Introduction
Excessive estrogen exposure is a critical risk
factor for breast cancer (1–7).
While the ovary is the major site of estrogen biosynthesis in
premenopausal women, adipose stromal cells (ASCs) in the breast are
an important source of locally produced estrogen. Estrogens
produced in distal adipose tissue and within the breast tissue
influence the growth of breast epithelial cells (8). Especially, excessive local estrogen
production in the breast promotes estrogen-dependent breast cancer.
At the molecular level, tumor cells secrete factors such as
prostaglandin E2 (PGE2). PGE2
stimulates stromal expression of aromatase, a key enzyme in
estrogen biosynthesis (9). The
breast quadrant bearing a malignant tumor shows consistently high
levels of aromatase activity (10), and breast adipose tissue adjacent
to the tumor shows a marked increase in aromatase expression and
activity (11–13). The clinically proven efficacy of
aromatase inhibitors (AIs) in treating estrogen receptor-positive
(ER+) post-menopausal breast cancer patients highlights
the important role of excessive local estrogen production in breast
cancer development. However, AIs indiscriminately reduce estrogen
synthesis throughout the body, causing major side-effects including
bone loss, increased fracture rates, and abnormal lipid metabolism
(14–16). Specific inhibitors that selectively
inhibit aromatase expression in the tumor micro-environment would
considerably benefit patients, by reducing side-effects. In
addition, the aromatase promoters I.3 and PII have been reported to
be activated in tumor, but not in healthy tissues (8). This implies that specific inhibition
of pathways that lead to the activation of these promoters may
specifically inhibit aromatase expression in the tumor tissue. In
order to identify these pathways, the mechanism of regulation of
aromatase expression needs to be elucidated.
Transcription of the aromatase gene is controlled by
a number of tissue- and cell type-specific promoters that are
located upstream of the aromatase coding region. The coding region
of the aromatase transcripts is identical in different tissues, but
a noncoding exon 1 is transcribed in a tissue-specific manner and
spliced with the common coding exons (9,17–19).
In cancer-free breast adipose tissue, the aromatase gene is mainly
transcribed under control of the relatively weak I.4 promoter, with
a small amount of aromatase mRNA coming from ovary-specific
promoters I.3 and PII. However, in ASCs adjacent to the breast
tumor, aromatase expression is activated via the proximally-located
promoters I.3 and PII (8). The
switch in promoter utilization from the weak I.4 to the stronger
I.3 and PII results in elevated aromatase expression and excessive
production of local estrogen (20,21).
Several lines of evidence indicate that
PGE2 is involved in breast cancer development and
progression (22–24). In the tumor microenvironment,
PGE2 activates the protein kinase A and C (PKA and PKC)
pathways and induces aromatase expression via promoters I.3 and PII
in adjacent ASCs (25). In
PGE2-treated ASCs, the aromatase mRNA level was found to
be markedly increased, and phosphorylation of a number of signaling
proteins was observed. These signaling proteins include PKA, PKC,
transforming growth factor-β-activated kinase-1 (TAK1),
mitogen-activated protein kinase kinase 4 (MMK4), c-Jun
NH2-terminal kinase 1 (JNK-1) and mitogen-activated
protein kinase (MAPK) p38 (26).
This leads to phosphorylation of members of the Jun family of
transcription factors and of the activating transcription factor
(ATF) 2 protein. The phosphorylated Jun and ATF transcription
factors directly bind to the promoter I.3 and PII to regulate
aromatase gene expression (26,27).
Inhibition of p38 or JNK1 by respective inhibitors effectively the
blocked PGE2-induced aromatase expression. Consistently,
knockdown of p38, JNK1, JunB or JunD by small interfering RNA
(siRNA) also blocked PGE2-induced aromatase expression
(28). Therefore,
PGE2-induced pathways play important roles in regulation
of aromatase expression in the tumor microenvironment.
It has been shown that mechanical changes, including
elevated extracellular matrix (ECM) stiffness and increased
interstitial pressures, are associated with epithelial carcinomas.
Moreover, mechanical force due to altered architecture in the
tissue microenvironment can affect gene expression patterns
(29–34). In breast cancer, mechanical force
significantly affects the invasive behavior of tumor cells, as well
as breast cancer incidence and mortality (35–39).
In this study, we used a collagen 3D culture system
to investigate the signaling pathways involved in regulation of
aromatase expression. Mammographically dense breast tissue is
linked to increased risk of breast carcinoma (40,41).
Areas of increased breast density are not only associated with
increased epithelial and stromal cellularity, but also
significantly increased fibrillar collagen deposition (42–45).
It was also shown that increased stromal collagen in mouse mammary
tissue significantly increases tumor formation and results in a
significantly enhanced invasive phenotype (46). However, the mechanisms driving
collagen-related breast tumor formation and progression remain
largely unknown. Initiation of collagen-induced signals is mediated
by a batch of cell-surface receptors, including integrins,
discoidin domain receptors, glycoprotein VI, leukocyte-associated
immunoglobin-like receptor-1, and members of the mannose receptor
family (47). We are interested in
two collagen receptors, α2β1 integrin and discoidin domain receptor
1. These two receptors propagate signals through separate, as well
as shared pathways. One pathway is initiated by the integrin-linked
kinase (ILK), which passes the signal to the IκB kinase (IKK) β via
phosphatidylinositide 3-kinase (PI3K)/AKT (48–51).
