Introduction
Glioma arises from glial cells and represents the
most prevalent diagnostic category of primary brain tumor in the
adult population (1). According to
the World Health Organization (WHO) classification, which is based
on histomorphological criteria, human gliomas are divided into
well-differentiated low grade astrocytomas (WHO grades I–II),
anaplastic astrocytomas (AA, WHO grade III) and glioblastoma (GBM,
WHO grade IV). Regardless of recent significant improvements in
therapeutic technologies, such as neurosurgery, radiotherapy,
chemotherapy and photodynamic therapy, the median survival time for
patients with GBM is only 12–15 months, and 2–5 years for patients
with anaplastic glioma (2,3). One of the predominant reasons for the
low survival rates is that the active cell migration and invasion
of GBM cells ultimately leads to ubiquitous tumor recurrence and
patient mortality (4). Previous
studies have identified numerous molecules that are involved in
glioma tumorigenesis and progression (5). Among these molecules, matrix
metalloproteinases (MMPs) have been shown to have an important
role, due to their ability to degrade extracellular matrix proteins
(6). It has previously been
reported that the expression levels of MMP-2 are increased in human
glioma and this increase is associated with advanced
histopathological grade, cell growth and apoptosis, tumor invasion
and metastasis, and radiotherapeutic sensitivity (7).
McroRNAs (miRNAs) are a class of naturally
occurring, small (21–25 nucleotides), noncoding RNAs. They bind
through partial sequence homology to the 3′-untranslated region of
target mRNAs, and may either block translation or promote mRNA
degradation (8). As well as their
involvement in diverse biological processes, including cell growth,
apoptosis, development, differentiation and endocrine homeostasis
(8), emerging evidence has
suggested that the deregulation or dysfunction of miRNAs may
contribute to human carcinogenesis and cancer progression (9). Aberrant expression of miRNAs or
mutations in miRNA genes have been well characterized in human
glioma (10,11). miR-218 has been identified as being
downregulated in numerous types of human malignancy (12–14).
Furthermore, Xia et al (15) and Zhang et al (16) identified the repressive role of
miR-218 in glioma as it was shown to inhibit cell growth and
invasion, and induce cell apoptosis.
Propofol (2,6-diisopropylphenol) is a commonly used
intravenous anesthetic agent, which has gained wide acceptance
since its introduction in the late 1980s (17). As well as its numerous anesthetic
advantages, propofol exerts certain non-anesthetic effects.
Previous studies have identified its tumor-suppressive functions in
various types of cancer (18–21).
Thus suggesting that propofol may be a better agent, as compared
with other anesthetics, for use in cancer surgery (22). However, the potential antitumor
effects of propofol in glioma remain unknown. The present study
aimed to determine the influence of propofol on the biological
behavior of GBM cells, and the role of miR-218 in these
effects.
Materials and methods
Cell culture and reagents
The U373 human GBM cell line was obtained from the
Shanghai Institute of Cell Biology, Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Invitrogen Life Technologies, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich,
St. Louis, MO, USA), 2 mM glutamine (Qiagen, Shanghai, China), 100
U/m penicillin (Qiagen) and 100 mg/ml streptomycin (Qiagen) at 37°C
in an atmosphere containing 5% CO2. Propofol was
purchased from Sigma-Aldrich and dissolved in dimethyl sulfoxide
(DMSO; Sigma-Aldrich) for in vitro analysis. An MMP-2 ELISA
kit was obtained from R&D Systems Europe, Ltd. (Abingdon, UK).
β-actin (1:300, sc-130656) and MMP-2 (1:2,000, sc-10736) polyclonal
rabbit antibodies were supplied from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The U373 cells were treated with control or
different concentrations of propofol (1, 5, 10μg/ml) in this
study.
Cell viability assay
Cell viability was measured in 96-well plates using
a quantitative colorimetric MTT assay (Sigma-Aldrich). Briefly, at
the indicated times after treatment with propofol, 20 μl MTT
(5 mg/ml) was added to each corresponding well and incubated at
37°C for 4 h in a humidified incubator. The MTT solution was then
removed and 200 μl DMSO was added to each well for 15 min,
in order to dissolve the colored formazan crystals. The absorbance
of each aliquot was measured at a wavelength of 570 nm using a
multidetection microplate reader (BMG LABTECH, Cary, NC, USA). The
experiments were independently repeated three times and the results
represent the mean ± standard deviation (SD).
