4°C) was obtained from Swant, Inc.
(Belinzona, Switzerland) and the AGT and AngII ELISA Assay kits
were purchased from R&D Systems Europe Ltd. (Abingdon, UK).
Construction of GPE-AGT-shRNA
nanoparticles
GPE-AGT-shRNA complexes were prepared by vigorous
mixing of GPE solution and plasmid solution of AGT shRNA or
negative shRNA at a weight ratio of 30:1. The mixture was incubated
at room temperature for 1 hour. Since the GPE/DNA weight ratio is a
major cytotoxic determinant of the nanoparticles, the optimal
GPE/DNA weight ratio (30:1) with the lowest cytotoxicity and the
highest transfection efficiency was determined in preliminary
experiments. All GPE-AGT-shRNA nanoparticles were freshly prepared
prior to the experiments. The nanoparticles were filtered through a
0.2-mm membrane (Millipore Corp., Billerica, MA, USA).
Experimental animals
The present study utilized sixty-one 16-week-old
male SHR weighing 350±30 g and twenty-one Wistar-Kyoto rats (WKY)
with similar features to those of SHR were purchased from the SLAC
Laboratory Animal Co., Ltd (Shanghai, China). Rats were housed at
three per cage, under a regular 12-hour diurnal light cycle, with
free access to food and water during the entire study. All
procedures of the animal study were approved by the local ethics
committee of the School of Medicine, Shanghai Jiao-Tong University
(Shanghai, China) (no. 0708253).
In vivo experiment
Sixty-three SHRs were randomly divided into three
groups (n=21, each group): 1) Blank control injected with 500
µl sterile water via the tail vein; 2) negative control with
i.v. injection of 500 µl of GPE/negative shRNA complex and
3) AGT-RNAi group with i.v. injection of 500 µl of
GPE-AGT-shRNA. WKY were used as normotensive controls that were
only treated with sterile water (500 µl) intravenously. All
rats received a series of nine injections during the experiment, in
which 10 consecutive days were considered a cycle. The injection
was administered on the first day of each cycle. Prior to and after
injection, SBP (systolic blood pressure) of the caudal artery was
measured by the standard tail-cuff method. At days 0, 3, 5, 7 and
10 after injection, the expression levels of AGT mRNA and protein
in tissue samples of the liver were determined by reverse
transcription quantitative polymerase chain reaction (RT-qPCR) and
western blot analyses. Furthermore, serum/plasma levels of AGT and
AngII were measured by ELISA. Liver and kidney functions of
experimental animals were also examined using the Fully Automatic
Biochemical Analyzer (Beckman Coulter, Inc.). Finally, experimental
rats were sacrificed by intraperitoneal injection of 50 mg/kg
pentobarbital solution (Sigma-Aldrich). Specimens of heart tissue
were taken for pathological examination. Ratios of HW (heart
weight)/BW (body weight) and LVW (left ventricle weight)/BW were
calculated.
RT-qPCR
Total RNA was extracted from tissue samples of the
liver using TRIzol reagent (Invitrogen Life Technologies, Shanghai,
China). Total RNA (5 µg) was reversely transcribed into cDNA
in a 20-µl mixture using Moloney murine leukemia virus
Reverse Transcriptase (Cell Bank of Pharmacology and Toxicology
Laboratory, New Medicine and Marine Medicine Research Center of
Nanjing University of Chinese Medicine, China., according to the
manufacturer's instructions. RT-qPCR was performed using the MX
3000P Sequence Detection system (Stratagene, La Jolla, CA, USA) and
SYBR Green PCR kit (Applied Biosystems, Life Technologies, Thermo
Fisher Scientific, Waltham, MA, USA). The specific primers for
specific genes were synthesized by Invitrogen Life Technologies.
Primer sequences for AGT were as follows: Forward,
5′-CATCTTCCCTCGCTCTCTG-3′ and reverse, 5′-GCCTCTCATCTTCCCTTGG-3′
(175 bp). The housekeeping gene, β-actin, was used as an internal
reference for the normalization of the target genes. β-actin
primers were: Forward, 5′-CAAGGTCATCCATGACAACTTTG-3′ and reverse,
5′-GTCCACCACCCTGTTGCTGTAG-3′ (217 bp). The PCR protocol included a
pre-denaturation at 95°C for 10 min and 40 cycles of denaturation
at 95°C for 30 sec, annealing at 60°C for 30 sec and extension at
72°C for 45 sec.
Western blot analysis
50 µg lysates of tissue samples were loaded
onto 10% polyacrylamide gels and separated by SDS-PAGE (Shanghai
Biological Technology Co., Ltd., Shanghai, China). The separated
proteins were transferred onto nitrocellulose membranes, the
membranes were blocked with 0.1% bovine serum albumin for 2 h at
room temperature and then incubated overnight with mouse anti-rat
AGT antibody (1:1,000) or mouse anti-rat β-actin antibody (1:5,000)
at 4°C. After washing three times (5 min each) with Tris-buffered
saline (10 Mm Tris/HCl, pH 7.5 and 150 mM NaCl) containing 0.05%
Tween 20 (TBS-T) (Cell Signaling Technology, Inc., Danvers, TX,
USA), membranes were incubated with a sheep anti-mouse IgG antibody
(cat. no. 0257; 1:1,000; Sigma-Aldrich) for 2 h at room temperature
and then rinsed three times with TBS-T. Finally, blots were
developed by enhanced chemiluminescence and exposed to X-ray film
(Cell Signaling Technology, Inc.). Quantification of signals was
performed by an image acquisition and analysis system (Odyssey v1.2
family of imaging systems; LI-COR Biosciences, Lincoln, NE, USA).
Results are presented as the ratio of AGT/β-actin.
Measurement of serum levels of AGT and
AngII
Following anesthesia with an intraperitoneal
injection of 1% sodium pentobarbital, blood samples were collected
from the femoral vein of the rats. Plasma and cells were then
separated by centrifugation for 10 min at 900 × g at room
temperature. The collected plasma was stored at −80°C prior to
measurement. Serum levels of AGT and AngII were determined by using
ELISA, according to the manufacturer's protocol.
Histological and ultrastructural
examination
Sections of heart samples were stained with
hematoxylin-eosin (Invitrogen Life Technologies) for the
examination of cardiac hypertrophy (36,37).
Myocardial ultrastructure was observed under an electron microscope
(CMI20; Philips, Amsterdam, Netherlands).