Another pathway is the MAPK pathway, including
extracellular-signal-regulated kinase (ERK)1/2, JNK and p38
(48–55). It has been shown that IKKβ is
involved in cell shape-induced aromatase expression, while MAPK
pathways are involved in PGE2-induced aromatase
expression (26,28,56).
Based on this, we hypothesized that collagen may induce aromatase
expression through these pathways and that there may be a crosstalk
between collagen- and PGE2-induced signaling pathways.
In this study, we investigated the signaling pathways involved in
collagen- and PGE2-induced aromatase expression. This
study will contribute to the understanding of the mechanism of
regulation of aromatase expression by collagen and PGE2.
Our study provides useful insights for the design of selective
inhibitors of aromatase in the context of breast cancer via the
inhibition of specific signaling pathways.
Materials and methods
Cell culture
Primary human ASCs were isolated from individuals
undergoing elective surgical procedures at the Department of Breast
Surgery, General Hospital of Nanjing Military Region (Fuzhou,
China). Informed consent was obtained and this study was approved
by the Ethics Committee of the General Hospital of Nanjing Military
Region (Fuzhou, China) The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM)/nutrient mixture F-12 (Gibco
Industries, Inc., Big Cabin, OK, USA), supplemented with 10% fetal
bovine serum (FBS; Hyclone, Lawrenceville, GA, USA) and 1%
antibiotic-antimycotic solution (Gibco Industries, Inc.). The 3D
culture was performed in 24-well plates using collagen (collagen
bovine type I; BD Biosciences, San Jose, CA, USA). Briefly,
2×105 cells were suspended in 125 µl medium and
mixed with 125 µl collagen. Following the formation of a
gel-like 3D structure, additional medium was added to the top.
Chemicals and treatment
PGE2 was purchased from Cayman Chemicals
(Ann Arbor, MI, USA). The PI3K inhibitor LY-294002 was purchased
from Sigma-Aldrich (St. Louis, MO, USA). The mitogen-activated
protein kinase kinase (MEK)1/2 inhibitor U0126, JNK inhibitor
AS601245, p38 inhibitor SB202190, and IKK inhibitor BAY11-7082 were
purchased from Calbiochem (La Jolla, CA, USA). The PKA inhibitor
H89 dihydrochloride was purchased from Cell Signaling Technology
(Danvers, MA, USA). ASCs were serum-starved for 16 h and exposed to
collagen or PGE2 with and without inhibitors for 12 h.
Samples were harvested for RNA analysis. Chemical concentrations
used in this study are shown in the figure legends.
Quantitative (q) and semi-quantitative
reverse transcription-polymerase chain reaction (RT-PCR)
Cells in collagen were released by using collagenase
(Sigma-Aldrich). Total RNA was isolated using the Invitrogen™
TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA)
according to the manufacturer's instructions. The RNA concentration
was measured using a NanoDrop2000 (Thermo Fisher Scientific), and
RNA was reverse-transcribed using the ImPrompII™ kit (Promega,
Madison, WI, USA). qPCR was carried out using the fluorescent dye
SYBR-Green (Applied Biosystems, Foster City, CA, USA) on an ABI
7900 Real-Time PCR system (Applied Biosystems). The primers for
amplification of aromatase were: forward,
5′-TGGAATTATGAGGGCACATCC-3′, and reverse,
5′-GTCCAATTCCCATGCAGTAGC-3′. Semi-quantitative RT-PCR was performed
using primer pairs that are specific to the promoters I.4, I.3 and
PII of the aromatase transcripts. The glyceraldehyde 3-phosphate
dehydrogenase gene (GAPDH) served as an internal control.
The forward primers used were: promoter I.3,
5′-CCTTGTTTTGACTTGTAACCA-3′; promoter I.4,
5′-GTAGAACGTGACCAACTGG-3′; and promoter PII,
5′-GCAACAGGAGCTATAGAT-3′. The reverse primer for all three
promoters was 5′-ATTCCCATGCAGTAGCCAGG-3′. The GAPDH primers were:
forward, 5′-CCATCAATGACCCCTTCATTG-3′, and reverse,
5′-GACGGTGCCATGGAATTT-3′. The PCR reaction was performed using
reagents from the PCR Master Mix kit (Promega). The PCR cycling
conditions were as follows: 94°C for 2 min (1 cycle), 94°C for 30
sec, 56°C for 30 sec, 72°C for 1 min (35 cycles), 72°C for 10 min
(1 cycle), and hold at 4°C.
siRNA knockdown
Gene-specific knockdown by siRNA oligonucleotides
was conducted using the Invitrogen™ Lipofectamine®
RNAiMAX reagent (Thermo Fisher Scientific). Briefly, cells at an
approximate density of 60% were transfected with siRNA oligos at a
final concentration of 10 nM. Cells were trypsinized 24 h after
transfection using 0.25% Trypsin-EDTA (Gibco Industries, Inc.),
plated in subconfluent condition, and cultured in collagen. Cells
were harvested for RNA analysis 12 h after plating. c-Jun, JunB,
AKT2 and control siRNA oligos were purchased from Dharmacon, Inc.