Apoptosis assay by Hoechst 33258 staining
and caspase-3 activity measurement
Nuclear morphology was analyzed using a confocal
laser scanning microscope (IX51; Olympus Corporation, Tokyo, Japan)
following staining of the U373 GBM cells with Hoechst 33258
(Sigma-Aldrich). Control and propofol-treated U373 cells were
washed in ice cold phosphate-buffered saline (Qiagen) and stained
with Hoechst 33258 for 5 min. The cells were mounted on poly
L-lysine coated slides (Qiagen), and dead cells and apoptotic
bodies were determined as those with condensed or fragmented nuclei
as observed using a LSM 5 Live microscope (Carl Zeiss, Shanghai,
China). In order to further detect apoptosis at the molecular
level, the activity of caspase-3, a key enzyme in the regulation of
apoptotic cascades, was also measured using a caspase colorimetric
protease assay. Briefly, the cells were cultured in 96-well plates
and treated with various concentrations of propofol, prior to being
assayed using a Caspase 3 Colorimetric Assay kit (Promega
Corporation, Madison, WI, USA), according to the manufacturer's
instructions.
Matrigel™ invasion assay
Invasion assays were performed in triplicate using a
24-well invasion chamber system coated with Matrigel™ (50 μl
per-filter; BD Biosciences, Franklin Lakes, NJ, USA). The cells
were seeded in the upper chamber (1×105 cells/well) in
serum-free DMEM, and DMEM containing 10% FBS was added to the lower
chambers as a chemoattractant. Following a 24 h incubation, the
non-migratory cells in the upper chamber were removed using a
cotton-tip applicator. The migrated cells on the lower surface were
fixed with methanol and stained with hematoxylin. The number of
migratory cells was determined by counting five random fields on
each membrane.
miRNA extraction and detection by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Approximately 5×l06 cells were collected
following treatment with or without (control) propofol for 24 h,
and miRNAs were extracted using TRIzol® reagent
(Invitrogen Life Technologies), according to the manufacturer's
instructions. miRNA-218 quantification was performed using the
Bio-Rad iQ5 Multicolor Real-Time PCR (RT-PCR) Detection system
(Bio-Rad Laboratories, Hercules, CA, USA) and the
TaqMan® MicroRNA Reverse Transcription kit (Invitrogen
Life Technologies). At the reverse transcription step, cDNA was
synthesized from total RNA (1μg) using specific miRNA
primers and reagents from the TaqMan® MicroRNA Reverse
Transcription kit. At the PCR step, PCR products were amplified
from cDNA using the TaqMan® MicroRNA
Assay/TaqMan® Universal PCR Master mix. The following
conditions were used for PCR: 40 Cycles of 95°C for 10 min; 95°C
for 15 sec; and 60°C for 60 sec. U6 small nuclear RNA was used as
an endogenous reference gene. The RT primers had the following
sequences: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTAT
TCGCACTGGATACGACTCTTAGG-3′ for miR-218 and 5′-TGGTGTCGTGGAGTCG-3′
for U6. The PCR primers were designed as follows: Forward:
5′-TGCGGC GGCCCCACGCACCAG-3′ andreverse: 5′-CCAGTGCAGGGT CCGAGGT-3′
for miR-218; and forward: 5′- TGCGGGTGCT CGCTTCGGCAGC-3′ and
reverse 5′-CCAGTGCAGGGTC CGAGGT-3′ for U6. The primers obtained
from Qiagen. The cycle threshold (Ct) was defined as the fractional
cycle number at which fluorescence passed the fixed threshold, and
the relative miR-218 expression levels were calculated using the
2‒∆∆Ct method.
Western blot analysis
The U373 cells were homogenized using a dounce
homogenizer (Corning, Shanghai, China) in ice-cold lysis buffer
containing 10 mM Tris (pH 8.0), 150 mM NaCl, 10% glycerol, 1%
NP-40, 5 mM EDTA and a protease inhibitor cocktail (Sigma-Aldrich).
The homogenates were then centrifuged at 13,200 × g for 20 min at
4°C, and the total protein concentrations were quantified using a
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology, Haimen, China). Equal quantities of protein (30
μg) were separated by SDS-PAGE using 12% gradient
Tris/glycine gels. The proteins were then transferred to
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). After
blocking in 5% non-fat dry milk for 1 h, the blots were incubated
with the affinity purified rabbit anti-MMP-2 (1:300) and β-actin
(1:2,000) antibodies at 4°C overnight. The membranes were then
washed and incubated for 2 h with peroxidase-labeled goat
anti-rabbit immunoglobulin G (1:5,000, sc-2445; Santa Cruz
Biotechnology, Inc.). The blots were visualized using an Enhanced
Chemiluminescence substrate (Applygen Technologies, Inc., Beijing,
China) and exposed to X-ray film (Sigma-Aldrich). β-actin was used
as an internal control for relative quantification. The grey value
analysis of immunoreactive bands was performed using Quantity One
software (Bio-Rad Laboratories).