Statistical analysis
All statistical analyses were performed using SPSS
software (version 19.0; SPSS, Inc, Chicago, IL). Values are
expressed as the mean ± standard deviation. Student's t-test
(two-tailed) was applied to test the significance of the
differences between two groups. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
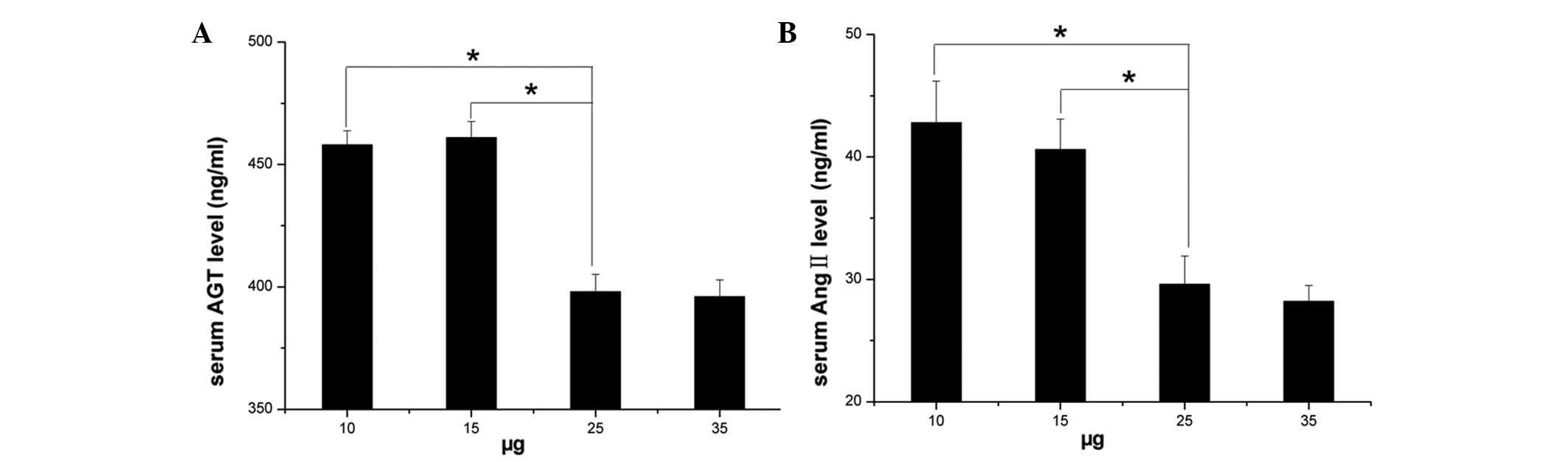
Dose-dependent effect of injection of
GPE-AGT-shRNA nanoparticles
To investigate the efficacy of GPE-AGT-shRNA
nanoparticles to inhibit plasma levels of AGT in vivo,
different doses of GPE-AGT-shRNA (AGT-shRNA plasmids coupled to GPE
carrier molecules, ranging from 10 to 35 µg) were
intravenously injected into SHR via the tail vein. As shown in
Fig. 1, at day 3 after injection
of GPE-AGT-shRNA, the plasma levels of angiotensinogen were
significantly decreased (P<0.05) in a dose-dependent manner, as
compared with pre-injection levels (day 0). Results showed a
significant inhibitory effect on the plasma levels of
angio-tensinogen by injection of GPE-AGT-shRNA at doses of 25 and
35 µg, but no difference was observed between these two
doses of GPE-AGT-shRNA (P<0.05). Therefore, 25 µg
GPE-AGT-shRNA was selected as an optimal dose for injection in
further SHR experiments.
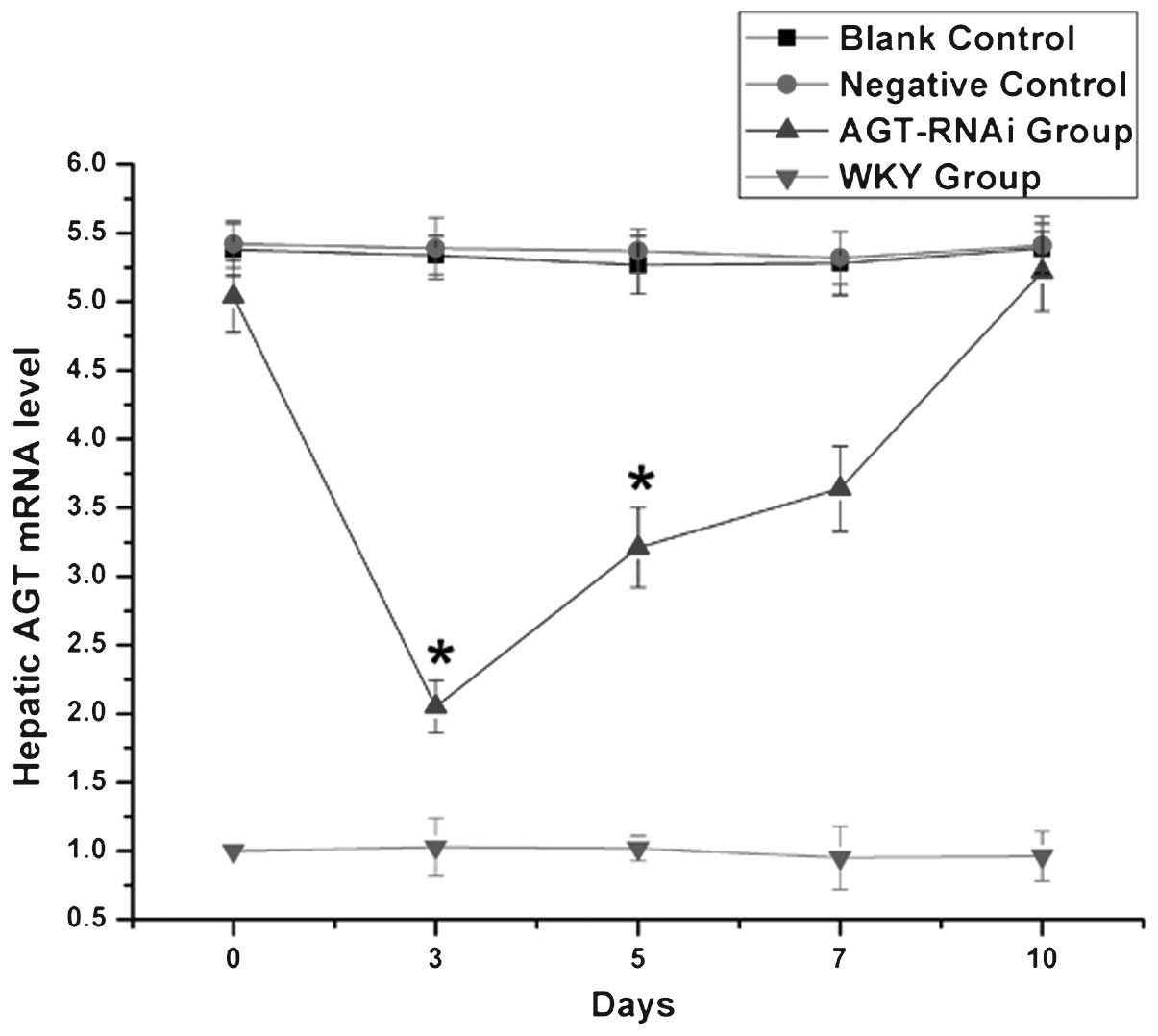
mRNA levels of AGT in experimental
rats
As shown in Fig. 2,
at days 3 and 5 after injection, hepatic mRNA levels of AGT in the
GPE-AGT-shRNA group were significantly lower than those in the
other SHR groups (P<0.05). However, from days 7–10 after
injection, hepatic AGT mRNA expression in GPE-AGT-shRNA group
gradually increased, eventually equaling the levels in the SHR
control groups. However, there was no significant difference in
blood pressure between the GPE-AGT-shRNA and control groups during
this period.