(Lafayette, CO, USA).
Statistics and data analysis
A paired t-test was used for pairwise comparisons of
chemical-treated cells to control cells. Aromatase mRNA levels from
independent measurements were collected for data analysis The data
were analyzed using the SPSS 18.0 software (International Business
Machines, Armonk, NY, USA).
Results
Collagen and PGE2 induce
aromatase expression in adipose stromal cells
Collagen is a major component of the ECM and
contributes to the formation of mechanical force in the tissue
microenvironment. Mechanical force influences cell shape, as well
as gene expression patterns (35,53,57,58).
Furthermore, IKKβ is a downstream signaling molecule of
collagen-induced signaling pathways that may play the same role in
regulating aromatase expression. Based on these findings, we
decided to test whether collagen can induce aromatase expression in
ASCs.
Collagen-induced aromatase expression was revealed
to be nearly 100-fold higher in ASCs compared to control ASCs that
grew on 2D dishes (Fig. 1A). Data
from semi-quantitative RT-PCR showed that collagen-induced
aromatase expression involves the promoters I.3 and PII (Fig. 1C). PGE2-treated ASCs
also showed induced aromatase expression through promoters I.3 and
PII (Fig. 1B and D), in accordance
with a previous study (26). In
conclusion, both collagen and PGE2 can induce aromatase
expression via promoters I.3 and PII in adipose stromal cells.
MAP kinase pathways are involved in
collagen- and PGE2-induced aromatase expression
It has been shown that collagen activates MAP
kinases, including ERK1/2, JNK and p38 (48–55).
In addition, MAP kinases are induced by PGE2 in ASCs
(26). Furthermore,
PGE2 induces aromatase expression in ASCs through JNK
and p38 (26). Therefore, we
hypothesized that collagen may induce aromatase expression by
activating MAP kinase pathways. Collagen-induced aromatase
expression was found significantly inhibited by the MEK inhibitor
U0126, which leads to reduced ERK phosphorylation (Fig. 2A). Collagen-induced aromatase
expression was also significantly inhibited by the JNK inhibitor
AS601245, but not by the p38 inhibitor SB202190. PGE2-induced
aromatase expression was significantly inhibited by all three
inhibitors (Fig. 2B).
Semi-quantitative RT-PCR confirmed these results (Fig. 2C and D). Therefore, we conclude
that the MAP kinases ERK1/2 and JNK are involved in
collagen-induced aromatase expression, while ERK1/2, JNK and p38
are all involved in PGE2-induced aromatase
expression.
 | Figure 2Collagen- and prostaglandin
E2 (PGE2)-induced aromatase expression is
inhibited by mitogen-activated protein (MAP) kinase inhibitors. (A
and B) Relative aromatase mRNA level was measured by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
after drug treatment. AS601245 (or AS; 7.5 µM), c-Jun
NH2-terminal kinase (JNK) inhibitor; SB202190 (or SB; 5
µM), p38 inhibitor; U0126 (10 µM), mitogen-activated
protein kinase kinase (MEK) inhibitor. Data come from three
independent measurements for each condition. (C and D) Promoter
utilization was tested by semi-quantitative RT-PCR. (E) siRNA
knockdown of JunB significantly reduces collagen-induced
aromatase expression, measured by RT-qPCR. The knockdown experiment
was performed in the presence of collagen. SC-OTP, subconfluent
control; OTP, collagen control; c-Jun, knockdown of c-Jun;
JunB, knockdown of JunB in adipose stromal cells. (F)
Semi-quantitative RT-PCR was performed using the knockdown samples.
I.3, aromatase promoter I.3; PII, aromatase promoter II. Bars in
(A, B and E) denote standard error (SE). *P<0.005
compared to the controls collagen™, dimethylsulfoxide
(DMSO) and PGE2, respectively. |
Since transcription factors of the Jun family act
downstream of the MAP kinase pathways, and previous data from our
laboratory, as well as other studies showed that the Jun family
plays important roles in regulating aromatase expression (26,28,59,60),
we knocked down c-Jun and JunB by siRNA to determine
whether these molecules have an effect on aromatase expression.
Knockdown of JunB significantly reduced collagen-induced
aromatase expression. Knockdown of c-Jun did not reduce
collagen-induced aromatase expression in our experiments (Fig. 2E). However, we observed that the
c-Jun knockdown was not as efficient as the JunB one
in terms of protein expression (data not shown). Semi-quantitative
RT-PCR confirmed these results and showed that the JunB
knockdown affects aromatase expression via both promoters I.3 and
PII (Fig. 2F). In conclusion, JunB
may be involved in collagen-induced aromatase expression by
regulating the aromatase promoters I.3 and PII.