Cell transfection procedures
The overexpression or knockdown of miR-218 was
conducted by transfection of the U373 cells with either 100 nM
miR-218 precursor or anti-miR-218 or negative control (NC)
RNA-oligonucleotides (Ambion Life Technologies, Carlsbad, CA, USA).
The transfections were conducted using Lipofectamine
2000® (Invitrogen Life Technologies) in antibiotic-free
medium, in 60-mm dishes, once the cells had reached 30–50%
confluence. Following a 24 h incubation, the cells were treated
with or without propofol.
Statistical analysis
All of the results represent the mean + SD.
Statistical analyses were conducted using either an analysis of
variance or Student's t-test. SPSS software v16 (SPSS Inc.,
Chicago, IL, USA) was used for statistical analysis. P<0.01 was
considered to indicate a statistically significant difference.
Results
Propofol suppresses cell proliferation
and invasion, and facilitates apoptosis
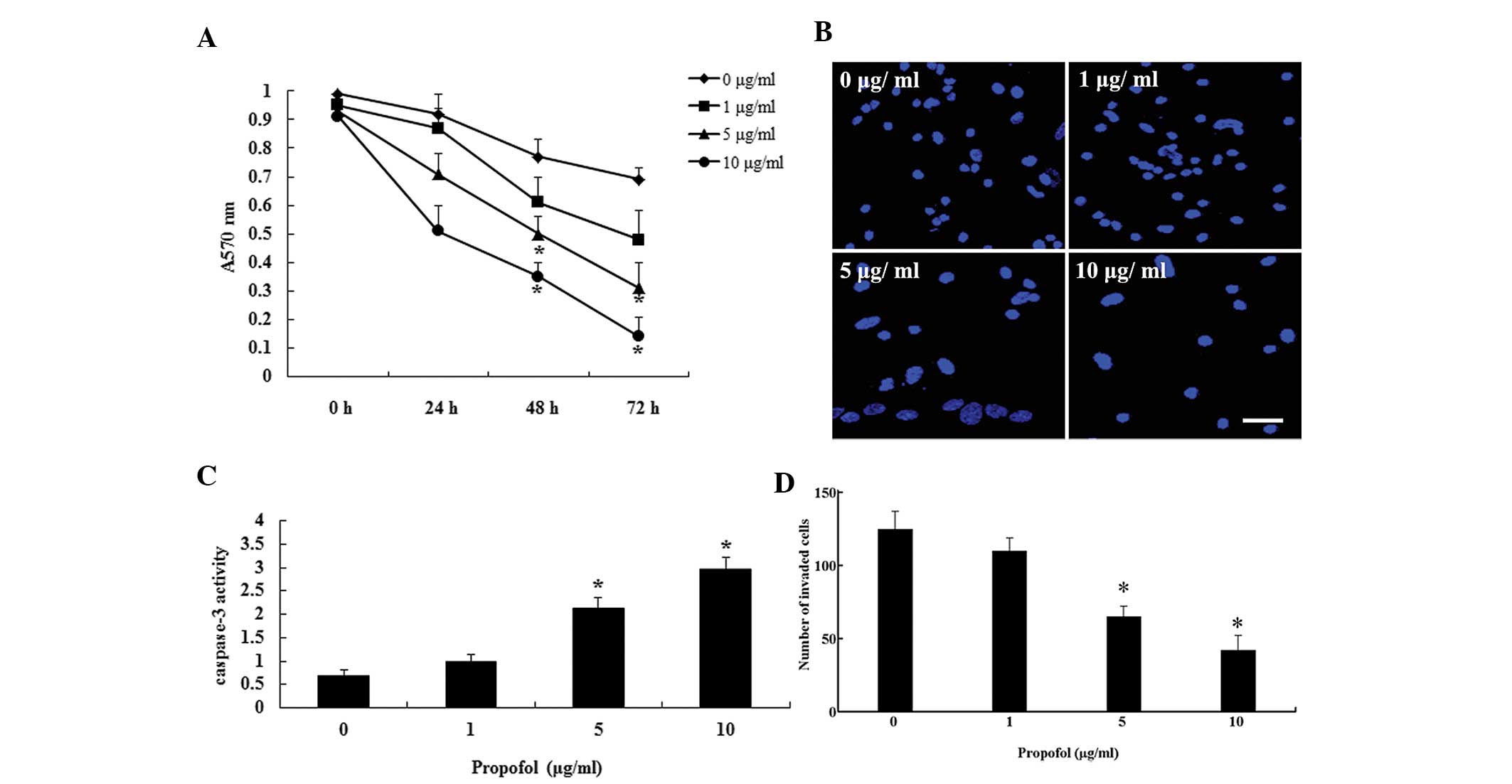
The present study aimed to determine the effects of
propofol on proliferation, apoptosis and invasion in the U373
glioma cell line. Following treatment with various concentrations
of propofol, the proliferation of U373 cells was measured using the
MTT method. The proliferation of U373 cells was markedly suppressed
by propofol in a dose- and time-dependent manner (Fig. 1A). Treatment with propofol at 5 and
10 μg/ml significantly inhibited the proliferation of the
cells at 48 and 72 h. Furthermore, the rate of cell apoptosis was
evaluated by Hoechst 33258 staining and caspase-3 activity
measurement. The U373 cells had an increased rate of apoptosis
following treatment with propofol for 48 h (Fig. 1B and C). A Matrigel™ invasion assay
also demonstrated that propofol significantly reduced cell invasion
when the cells were treated with 5 and 10 μg/ml (Fig. 1D). These results indicate that
propofol inhibits proliferation and invasion, and promotes
apoptosis of U373 cells.