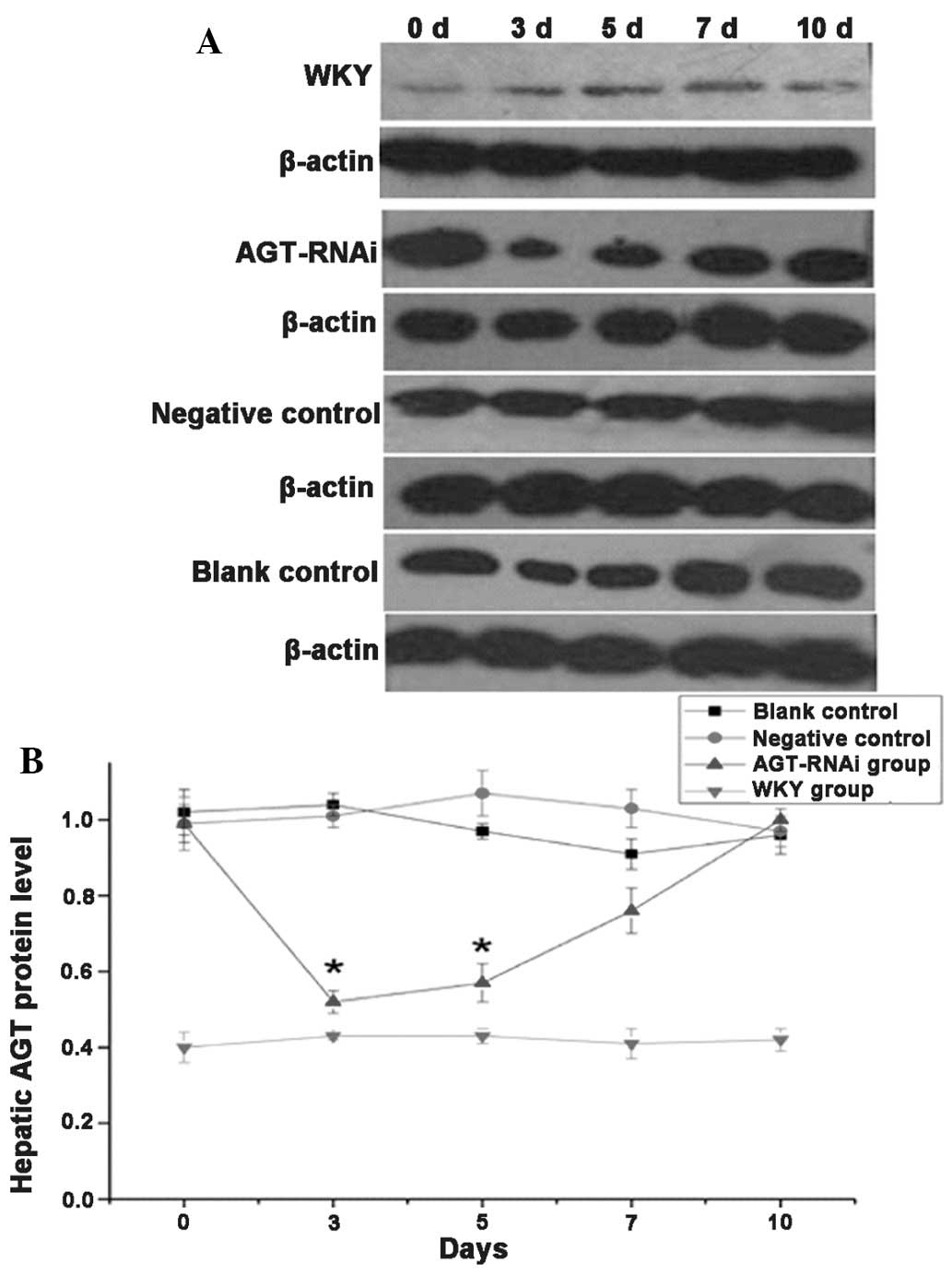
Protein levels of AGT in experimental
rats
As shown in Fig.
3A, at days 3 and 5 after injection, AGT protein levels in the
liver of the GPE-AGT-shRNA group were significantly lower than
those in the other SHR groups (P<0.05), but at days 7 and 10
after injection, its levels in the GPE-AGT-shRNA group were equal
to those in the other SHR groups. No significant difference in AGT
protein expression was found among SHR groups at days 7 and 10
after injection. Quantified results of AGT protein expression in
the different SHR groups are shown in Fig. 3B.