Cell-ECM communications are predominantly mediated
by integrins, and integrin-mediated signaling events can activate
PI3K/AKT (61,62). On the other hand, AKT is physically
associated with IKKβ and can trigger phosphorylation of IKK in
response to inflammatory factors such as tumor necrosis factor-α
(63,64). We therefore hypothesized that AKT
may play an important role in passing signals from ECM to
downstream IKK, and thus, in activating aromatase expression. There
are three AKT genes in the human genome: AKT1,
AKT2 and AKT3. These genes code for enzymes that
belong to the serine/threonine-specific protein kinase family
(65–67). Knockdown of AKT2
significantly reduced collagen-induced aromatase expression
(Fig. 3E). Data from
semi-quantitative RT-PCR confirmed these results and showed that
knockdown of AKT2 affects both promoters I.3 and II
(Fig. 3F). These data indicates
that collagen induces aromatase expression at least partly via the
PI3K/AKT/IKK pathway.
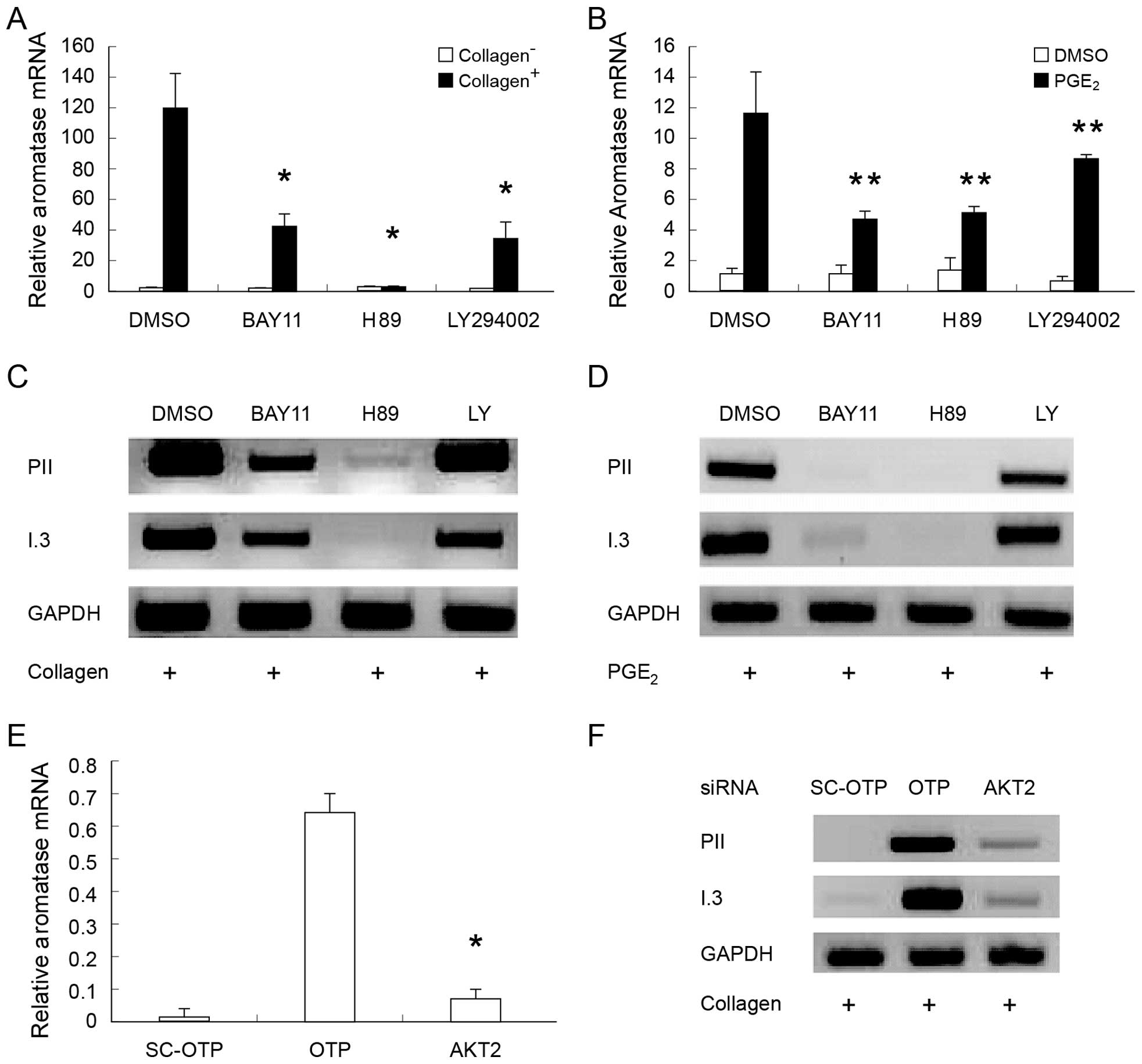 | Figure 3Collagen- and prostaglandin
E2 (PGE2)-induced aromatase expression is
inhibited by the IκB kinase (IKK), protein kinase A (PKA) and
phosphati-dylinositide 3-kinase (PI3K) inhibitors. (A and B) The
relative aromatase mRNA level was measured by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
after drug treatment. Data come from from three independent
measurements for each condition. BAY11-7082 (5 µM), IKK
inhibitor; LY294002 (or LY; 20 µM), PI3K inhibitor; H89 (20
µM), PKA inhibitor. (C and D) Promoter utilization was
tested by semi-quantitative RT-PCR. (E) AKT2 knockdown
significantly reduces collagen-induced aromatase expression,
measured by RT-qPCR. The knockdown experiment was performed in the
presence of collagen. SC-OTP, subconfluent control; OTP, collagen
control; AKT2, knockdown of AKT2 in adipose stromal cells.