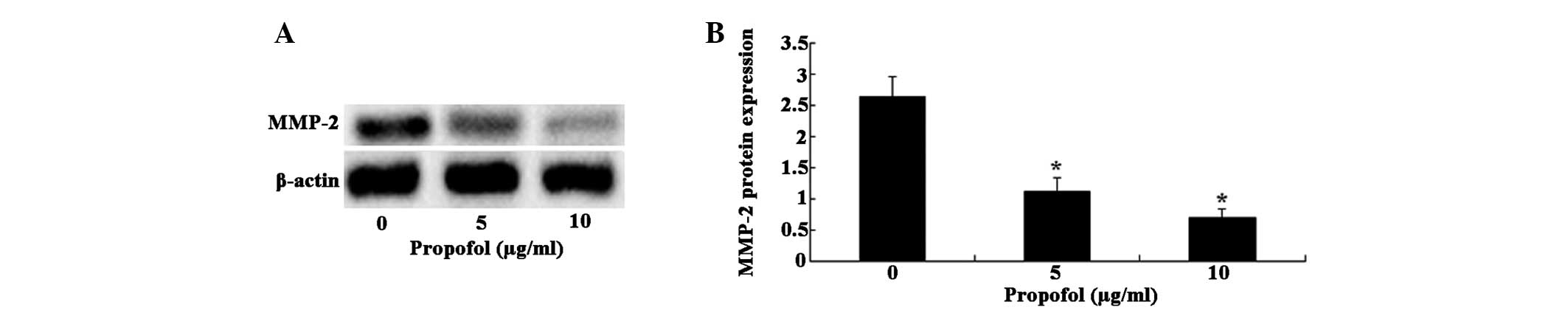
Inhibitory effects of propofol on MMP-2
protein expression levels
To confirm the inhibitory effects of propofol on
MMP-2, the MMP-2 protein expression levels were examined in cells
following treatment with propofol by western blot analysis
(Fig. 2A). A marked reduction in
MMP-2 protein expression levels were observed in the U373 cells
treated with 5 μg/ml propofol, as compared with the control
cells (P<0.01). In addition, propofol dose-dependently
suppressed MMP-2 protein expression, with a 57.58% reduction at 5
μg/ml and a 73.86% decrease at 10 μg/ml (Fig. 2B).
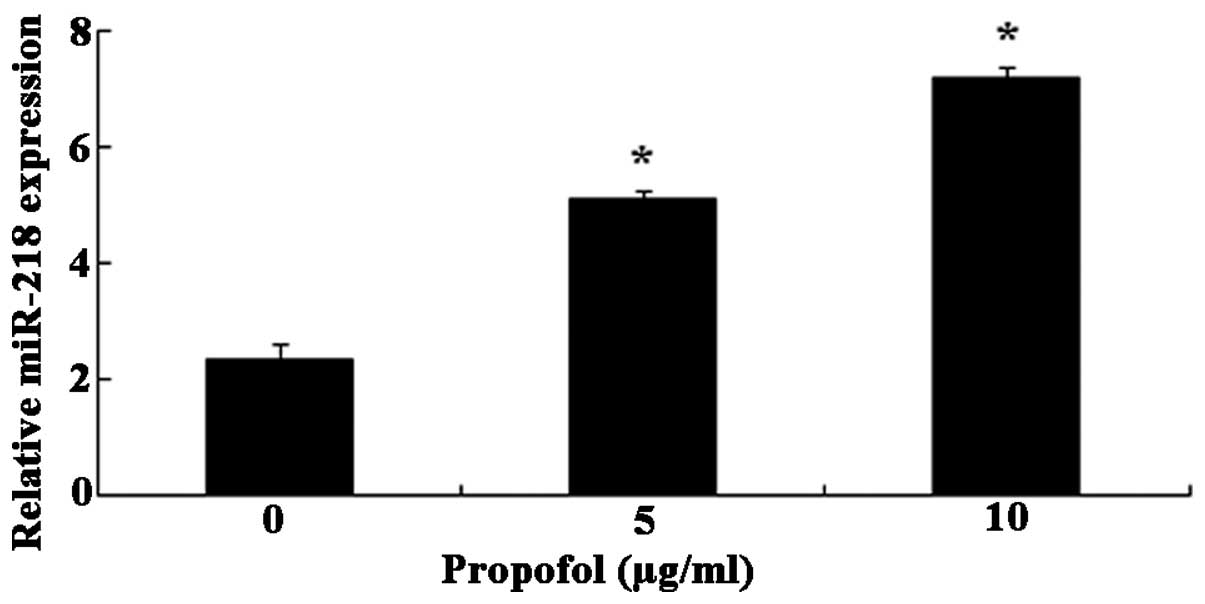
Propofol stimulates miR-218
expression
Treatment with propofol dose-dependently augmented
miR-218 expression in U373 cells (Fig.