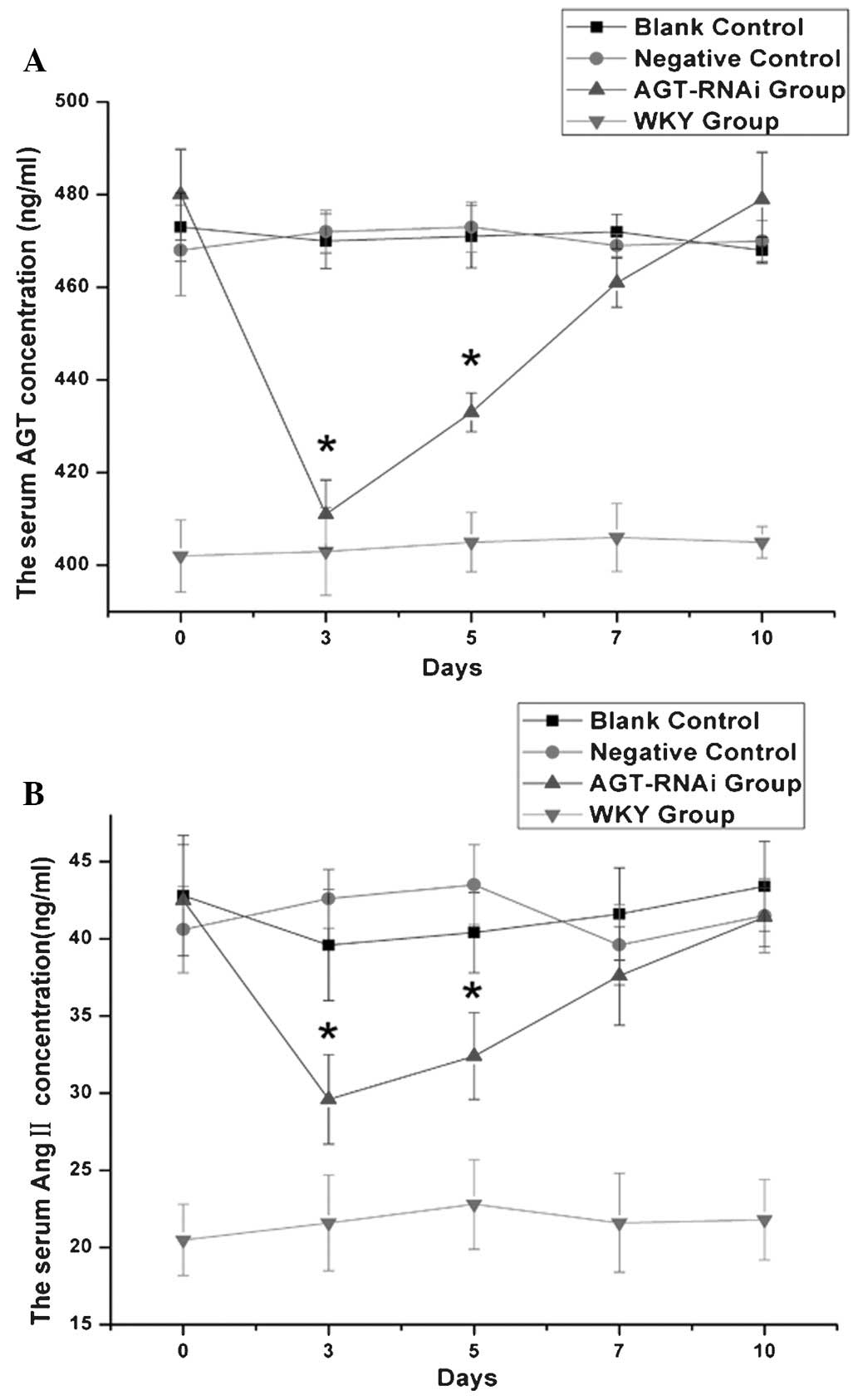
Serum levels of AGT and AngII in
experimental rats
As shown in Fig. 4,
at days 3 and 5 after injection, serum levels of AGT (Fig. 4A) and AngII (Fig. 4B) in the GPE-AGT-shRNA group were
significantly reduced, and lower than the levels in the other SHR
groups (P<0.05). However, from days 5 to 10 after injection, AGT
and AngII levels in the GPE-AGT-shRNA group were gradually
increased, eventually equal to those in other SHR groups. No
significant differences in serum AGT and AngII levels were observed
within the other SHR groups and WKY control at different time
points.
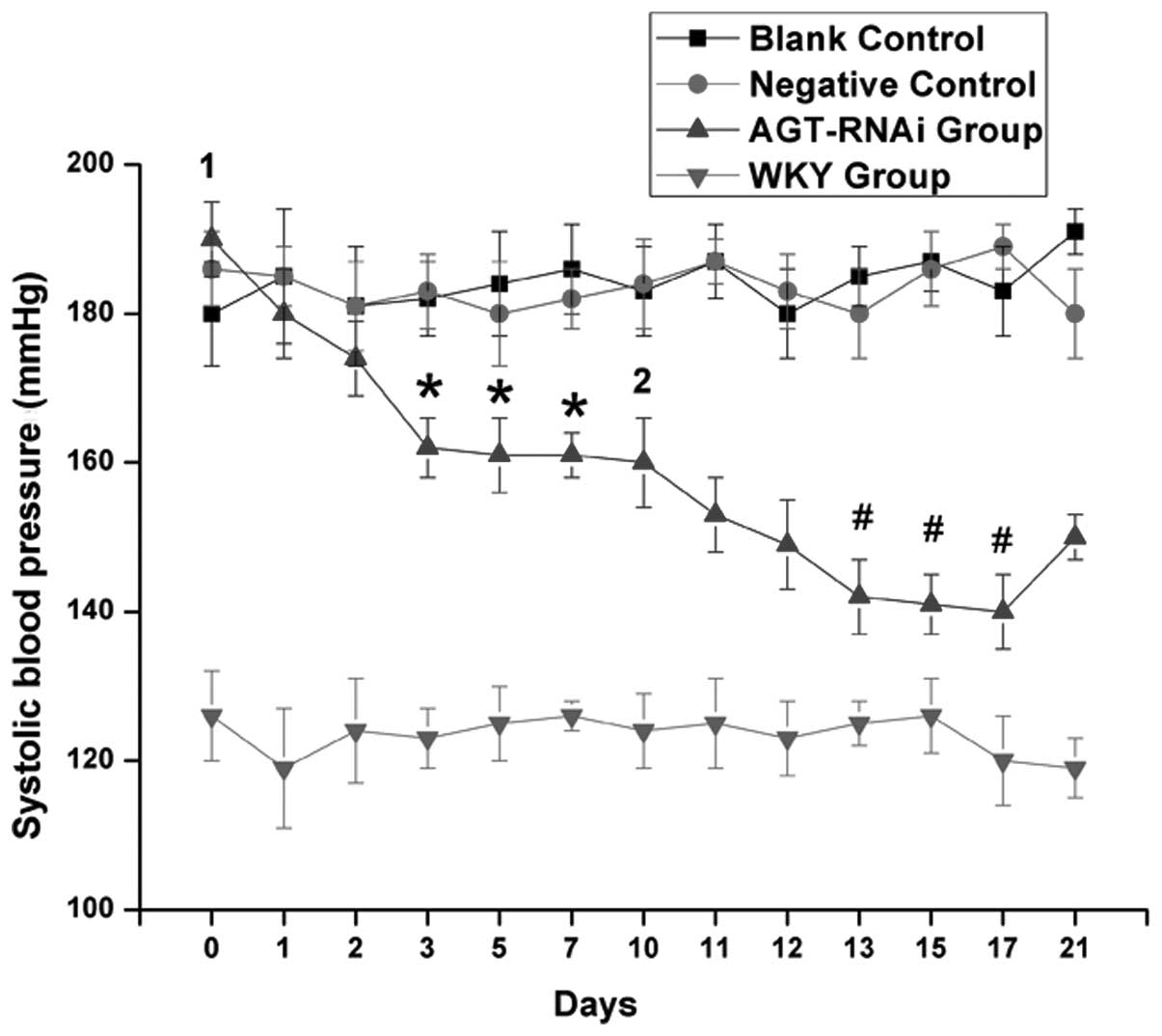
Effects of AGT-RNAi on blood
pressure
As indicated in Fig.