(F) Semi-quantitative RT-PCR was performed using the knockdown
samples. I.3, aromatase promoter I.3; PII, aromatase promoter II.
Bars in (A, B and E) denote standard error (SE).
*P<0.005 and **P<0.05, compared to the
controls collagen™, dimethylsulfoxide (DMSO) and PGE2,
respectively. |
Next, we tested whether PKA is involved in
collagen-induced aromatase expression, since it has been shown to
be involved in PGE2-induced aromatase expression
(26,68). Our data confirmed the important
role of PKA in regulating PGE2-induced aromatase
expression (Fig. 3B). In addition,
our data showed that the PKA inhibitor H89 significantly inhibits
collagen-induced aromatase expression (Fig. 3A). The RT-qPCR results were
confirmed by semi-quantitative RT-PCR (Fig. 3C and D). Taken together, these
findings strongly suggest that PKA plays an important role in
PGE2-, as well as in collagen-induced aromatase
expression.
Discussion
Excessive exposure to estrogen is a critical risk
factor for breast cancer, while aromatase is a key enzyme
controlling estrogen production. Currently, AIs are the most
effective endocrine treatment for breast cancer (8,69).
However, AIs indiscriminately reduce estrogen synthesis throughout
the body, causing major side-effects (14–16).
To address this issue, new drug targets need to be identified,
allowing to selectively block aromatase expression in the tumor
microenvironment. To this end, it is important to dissect the
mechanisms by which aromatase expression is regulated. Data from
this study showed that collagen-induced aromatase expression in
ASCs is significantly reduced by inhibitors of PI3K, IKK, MEK, JNK,
PKA and the knockdown of the JunB and AKT2 genes.
These findings indicate that the PI3K/AKT/IKK, as well as the MAP
kinase pathways play important roles in collagen-induced aromatase
expression. On the other hand, PGE2-induced aromatase
expression was significantly reduced by inhibitors of IKK, MEK,
JNK, p38 and PKA. Data from semi-quantitative RT-PCR showed that
collagen and PGE2 induce aromatase expression through
the aromatase promoters I.3 and PII. In addition, the
semi-quantitative data were consistent with quantitative RT-PCR
data in the drug treatment and the knockdown experiments.
Data from this study also indicated that there is a
crosstalk between collagen and PGE2-induced signaling
pathways in regulating aromatase expression. Signaling molecules
that are responsible for this crosstalk could serve as potential
drug targets. Signals induced by both PGE2 and collagen
could be blocked by hitting the targets that are common to both
pathways involved in regulating collagen and
PGE2-induced expression, which may effectively silence
the aromatase promoters. In addition to these
shared-between-pathways molecules, our data also highlighted a
number of proteins that are unique to one of the two
aromatase-activating pathways. For example, p38 plays a critical
role in PGE2-induced but not in collagen-induced
aromatase expression. PI3K is another example, since this protein
plays a critical role in collagen-induced, but not in
PGE2-induced aromatase expression.
In the present study, PGE2-induced
aromatase expression was inhibited by a MEK inhibitor. This
indicates that the ERK pathway may be involved in regulating
aromatase expression in response to PGE2 induction. Data
from another study showed that the same MEK inhibitor has no
effects on PGE2-induced aromatase expression (26). This discrepancy could be due to the
use of different cells in the two studies. Since regulation of gene
expression is complex, the signaling pathways involved in
regulating expression are only one facet of the process. Other
factors likely contribute to aromatase expression as well. For
instance, the DNA methylation status of the aromatase promoter
region also plays important roles in regulating aromatase
expression. In another project of our research team, a DNA
methylation assay was performed to test the methylation status of
aromatase promoters in a batch of adipose stromal cells. Our data
showed that the highest DNA methylation load of certain CpG sites
corresponds to the lowest aromatase activation. Reduction of the
DNA methylation load by drug treatment restored aromatase
responsiveness to forskolin activation (unpublished data). Another
study also showed that CpG dinucleotide methylation of the
aromatase promoter modulates cAMP-stimulated aromatase activity
(70).