3). Specifically, treatment with 5μg/ml propofol
enhanced the miR-218 expression in U373 cells 2.19 fold and 10
μg/ml propofol elevated the miR-218 expression in U373 cells
3.07 fold.
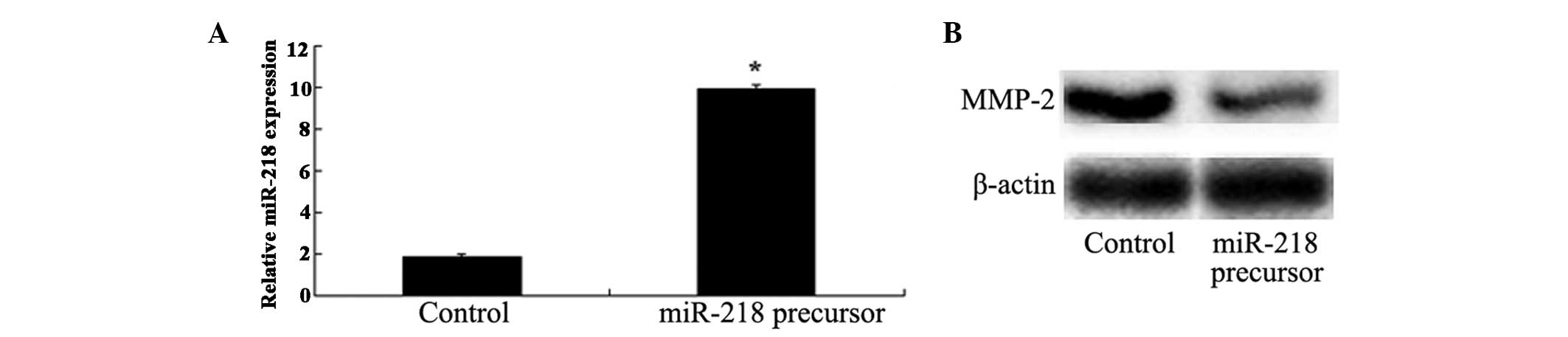
Overexpression of miR-218 suppresses
MMP-2 expression levels
The present study also investigated whether MMP-2
expression was manipulated by miR-218 in U373 cells. qPCR was
performed to detect miR-218 expression levels, following
transfection with miR-218 precursor. Notably, transfection of the
U373 cells with miR-218 precursor significantly elevated the
expression of miR-218 (Fig. 4A),
and markedly decreased MMP-2 protein expression levels (Fig. 4B).