5, there was a marked decrease in tail arterial pressure in
GPE-AGT-shRNA-injected rats, whereas tail arterial pressure
remained stable in the blank and negative controls. However, no
significant changes in BP in the WKY group were observed. The
largest decrease in BP was after the 2nd injection accounting up to
53 mmHg. The anti-hypertensive effect induced by injection of
AGT-shRNA nanoparticles lasted for >10 days after each
injection.
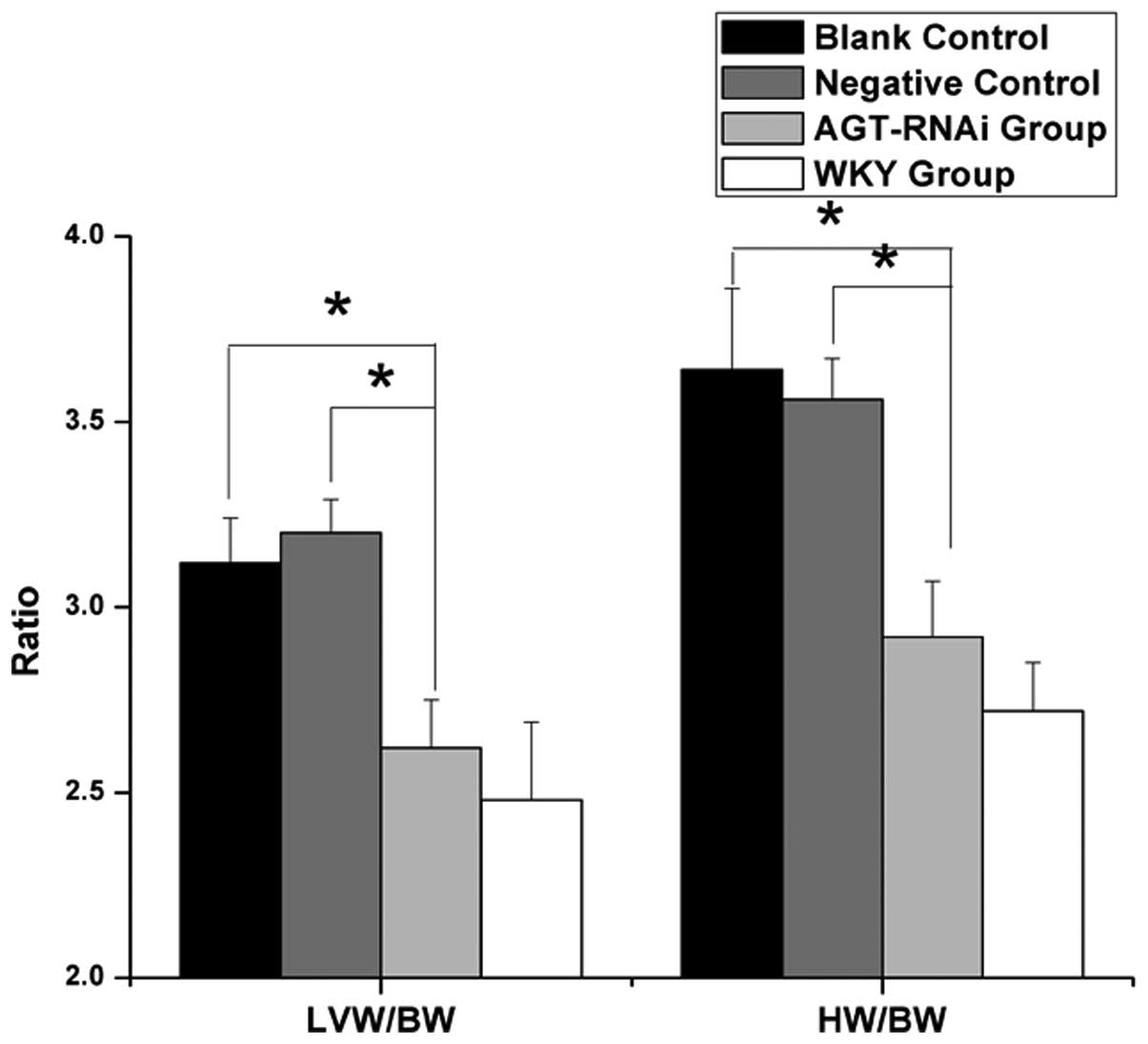
Changes of HW/BW and LVW/BW in
experimental rats
As shown in Fig. 6,
the ratios of HW/BW and LVW/BW in experimental rats were compared.
Among them, ratios of HW/BW and LVW/BW in the GPE-AGT-shRNA group
(2.92±0.15 and 2.62±0.13 mg/g respectively) were significantly
lower than those in the SHR blank controls (3.64±0.22 and 3.12±0.12
mg/g; both P<0.05) or negative controls (3.56±0.11 and 3.2±0.09
mg/g; both P<0.05), but marginally higher than those in the WKY
group (2.72±0.13 and 2.48±0.21 mg/g; both P>0.05).