This study elucidated the signaling pathways
involved in collagen- and PGE2-induced aromatase
expression in adipose stromal cells. It is thus expected to shed
light on the mechanisms of aromatase activation in response to
collagen, as well as PGE2. This study provides an
opportunity to test whether breast cancer can be effectively
treated by selectively blocking aromatase expression via the
inhibition of the specific signaling pathways identified herein.
Further investigation is however needed to identify the downstream
transcription factors and additional molecular targets for
selective inhibition of aromatase expression.
References
|
1
|
Ewertz M: Influence of non-contraceptive
exogenous and endogenous sex hormones on breast cancer risk in
Denmark. Int J Cancer. 42:832–838. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nass SJ and Davidson NE: The biology of
breast cancer. Hematol Oncol Clin North Am. 13:311–332. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okobia MN and Bunker CH: Estrogen
metabolism and breast cancer risk - a review. Afr J Reprod Health.
10:13–25. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meyer F, Brown JB, Morrison AS and
MacMahon B: Endogenous sex hormones, prolactin, and breast cancer
in premenopausal women. J Natl Cancer Inst. 77:613–616.
1986.PubMed/NCBI
|
|
5
|
Key T, Appleby P, Barnes I and Reeves G;
Endogenous Hormones and Breast Cancer Collaborative Group:
Endogenous sex hormones and breast cancer in postmenopausal women:
reanalysis of nine prospective studies. J Natl Cancer Inst.
94:606–616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Onland-Moret NC, Kaaks R, van Noord PA, et
al: Urinary endogenous sex hormone levels and the risk of
postmenopausal breast cancer. Br J Cancer. 88:1394–1399. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Biggar RJ: Molecular pathways: digoxin use
and estrogen-sensitive cancers - risks and possible therapeutic
implications. Clin Cancer Res. 18:2133–2137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bulun SE, Lin Z, Imir G, et al: Regulation
of aromatase expression in estrogen-responsive breast and uterine
disease: from bench to treatment. Pharmacol Rev. 57:359–383. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simpson ER and Davis SR: Minireview:
aromatase and the regulation of estrogen biosynthesis - some new
perspectives. Endocrinology. 142:4589–4594. 2001.PubMed/NCBI
|
|
10
|
O'Neill JS, Elton RA and Miller WR:
Aromatase activity in adipose tissue from breast quadrants: a link
with tumour site. Br Med J (Clin Res Ed). 296:741–743. 1988.
View Article : Google Scholar
|
|
11
|
Bulun SE, Price TM, Aitken J, Mahendroo MS
and Simpson ER: A link between breast cancer and local estrogen
biosynthesis suggested by quantification of breast adipose tissue
aromatase cytochrome P450 transcripts using competitive polymerase
chain reaction after reverse transcription. J Clin Endocrinol
Metab. 77:1622–1628. 1993.PubMed/NCBI
|
|
12
|
Harada N: Aberrant expression of aromatase
in breast cancer tissues. J Steroid Biochem Mol Biol. 61:175–184.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sasano H and Harada N: Intratumoral
aromatase in human breast, endometrial, and ovarian malignancies.
Endocr Rev. 19:593–607. 1998.PubMed/NCBI
|
|
14
|
Morales L, Neven P and Paridaens R:
Choosing between an aromatase inhibitor and tamoxifen in the
adjuvant setting. Curr Opin Oncol. 17:559–565. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Umar A, Dunn BK and Greenwald P: Future
directions in cancer prevention. Nat Rev Cancer. 12:835–848. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tomao F, Spinelli G, Vici P, et al:
Current role and safety profile of aromatase inhibitors in early
breast cancer. Expert Rev Anticancer Ther. 11:1253–1263. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamat A, Hinshelwood MM, Murry BA and
Mendelson CR: Mechanisms in tissue-specific regulation of estrogen
biosynthesis in humans. Trends Endocrinol Metab. 13:122–128. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bulun SE, Sebastian S, Takayama K, Suzuki
T, Sasano H and Shozu M: The human CYP19 (aromatase P450) gene:
update on physiologic roles and genomic organization of promoters.
J Steroid Biochem Mol Biol. 86:219–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stocco C: Tissue physiology and pathology
of aromatase. Steroids. 77:27–35. 2012. View Article : Google Scholar :
|
|
20
|
Meng L, Zhou J, Sasano H, Suzuki T,
Zeitoun KM and Bulun SE: Tumor necrosis factor alpha and
interleukin 11 secreted by malignant breast epithelial cells
inhibit adipocyte differentiation by selectively down-regulating
CCAAT/enhancer binding protein alpha and peroxisome
proliferator-activated receptor gamma: mechanism of desmoplastic
reaction. Cancer Res. 61:2250–2255. 2001.PubMed/NCBI
|
|
21
|
Zhou J, Gurates B, Yang S, Sebastian S and
Bulun SE: Malignant breast epithelial cells stimulate aromatase
expression via promoter II in human adipose fibroblasts: an
epithelial-stromal interaction in breast tumors mediated by
CCAAT/enhancer binding protein beta. Cancer Res. 61:2328–2334.