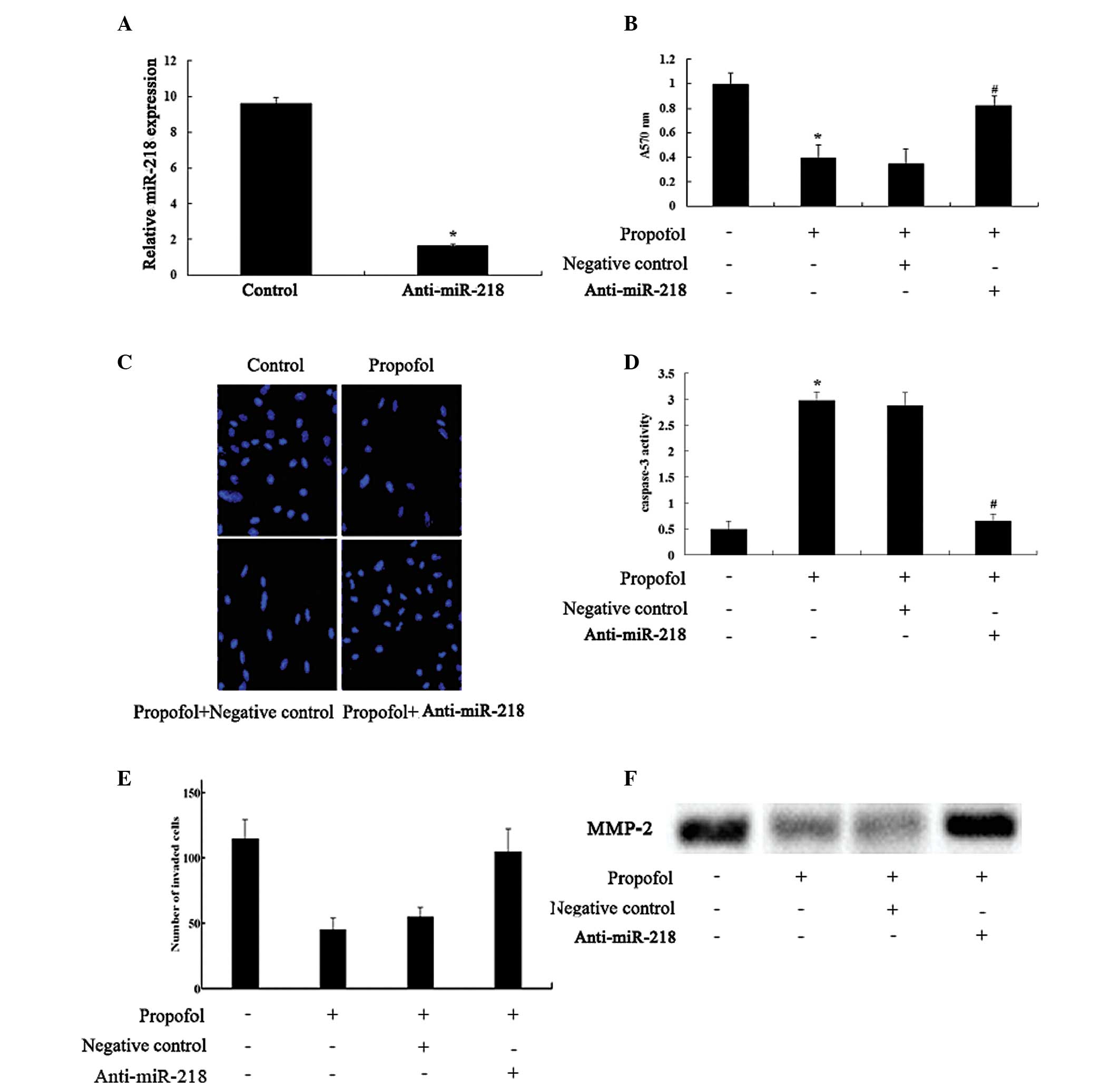
Effects of knockdown of miR-218
expression on cell proliferation, apoptosis and invasion
The role of miR-218 in the effects of propofol was
further explored in U373 cells. Transfection of the cells with
anti-miR-218 antibody was performed to knockdown miR-218
expression. The anti-miR-218 antibody significantly reduced miR-218
expression levels, suggesting the successful introduction of
anti-miR-218 into the U373 cells (Fig.
5A). Furthermore, transfection with the anti-miR-218 antibody
significantly reversed the inhibition of cell proliferation and
invasion, and facilitation of apoptosis caused by propofol
(Fig. 5B–E). In addition, the
inhibitory effects of propofol on the protein expression levels of
MMP-2 were markedly neutralized following transfection of the U373
cells with anti-miR-218 (Fig.
5F).
Discussion
Malignant glioma is the most common type of brain
tumor worldwide and is often fatal (23). The present study investigated the
effects of propofol on the behavior of human GBM cells and
demonstrated that treatment with propofol suppressed the
proliferation and invasion of U373 cells, and induced cell
apoptosis. These results are concordant with numerous previous
findings. Siddiqui et al (24) demonstrated that propofol could
inhibit breast cancer cell growth, and Zhang et al (21,25)
showed that propofol effectively induced apoptosis and suppressed
invasiveness of hepatocellular carcinoma cells. In addition, Miao
et al (20) confirmed the
inhibitory effects of propofol on the invasive activity of colon
carcinoma cells. These results indicate that propofol may be a
particularly suitable anesthetic for use in tumor surgery (22,26,27).