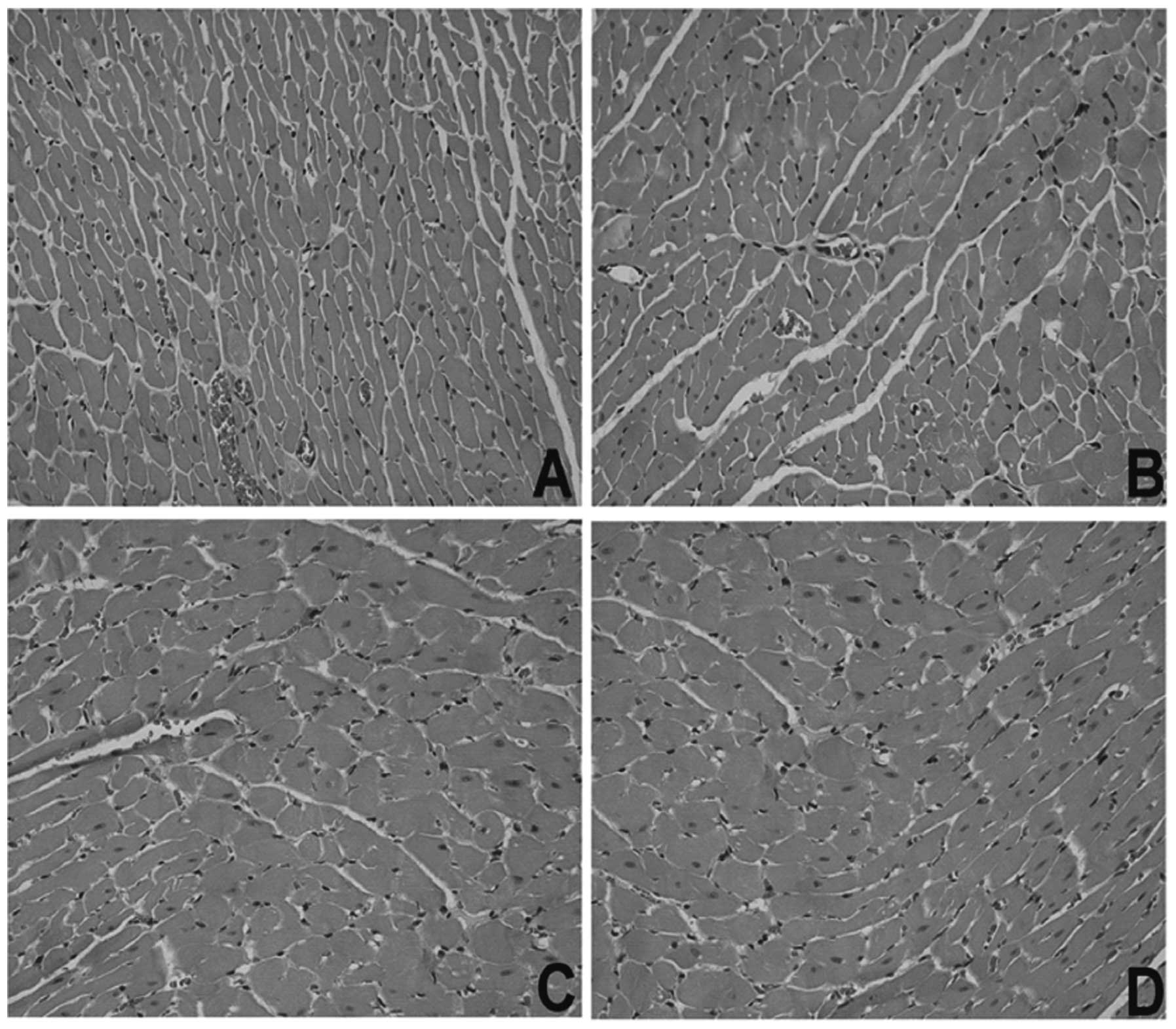
Pathological changes of myocardial
structure by microscopic examination
Pathological changes in myocardial structure in
experimental rats were observed under an optical microscope. As
shown in Fig. 7, hypertrophy of
cardiac muscle cells was found in the blank and negative control
SHR groups, but a significant improvement of cardiac hypertrophy
was observed in the GPE-AGT-shRNA group.
Pathological changes of myocardial
ultrastructure
Pathological changes of myocardial ultrastructure in
experimental rats were observed under an electron microscope.
Compared to the SHR control groups, integrity of the myocardial
cell membrane as well as clear myofibrillar structures with tidy
and defined cross striations were observed in the GPE-AGT-shRNA
group. In this group, mitochondria without swelling and a small
amount of local increase in myocardial interstitial collagen fibers
with no obvious hyperplasia were observed, suggesting that GPE-AGT
shRNA nanoparticles improved the myocardial ultrastructure in
SHR.
Discussion
Hypertension is a lifelong disease, which is not
only a cause of premature death and disability, but also a threat
to social and economic development. It is estimated that,
worldwide, the number of patients with hypertension will reach 1.5
billion in 2025, but only one-third of patients may be successfully
treated (38). Reasons for the low
control rate of hypertension include the short half-life of
antihypertensive drugs, as well as their short duration of action,
non-specific effects and side effects. Furthermore, poor compliance
of hypertensive patients often occurs in clinical practice.
Therefore, a therapy with long-term effects and reduced side
effects is desirable for a lifelong treatment of hypertension.
RNAi technology is a recent breakthrough in
post-transcriptional gene silencing. With this novel technique,
expression of specific genes in mammalian cells can be inhibited
(39–44). The formation of RNAi depends on the
enzyme Dicer, which cleaves long double-stranded RNA (dsRNA)
molecules into short double-stranded fragments of ~20 nucleotides
which are called siRNAs. siRNA is capable of blocking the mRNA
expression of a specific gene, resulting in inhibition of
subsequent protein expression, but avoiding non-specific
degradation of genes and long double strand RNA-mediated cell
death. Compared with technology of gene knockout, RNAi has the
advantages of a short turnover time and low cost. Relative to the
anti-sense nucleotide technique, RNAi has high efficiency, strong
specificity and long-lasting duration. Therefore, RNAi has been
widely applied in functional studies of specific genes in mammals
(45), including cancer gene
therapy (46,47), viral infections (48–51)
and monogenetic disorders (52).
The present study reported that injection of
GPE-AGT-shRNA nanoparticles was able to significantly reduce the
hepatic AGT mRNA expression, plasma levels of AGT and systolic
blood pressure in SHR. In a control group, injection of GPE shRNA
nanoparticles did not produce any similar effects. In the present
study, no obvious side effects were present within 10 days
following injection of nanopar-ticles. The intravenous injection of
GPE-AGT-shRNA nanoparticles resulted in a significant decrease in
blood pressure from days 1–7 after injection, as compared to the
other treatments (P<0.05). The anti-hypertensive effect induced
by GPE-AGT-shRNA nanoparticles lasted >10 days after a single
injection. Administration of GPE-AGT-shRNA nanoparticles in SHR
also had favorable effects on hypertension-induced cardiac
hypertrophy. The reduction in blood pressure in SHR was ~30 mmHg;
however, the blood pressure did not normalize. Therefore, it may be
assumed that the downregulation of hypertension in SHR was affected
not only by AGT silencing, but also by other diverse factors in
vivo. In addition, the results of the present study indicated
that circulating AGT also has an important role in the pathogenesis
of hypertension of SHR. Furthermore, the results showed that the
reduction of circulating AGT following RNAi led to a decrease in
hypertension in the SHR model. These findings are consistent with a
previous study on AGT-knockout mice (53) or a study using anti-sense
oligodeoxynucleotides (13). Due
to the short period of time for observation of only 10 days after
the injection in the present study, the accuracy of the results may
have been affected. Therefore, to elucidate the exact effect of AGT
on the development of hypertension and hypertrophy, further
long-term studies are warranted.