2001.PubMed/NCBI
|
|
22
|
Purohit A, Ghilchik MW, Duncan L, et al:
Aromatase activity and interleukin-6 production by normal and
malignant breast tissues. J Clin Endocrinol Metab. 80:3052–3058.
1995.PubMed/NCBI
|
|
23
|
Schrey MP and Patel KV: Prostaglandin
E2 production and metabolism in human breast cancer
cells and breast fibroblasts. Regulation by inflammatory mediators.
Br J Cancer. 72:1412–1419. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang SH, Liu CH, Conway R, et al: Role of
prostaglandin E2-dependent angiogenic switch in cyclooxygenase
2-induced breast cancer progression. Proc Natl Acad Sci USA.
101:591–596. 2004. View Article : Google Scholar :
|
|
25
|
Zhao Y, Agarwal VR, Mendelson CR and
Simpson ER: Estrogen biosynthesis proximal to a breast tumor is
stimulated by PGE2 via cyclic AMP, leading to activation
of promoter II of the CYP19 (aromatase) gene. Endocrinology.
137:5739–5742. 1996.PubMed/NCBI
|
|
26
|
Chen D, Reierstad S, Lin Z, et al:
Prostaglandin E2 induces breast cancer related aromatase
promoters via activation of p38 and c-Jun NH-terminal kinase in
adipose fibroblasts. Cancer Res. 67:8914–8922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bulun SE, Lin Z, Zhao H, et al: Regulation
of aromatase expression in breast cancer tissue. Ann NY Acad Sci.
1155:121–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen D, Reierstad S, Fang F and Bulun SE:
JunD and JunB integrate prostaglandin E activation of breast
cancer-associated proximal aromatase promoters. Mol Endocrinol.
25:767–775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Avvisato CL, Yang X, Shah S, et al:
Mechanical force modulates global gene expression and beta-catenin
signaling in colon cancer cells. J Cell Sci. 120:2672–2682. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shyy JY and Chien S: Role of integrins in
endothelial mechanosensing of shear stress. Circ Res. 91:769–775.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alenghat FJ and Ingber DE:
Mechanotransduction: all signals point to cytoskeleton, matrix, and
integrins. Sci STKE. 119:pe62002.
|
|
32
|
Lopez JI, Mouw JK and Weaver VM:
Biomechanical regulation of cell orientation and fate. Oncogene.
27:6981–6993. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nelson CM and Bissell MJ: Of extracellular
matrix, scaffolds, and signaling: tissue architecture regulates
development, homeostasis, and cancer. Annu Rev Cell Dev Biol.
22:287–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Provenzano PP and Keely PJ: Mechanical
signaling through the cytoskeleton regulates cell proliferation by
coordinated focal adhesion and Rho GTPase signaling. J Cell Sci.
124:1195–1205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Paszek MJ and Weaver VM: The tension
mounts: mechanics meets morphogenesis and malignancy. J Mammary
Gland Biol Neoplasia. 9:325–342. 2004. View Article : Google Scholar
|
|
36
|
Ingber DE: Can cancer be reversed by
engineering the tumor microenvironment? Semin Cancer Biol.
18:356–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Le Beyec J, Xu R, Lee SY, et al: Cell
shape regulates global histone acetylation in human mammary
epithelial cells. Exp Cell Res. 313:3066–3075. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wiseman BS and Werb Z: Stromal effects on
mammary gland development and breast cancer. Science.
296:1046–1049. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Plodinec M and Schoenenberger CA: Spatial
organization acts on cell signaling: how physical force contributes
to the development of cancer. Breast Cancer Res. 12:3082010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McCormack VA and dos Santos Silva I:
Breast density and parenchymal patterns as markers of breast cancer
risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev.
15:1159–1169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boyd NF, Martin LJ, Stone J, Greenberg C,
Minkin S and Yaffe MJ: Mammographic densities as a marker of human
breast cancer risk and their use in chemoprevention. Curr Oncol
Rep. 3:314–321. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hawes D, Downey S, Pearce CL, et al: Dense
breast stromal tissue shows greatly increased concentration of
breast epithelium but no increase in its proliferative activity.
Breast Cancer Res. 8:R242006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo YP, Martin LJ, Hanna W, et al: Growth
factors and stromal matrix proteins associated with mammographic
densities. Cancer Epidemiol Biomarkers Prev. 10:243–248.
2001.PubMed/NCBI
|
|
44
|
Alowami S, Troup S, Al-Haddad S,
Kirkpatrick I and Watson PH: Mammographic density is related to
stroma and stromal proteoglycan expression. Breast Cancer Res.
5:R129–R135. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li T, Sun L, Miller N, et al: The
association of measured breast tissue characteristics with
mammographic density and other risk factors for breast cancer.