The present study also analyzed the underlying
mechanisms of the effects of propofol on U373 cell growth and
invasion. The identification of miRNAs has substantially altered
views in regards to gene regulation, and recent findings over the
past few years have increased focus on miRNAs in cancer molecular
biology. The anti-tumor functions of miR-218 have been demonstrated
in osteosarcoma (13),
gastrointestinal stromal tumor (28), gastric cancer (29), colon cancer (30), head and neck squamous cell
carcinoma (31), cervical squamous
cell carcinoma (32) and renal
cell carcinoma (33). A previous
study also identified miR-218 as a tumor-suppressor in glioma
(34). The present study observed
that treatment with propofol stimulated the expression of miR-218
in U373 cells. Furthermore, neutralizing miR-218 with anti-miR-218
antibody reversed the effects of propofol on U373 cells. These
results indicate that propofol-induced miR-218 upregulation may
contribute to the anti-proliferative and anti-invasive effects of
propofol. Using TaqMan® low-density arrays and RT-PCR,
Ishikawa et al (35)
corroborated the changes to miRNA expression profiles following
treatment with propofol in animal models. Furthermore, Zhang et
al (21,25) showed that treatment with propofol
promoted apoptosis and suppressed adhesion of hepatocellular
carcinoma cells, by upregulation of miR-199a. However, the
underlying mechanisms regarding how propofol influences miRNA
expression remain unclear, and require further clarification.
It is clear that miRNAs perform their functions
through regulating target gene expression. Identifying the
downstream genes of miR-218 may aid in explaining its roles in
tumor initiation and development. MMP-2, which degrades gelatin and
type IV collagen, is closely associated with the invasive activity
of numerous human malignancies, including glioma (36,37).
Recently, Jin et al (13)
verified the regulatory function of miR-218 on MMP-2 expression.
The present study showed that treatment with propofol and
transfection with miR-218 decreased MMP-2 protein expression
levels, and transfection with anti-miR-218 reversed the inhibitory
effects of propofol on MMP-2 expression. However, it is predicted
that an average miRNA may have >100 targets (38). Therefore, it is probable that there
are other molecules that are targeted by miR-218, which may be
involved in miR-218-mediated effects of propofol on glioma cell
behavior.
In conclusion, the present study demonstrated that
treatment with propofol may suppress proliferation and invasion,
and induce apoptosis of glioma cells, at least partly through
upregulation of miR-218 expression. However, further studies are
required, in order to validate its clinical relevance.
References
|
1
|
Vescovi AL, Galli R and Reynolds BA: Brain
tumour stem cells. Nat Rev Cancer. 6:425–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia H, Qi Y, Ng SS, Chen X, Li D, Chen S,
Ge R, Jiang S, Li G, Chen Y, et al: microRNA-146b inhibits glioma
cell migration and invasion by targeting MMPs. Brain Res.
1269:158–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakada M, Nakada S, Demuth T, Tran NL,
Hoelzinger DB and Berens ME: Molecular targets of glioma invasion.
Cell Mol Life Sci. 64:458–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Badiga AV, Chetty C and Kesanakurti D:
MMP-2 siRNA inhibits radiation-enhanced invasiveness in glioma
cells. PloS One. 6:e206142011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
10
|
Xiao B, Tan L, He B, Liu Z and Xu R:
MiRNA-329 targeting E2F1 inhibits cell proliferation in glioma
cells. J Transi Med. 11:1722013. View Article : Google Scholar
|
|
11
|
Hui W, Yuntao L and Lun L: MicroRNA-195
inhibits the proliferation of human glioma cells by directly
targeting cyclin D1 and cyclin E1. PloS One. 8:e549322013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang C, Ge S, Hu C, Yang N and Zhang J:
MiRNA-218, a new regulator of HMGB1, suppresses cell migration and
invasion in non-small cell lung cancer. Acta Biochim Biophys Sin
(Shanghai). 45:1055–1061. 2013. View Article : Google Scholar
|
|
13
|
Jin J, Cai L, Liu ZM and Zhou XS:
miRNA-218 inhibits osteosarcoma cell migration and invasion by
down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev.
14:3681–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prudnikova TY, Mostovich LA, Kashuba VI,
Ernberg I, Zabarovsky ER and Grigorieva EV: miRNA-218 contributes
to the regulation of D-glucuronyl C5-epimerase expression in normal
and tumor breast tissues. Epigenetics. 7:1109–1114. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia H, Yan Y, Hu M, et al: MiR-218
sensitizes glioma cells to apoptosis and inhibits tumorigenicity by
regulating ECOP-mediated suppression of nf-κb activity. Neuro
Oncol. 15:413–422. 2013. View Article : Google Scholar :
|
|
16
|
Zhang JM, Sun CY, Yu SZ, et al:
Relationship between miR-218 and CDK6 expression and their
biological impact on glioma cell proliferation and apoptosis.
Zhonghua Bing Li Xue Za Zhi. 40:454–459. 2011.PubMed/NCBI
|
|
17
|
Hertzog JH, Campbell JK, Dalton HJ and
Hauser GJ: Propofol anesthesia for invasive procedures in
ambulatory and hospitalized children: experience in the pediatric
intensive care unit. Pediatrics. 103:E301999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altenburg JD, Harvey KA, McCray S, Xu Z
and Siddiqui RA: A novel 2,6-diisopropylphenyl-docosahexaenoamide
conjugate induces apoptosis in T cell acute lymphoblastic leukemia
cell lines. Biochem Biophys Res Commun. 411:427–432. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mammoto T, Mukai M, Mammoto A, et al:
Intravenous anesthetic, propofol inhibits invasion of cancer cells.