In conclusion, intravenous injection with shRNA
targeting the AGT gene coupled to the hepatic-specific GPE carrier
decreased the plasma levels of AGT. The present study demonstrated
that injection of AGT shRNA nanoparticles attenuated blood
pressure, accompanied by improvement of the hypertension-induced
cardiac hypertrophy in SHR. These findings suggested that RNAi may
provide a potential therapeutic strategy for hypertension with
cardiac hypertrophy.
Acknowledgments
This study was supported by grants from three
projects, including the major scientific and technological
specialized project sponsored by the National Natural Science
Foundation of China (no. 81001416); the Shanghai Science and
Technology Committee, China (no. 10JC1408902); the Research Fund
for Integrated Medicine and Engineering of Shanghai Jiao Tong
University (no. YG2011MS21); and the College subject of Shanghai
Ninth People's Hospital Affiliated to Shanghai Jiao Tong University
School of Medicine (no. 2012A13). The authors would also like to
thank the Shanghai Key Laboratory of Tissue Engineering (Shanghai,
China).
References
|
1
|
Wang Z, Hao G, Zhang L, Chen Z, Wang X,
Guo M, Tian Y, Shao L and Zhu M: Central systolic blood pressure is
associated with ethnicity and cardiovascular disease risk factors
in Chinese middle-aged population. Eur J Prev Cardiol. Mar
27–2015.Epub ahead of print. View Article : Google Scholar
|
|
2
|
Cano-Gutierrez C, Reyes-Ortiz CA,
Samper-Ternent R, Gélvez-Rueda JS and Borda MG: Prevalence and
factors associated to hypertension among older adults in Bogotá,
Colombia. J Aging Health. Mar 24–2015.Epub ahead of print.
View Article : Google Scholar
|
|
3
|
Mahn JJ, Dubey E, Brody A, Welch R,
Zalenski R, Flack JM, Ference B and Levy PD: Test characteristics
of electrocardiography for detection of left ventricular
hypertrophy in asymptomatic emergency department patients with
hypertension. Acad Emerg Med. 21:996–1002. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luft FC: Present status of genetic
mechanisms in hypertension. Med Clin North Am. 88:1–18. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kern G, Mair SM, Noppert SJ, Jennings P,
Schramek H, Rudnicki M, Mueller GA, Mayer G and Koppelstaetter C:
Tacrolimus increases Nox4 expression in human renal fibroblasts and
induces fibrosis-related genes by aberrant TGF-beta receptor
signalling. PLoS One. 9:e963772014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pandey VG, Jain S, Rana A, Puri N, Arudra
SK, Mopidevi B, Kaw M, Nasjletti A and Kumar A: Dexamethasone
promotes hypertension by allele-specific regulation of the human
angioten-sinogen gene. J Biol Chem. 290:5749–5758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qi Y, Zhang K, Wu Y, Xu Z, Yong QC, Kumar
R, Baker KM, Zhu Q, Chen S and Guo S: Novel mechanism of blood
pressure regulation by forkhead box class O1-mediated
transcriptional control of hepatic angiotensinogen. Hypertension.
64:1131–1140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alves M, Souza e Silva NA, Salis LH,
Pereira Bde B, Godoy PH, Nascimento EM and Oliveira JM: Survival
and predictive factors of lethality in hemodialysis: D/I
polymorphism of the angio-tensin I-converting enzyme and of the
angiotensinogen M235T genes. Arq Bras Cardiol. 103:209–219.
2014.PubMed/NCBI
|
|
9
|
Zhu X, Chang YP, Yan D, Weder A, Cooper R,
Luke A, Kan D and Chakravarti A: Associations between hypertension
and genes in the reninangiotensin system. Hypertension.
41:1027–1034. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kolder IC, Michels M, Christiaans I, Ten
Cate FJ, Majoor-Krakauer D, Danser AH, Lekanne Deprez RH, Tanck M,
Wilde AA, Bezzina CR, et al: The role of
renin-angiotensin-aldo-sterone system polymorphisms in phenotypic
expression of MYBPC3-related hypertrophic cardiomyopathy. Eur J Hum
Genet. 20:1071–1077. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu P, Wang Y, Sterner-Kock A, Bader M,
Schultheiss HP and Walther T: Excessive hypertension and end-organ
damage in a transgenic mouse line carrying the rat angiotensinogen
gene. J Cardiovasc Pharmacol. 53:38–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Makino N, Sugano M, Ohtsuka S and Sawada
S: Intravenous injection with antisense oligodeoxynucleotides
against angioten-sinogen decreases blood pressure in spontaneously
hypertensive rats. Hypertension. 31:1166–1170. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caulfield M, Lavender P, Farrall M, Munroe
P, Lawson M, Turner P and Clark AJ: Linkage of the angiotensinogen
gene to essential hypertension. N Engl J Med. 330:1629–1633. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng PF, Liu Y and Qin NP: Effect of sanwu
hypotensive decoction on blood pressure and lymphokine activated
killer cell in patient of primary hypertension and spontaneously
hypotensive rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 21:342–345.
2001.
|
|
15
|
Pinto YM, Paul M and Ganten D: Lessons
from rat models of hypertension: From Goldblatt to genetic
engineering. Cardiovasc Res. 39:77–88. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wright JW, Mizutani S, Murray CE, Amir HZ
and Harding JW: Aminopeptidase-induced elevations and reductions in
blood pressure in the spontaneously hypertensive rat. J Hypertens.
8:969–974. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lemmer B, Mattes A, Böhm M and Ganten D:
Circadian blood pressure variation in transgenic hypertensive rats.
Hypertension. 22:97–101. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee RM: Structural alterations of blood
vessels in hypertensive rats. Can J Physiol Pharmacol.
65:1528–1535. 1987. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rippe B, Lundin S and Folkow B: Plasma
volume, blood volume and transcapillary escape rate (TER) of
albumin in young spontaneously hypertensive rats (SHR) as compared
with normotensive controls (NCR). Clin Exp Hypertens. 1:39–50.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andresen MC, Krauhs JM and Brown AM:
Relationship of aortic wall and baroreceptor properties during
development in normotensive and spontaneously hypertensive rats.
Circ Res. 43:728–738. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xi-Ye Fang: Medical Experimental Zoology.
1st edition. People's Medical Publishing House; Beijing: pp.
p1211995
|
|
22
|
Wakui H, Tamura K, Masuda S, Tsurumi-Ikeya
Y, Fujita M, Maeda A, Ohsawa M, Azushima K, Uneda K, Matsuda M, et
al: Enhanced angiotensin receptor-associated protein in renal
tubule suppresses angiotensin-dependent hypertension. Hypertension.