Cancer Epidemiol Biomarkers Prev. 14:343–349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Provenzano PP, Inman DR, Eliceiri KW, et
al: Collagen density promotes mammary tumor initiation and
progression. BMC Med. 6:112008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Leitinger B and Hohenester E: Mammalian
collagen receptors. Matrix Biol. 26:146–155. 2007. View Article : Google Scholar
|
|
48
|
Shintani Y, Fukumoto Y, Chaika N, Svoboda
R, Wheelock MJ and Johnson KR: Collagen I-mediated up-regulation of
N-cadherin requires cooperative signals from integrins and
discoidin domain receptor 1. J Cell Biol. 180:1277–1289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shintani Y, Maeda M, Chaika N, Johnson KR
and Wheelock MJ: Collagen I promotes epithelial-to-mesenchymal
transition in lung cancer cells via transforming growth factor-beta
signaling. Am J Respir Cell Mol Biol. 38:95–104. 2008. View Article : Google Scholar
|
|
50
|
Shintani Y, Hollingsworth MA, Wheelock MJ
and Johnson KR: Collagen I promotes metastasis in pancreatic cancer
by activating c-Jun NH2-terminal kinase 1 and
up-regulating N-cadherin expression. Cancer Res. 66:11745–11753.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shintani Y, Wheelock MJ and Johnson KR:
Phosphoinositide-3 kinase-Rac1-c-Jun NH -terminal kinase signaling
mediates collagen I-induced cell scattering and up-regulation of
N-cadherin expression in mouse mammary epithelial cells. Mol Biol
Cell. 17:2963–2975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wheelock MJ and Johnson KR:
Cadherin-mediated cellular signaling. Curr Opin Cell Biol.
15:509–514. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Plant AL, Bhadriraju K, Spurlin TA and
Elliott JT: Cell response to matrix mechanics: focus on collagen.
Biochim Biophys Acta. 1793:893–902. 2009. View Article : Google Scholar
|
|
54
|
Zhu X and Assoian RK: Integrin-dependent
activation of MAP kinase: a link to shape-dependent cell
proliferation. Mol Biol Cell. 6:273–282. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang L, Bewick M and Lafrenie RM: Role of
Raf-1 and FAK in cell density-dependent regulation of
integrin-dependent activation of MAP kinase. Carcinogenesis.
23:1251–1258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ghosh S, Choudary A, Musi N, Hu Y and Li
R: IKKbeta mediates cell shape-induced aromatase expression and
estrogen biosynthesis in adipose stromal cells. Mol Endocrinol.
23:662–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee EY, Parry G and Bissell MJ: Modulation
of secreted proteins of mouse mammary epithelial cells by the
collagenous substrata. J Cell Biol. 98:146–155. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kass L, Erler JT, Dembo M and Weaver VM:
Mammary epithelial cell: influence of extracellular matrix
composition and organization during development and tumorigenesis.
Int J Biochem Cell Biol. 39:1987–1994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ghosh S, Wu Y, Li R and Hu Y: Jun proteins
modulate the ovary-specific promoter of aromatase gene in ovarian
granulosa cells via a cAMP-responsive element. Oncogene.
24:2236–2246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hu YF and Li R: JunB potentiates function
of BRCA1 activation domain 1 (AD1) through a coiled-coil-mediated
interaction. Genes Dev. 16:1509–1517. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Basson MD: An intracellular signal pathway
that regulates cancer cell adhesion in response to extracellular
forces. Cancer Res. 68:2–4. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Brancaccio M, Hirsch E, Notte A,
Selvetella G, Lembo G and Tarone G: Integrin signalling: the
tug-of-war in heart hypertrophy. Cardiovasc Res. 70:422–433. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Häcker H and Karin M: Regulation and
function of IKK and IKK-related kinases. Sci STKE.
357:re132006.
|
|
64
|
Bai D, Ueno L and Vogt PK: Akt-mediated
regulation of NFkappaB and the essentialness of NFkappaB for the
oncogenicity of PI3K and Akt. Int J Cancer. 125:2863–2870. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Staal SP, Hartley JW and Rowe WP:
Isolation of transforming murine leukemia viruses from mice with a
high incidence of spontaneous lymphoma. Proc Natl Acad Sci USA.
74:3065–3067. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Staal SP: Molecular cloning of the akt
oncogene and its human homologues AKT1 and AKT2: amplification of
AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci
USA. 84:5034–5037. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang ZZ, Tschopp O, Baudry A, Dümmler B,
Hynx D and Hemmings BA: Physiological functions of protein kinase
B/Akt. Biochem Soc Trans. 32:350–354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bulun SE, Chen D, Lu M, et al: Aromatase
excess in cancers of breast, endometrium and ovary. J Steroid
Biochem Mol Biol. 106:81–96. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Baum M, Budzar AU, Cuzick J, et al:
Anastrozole alone or in combination with tamoxifen versus tamoxifen
alone for adjuvant treatment of postmenopausal women with early
breast cancer: first results of the ATAC randomised trial. Lancet.
359:2131–2139. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Demura M and Bulun SE: CpG dinucleotide
methylation of the CYP19 I.3/II promoter modulates cAMP-stimulated
aromatase activity. Mol Cell Endocrinol. 283:127–132. 2008.
View Article : Google Scholar : PubMed/NCBI
|

