Cancer Lett. 184:165–170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miao Y, Zhang Y, Wan H, Chen L and Wang F:
GABA-receptor agonist, propofol inhibits invasion of colon
carcinoma cells. Biomed Pharmacother. 64:583–588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Zhang D, Wu GQ, Feng ZY and Zhu
SM: Propofol inhibits the adhesion of hepatocellular carcinoma
cells by upregulating microRNA-199a and downregulating MMP-9
expression. Hepatobiliary & Pancreatic Diseases International.
12:305–309. 2013. View Article : Google Scholar
|
|
22
|
Inada T, Kubo K and Shingu K: Possible
link between cyclooxygenase-inhibiting and antitumor properties of
propofol. J Anesth. 25:569–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parsons DW, Jones S, Zhang X, et al: An
integrated genomic analysis of human glioblastoma multiforme.
Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siddiqui RA, Zerouga M, Wu M, et al:
Anticancer properties of propofol-docosahexaenoate and
propofol-eicosapentaenoate on breast cancer cells. Breast Cancer
Res. 7:R645–R654. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Wu GQ, Zhang Y, Feng ZY and Zhu
SM: Propofol induces apoptosis of hepatocellular carcinoma cells by
upregulation of microRNA-199a expression. Cell Biol Int.
37:227–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siddiqui RA, Zerouga M, Wu M, et al:
Anticancer properties of propofol-docosahexaenoate and
propofol-eicosapentaenoate on breast cancer cells. Breast Cancer
Res. 7:R645–R654. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu RS, Liu KC, Tang NY, et al: cDNA
microarray analysis of the gene expression of murine leukemia RAW
264.7 cells after exposure to propofol. Environ Toxicol.
28:471–478. 2013. View Article : Google Scholar
|
|
28
|
Fan R, Zhong J, Zheng S, et al:
MicroRNA-218 inhibits gastrointestinal stromal tumor cell and
invasion by targeting KIT. Tumour Biol. 35:4209–4217. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao C, Zhang Z, Liu W, Xiao S, Gu W and Lu
H: Reduced microRNA-218 expression is associated with high nuclear
factor kappaB activation in gastric cancer. Cancer. 116:41–49.
2010.
|
|
30
|
He X, Dong Y, Wu CW, et al: MicroRNA-218
inhibits cell cycle progression and promotes apoptosis in colon
cancer by downregulating BMI1 polycomb ring finger oncogene. Mol
Med. 18:1491–1498. 2013.
|
|
31
|
Kinoshita T, Hanazawa T, Nohata N, et al:
Tumor suppressive microRNA-218 inhibits cancer cell migration and
invasion through targeting laminin-332 in head and neck squamous
cell carcinoma. Oncotarget. 3:1386–1400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamamoto N, Kinoshita T, Nohata N, et al:
Tumor suppressive microRNA-218 inhibits cancer cell migration and
invasion by targeting focal adhesion pathways in cervical squamous
cell carcinoma. Int J Oncol. 42:1523–1532. 2013.PubMed/NCBI
|
|
33
|
Yamasaki T, Seki N, Yoshino H, et al:
MicroRNA-218 inhibits cell migration and invasion in renal cell
carcinoma through targeting caveolin-2 involved in focal adhesion
pathway. J Urol. 190:1059–1068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tu Y, Gao X, Li G, et al: MicroRNA-218
inhibits glioma invasion, migration, proliferation and cancer
stem-like cell self-renewal by targeting the polycomb group gene
Bmil. Cancer Res. 73:6046–6055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishikawa M, Tanaka S, Arai M, Genda Y and
Sakamoto A: Differences in microRNA changes of healthy rat liver
between sevoflurane and propofol anesthesia. Anesthesiology.
117:1245–1252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rundhaug JE: Matrix metalloproteinases,
angiogenesis and cancer: commentary re: A. C. Lockhart et al:
Reduction of wound angiogenesis in patients treated with
BMS-275291, a broad spectrum matrix metalloproteinase inhibitor.
Clin Cancer Res. 9:551–554. 2003.PubMed/NCBI
|
|
37
|
Deryugina EI, Bourdon MA, Luo GX, Reisfeld
RA and Strongin A: Matrix metalloproteinase-2 activation modulates
glioma cell migration. J Cell Sci. 110:2473–2482. 1997.PubMed/NCBI
|
|
38
|
Brennecke J, Stark A, Russell RB and Cohen
SM: Principles of microRNA-target recognition. PLoS Biol.
3:e852005. View Article : Google Scholar : PubMed/NCBI
|