61:1203–1210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nabeshima Y, Tazuma S, Kanno K, Hyogo H
and Chayama K: Deletion of angiotensin II type I receptor reduces
hepatic steatosis. J Hepatol. 50:1226–1235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yue H, Li W, Desnoyer R and Karnik SS:
Role of nuclear unphos-phorylated STAT3 in angiotensin II type 1
receptor-induced cardiac hypertrophy. Cardiovasc Res. 85:90–99.
2010. View Article : Google Scholar
|
|
25
|
Chen X, Qiu Z, Yang S, Ding D, Chen F,
Zhou Y, Wang M, Lin J, Yu X, Zhou Z, et al: Effectiveness and
safety of a therapeutic vaccine against angiotensin II receptor
type 1 in hypertensive animals. Hypertension. 61:408–416. 2013.
View Article : Google Scholar
|
|
26
|
He J, Bian Y, Gao F, Li M, Qiu L, Wu W,
Zhou H, Liu G and Xiao C: RNA interference targeting the ACE gene
reduced blood pressure and improved myocardial remodelling in SHRs.
Clin Sci (Lond). 116:249–255. 2009. View Article : Google Scholar
|
|
27
|
Lu P, Yuan L, Wang Y, Du Q and Sheng J:
Effect of GPE-AGT nanoparticle shRNA transfection system mediated
RNAi on early atherosclerotic lesion. Int J Clin Exp Pathol.
5:698–706. 2012.PubMed/NCBI
|
|
28
|
Gong H, Liu CM, Liu DP and Liang CC: The
role of small RNAs in human diseases: Potential troublemaker and
therapeutic tools. Med Res Rev. 25:361–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park TG, Jeong JH and Kim SW: Current
status of polymeric gene delivery systems. Adv Drug Deliv Rev.
58:467–486. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Srinivas R, Samanta S and Chaudhuri A:
Cationic amphiphiles: Promising carriers of genetic materials in
gene therapy. Chem Soc Rev. 38:3326–3338. 2009. View Article : Google Scholar
|
|
31
|
Mintzer MA and Simanek EE: Nonviral
vectors for gene delivery. Chem Rev. 109:259–302. 2009. View Article : Google Scholar
|
|
32
|
Yu H and Wagner E: Bioresponsive polymers
for nonviral gene delivery. Curr Opin Mol Ther. 11:165–178.
2009.PubMed/NCBI
|
|
33
|
Wang YQ, Su J, Wu F, Lu P, Yuan LF, Yuan
WE, Sheng J and Jin T: Biscarbamate cross-linked polyethylenimine
derivative with low molecular weight, low cytotoxicity, and high
efficiency for gene delivery. Int J Nanomedicine. 7:693–704.
2012.PubMed/NCBI
|
|
34
|
Lu P, Yuan L, Wang Y, Du Q and Sheng J:
Effect of GPE-AGT nanoparticle shRNA transfection system mediated
RNAi on early atherosclerotic lesion. Int J Clin Exp Pathol.
5:698–706. 2012.PubMed/NCBI
|
|
35
|
Wang Y, Su J, Cai W, Lu P, Yuan L, Jin T,
Chen S and Sheng J: Hepatocyte-targeting gene transfer mediated by
galactosylated poly(ethylene glycol)-graft-polyethylenimine
derivative. Drug Des Devel Ther. 7:211–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang X, Gao Y, Dong J, Wang S, Yao B,
Zhang J, Hu S, Xu X, Zuo H, Wang L, et al: The compound Chinese
medicine 'Kang Fu Ling' protects against high power
microwave-induced myocardial injury. PLoS One. 9:e1015322014.
View Article : Google Scholar
|
|
37
|
Silvani A, Bastianini S, Berteotti C,
Cenacchi G, Leone O, Lo Martire V, Papa V and Zoccoli G: Sleep and
cardiovascular phenotype in middle-aged hypocretin-deficient
narcoleptic mice. J Sleep Res. 23:98–106. 2014. View Article : Google Scholar
|
|
38
|
Li D, Lv J, Liu F, Liu P, Yang X, Feng Y,
Chen G and Hao M: Hypertension burden and control in mainland
China: Analysis of nationwide data 2003–2012. Int J Cardiol.
184:637–644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khandelia P, Yap K and Makeyev EV:
Streamlined platform for short hairpin RNA interference and
transgenesis in cultured mammalian cells. Proc Natl Acad Sci USA.
108:12799–12804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maillard PV, Ciaudo C, Marchais A, Li Y,
Jay F, Ding SW and Voinnet O: Antiviral RNA interference in
mammalian cells. Science. 342:235–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, Lu J, Han Y, Fan X and Ding SW: RNA
interference functions as an antiviral immunity mechanism in
mammals. Science. 342:231–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lebedev TD, Spirin PV and Prassolov VS:
Transfer and expression of small interfering RNAs in mammalian
cells using lentiviral vectors. Acta Naturae. 5:7–18.
2013.PubMed/NCBI
|
|
43
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pare JM, LaPointe P and Hobman TC: Hsp90
cochaperones p23 and FKBP4 physically interact with hAgo2 and
activate RNA interference-mediated silencing in mammalian cells.
Mol Biol Cell. 24:2303–2310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Paddison PJ: RNA interference in mammalian
cell systems. Curr Top Microbiol Immunol. 320:1–19. 2008.PubMed/NCBI
|
|
46
|
Dalmay T: MicroRNAs and cancer. J Intern
Med. 263:366–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ohkumo T, Masutani C, Eki T and Hanaoka F:
Use of RNAi in C. elegans. Methods Mol Biol. 442:129–137. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang DD: The potential of RNA
interference-based therapies for viral infections. Curr HIV/AIDS
Rep. 5:33–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen Y, Cheng G and Mahato RI: RNAi for
treating hepatitis B viral infection. Pharm Res. 25:72–86. 2008.
View Article : Google Scholar :
|
|
50
|
Volarevic M, Smolic R, Wu CH and Wu GY:
Potential role of RNAi in the treatment of HCV infection. Expert
Rev Anti Infect Ther. 5:823–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Singh SK: RNA interference and its
therapeutic potential against HIV infection. Expert Opin Biol Ther.
8:449–461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ying SY and Lin SL: Current perspectives
in intronic micro RNAs (miRNAs). J Biomed Sci. 13:5–15. 2006.
View Article : Google Scholar
|
|
53
|
Chen D, Hazelwood L, Walker LL, Oldfield
BJ, McKinley MJ and Allen AM: Changes in angiotensin type 1
receptor binding and angiotensin-induced pressor responses in the
rostral ventrolateral medulla of angiotensinogen knockout mice. Am
J Physiol Regul Integr Comp Physiol. 298:R411–R418. 2010.
View Article : Google Scholar
|