Introduction
Anal papillomas are common, benign anal cysts.
Previous studies have indicated that hypertrophied anal papillae
arise as a result of proliferative inflammatory disease (1–3).
Hypertrophied anal papillae are, in essence, skin tags that project
up from the dentate line, or from the junction between the skin and
the epithelial lining of the anus. They are often found as part of
the classic triad of a chronic fissure; namely the fissure itself,
with hypertrophied papilla above and a skin tag below (1–3).
However, the mechanisms underlying the development of human
hypertrophied anal papillae are poorly understood.
Epidermal stem cells (EpSCs) are skin
tissue-specific adult stem cells with a strong proliferative
capacity, which undergo asymmetric division (4–7).
Following induction, EpSCs are able to differentiate into a variety
of epidermal lineages in order to promote self-renewal and
regeneration of the epidermis, and to promote wound healing
(5,6,8,9).
Proliferation and differentiation of EpSCs may be regulated by a
number of factors, including the stem cell microenvironment, or
cellular factors and cell surface receptors (5,6).
Previous studies have found that the integrin family of proteins
has a significant effect on EpSC proliferation and differentiation.
Integrins have been utilized for the isolation and enrichment of
EpSCs from tissues in vitro (5,6,8–10).
Integrins are cell surface receptors composed of α and β subunits.
To date, 18 different α subunits and 8 β subunits have been
identified that may be combined to form a total of 24 different
integrin receptors in mammals (9).
The N-terminal region of the α subunit forms a domain that binds
divalent cations and contains a highly conserved sequence,
'KXGFFKR', which is proximal to the cytoplasmic membrane and is
involved in regulation of integrin activity (9). β1 integrins form the largest subgroup
of integrins. The 12 members of this group bind a variety of
ligands. α1β1, α2β1, α10β1 and α11β1 primarily interact with
collagen, an interaction that is conducive to cell proliferation.
α1β1, α2β1, α3β1, α6β1 and α7β1 interact primarily with laminins,
which are involved in adhesion to the basement membrane. α4β1,
α5β1, α8β1 and αvβ1 bind fibronectin, and α9β1 binds tenascins
(9). In the intact epidermis,
integrin expression occurs in the basal layer and outer root sheath
of hair follicles. β1 expression is predominantly confined to
regions of the hair follicle bulge and epidermal prominences, while
α6 integrin expression occurs in the outer root sheath of hair
follicles and the outermost basal layer of the interfollicular
epithelium, which is composed of hemidesmosomes. Integrins α6 and
β1 are used as molecular biomarkers of EpSCs (9,10).
MicroRNAs (miRNAs) are a recently discovered class
of naturally occurring, single-stranded, 21–23 nucleotide,
non-coding RNAs (11,12), which exist in a wide range of
eukaryotic organisms (11–16). Each mammalian miRNA may prevent the
translation of a number of downstream target mRNAs, which
ultimately results in the inhibition of target gene expression
(17–20). let-7 is a well-studied
miRNA, known to be involved in cell cycle regulation and
development, which is underexpressed in various cancers (21). Restoration of let-7
expression has been found to inhibit cancer growth by targeting
various oncogenes and inhibiting key regulators of numerous
mitogenic pathways (21–24). Yu et al (24) found that let-7 suppressed
self-renewal and tumorigenicity of breast cancer cells by reducing
H-RAS and high-mobility group AT-hook 2 (HMGA2) expression.
Furthermore, Schultz et al (22) reported that let-7b, a member
of the let-7 microRNA family, interfered with the
proliferation and growth of primary malignant melanoma cells by
targeting and suppressing important cell cycle molecules, such as
cyclin D1 (CCND1). In addition, Dangi-Garimella et al
(23) revealed that elevated
let-7 expression inhibited HMGA2 expression and suppressed
metastasis in breast cancer cells.
The present study aimed to establish the role of
miRNA let-7b in regulation of integrin α6+/β1+EpSC
proliferation and define its role in the formation of human
hypertrophied anal papillae.
Materials and methods
Patients and ethics
Hypertrophic anal papilla tissue samples were
obtained during surgery from five patients who had been diagnosed
with mixed hemorrhoids or anal fistula. Two patients (1 male and 1
female) had archosyrinx, and three patients (2 males and 1 female)
had mixed hemorrhoids. The median age of this population was 39
years old. All human materials were obtained from the Department of
Anorectal Dermatology (Shuguang Hospital, Shanghai University of
Traditional Chinese Medicine, Shanghai, China). All of the patients
in the present study provided written informed consent. The study
was approved by the ethics committee of Shanghai Geriatric
Institute of Chinese Medicine, Longhua Hospital, Shanghai
University of Traditional Chinese Medicine (Shanghai, China).
Isolation of integrin α6 and integrin β1
phenotype cells by a magnetic activated cell sorting system
Integrin α6 and Integrin β1 subpopulation cells were
isolated from primary cells from hypertrophic anal papilla tissues,
using 4 µl primary monoclonal antibody (rabbit anti-human
Integrin α6-FITC, rabbit anti-human Integrin β1-PE, eBioscience,
Inc., San Diego, CA, USA), stored at 4°C in phosphate-buffered
saline (PBS) for 30 min in a volume of 1 ml, as previously
described (4,25). Following this reaction, the cells
were washed twice in PBS, and goat anti-rabbit secondary monoclonal
antibodies conjugated to magnetic microbeads (Miltenyi Biotec,
Auburn, CA, USA) were added, incubated at 10°C in PBS for 15 min
and then washed twice in PBS. Single cells were plated at 1,000
cells/ml in Dulbecco's modified Eagle's medium (DMEM:F12; HyClone
Laboratories, Inc., Logan, UT, USA), supplemented with 10% fetal
bovine serum (FBS), 10 ng/ml basic fibroblast growth factor (bFGF),
10 ng/ml epidermal growth factor (EGF), 5 µg/ml insulin and
0.5% bovine serum albumin (BSA; all Sigma-Aldrich, St. Louis, MO,
USA). All cells had been cultured under the same conditions until
passage three, prior to subsequent experiments.
Recombinant lentivirus second generation
vector construction
All steps of recombinant lentivirus packaging were
conducted as previously described (17,20).
An RNAi pLL3.7 (LentiLox 3.7) letroviral system was used to create
lentiviral virus vectors (GenePharma Co., Ltd., Shanghai, China).
For vector pLL3.7-miR-let-7 (pre-microRNA let-7), an
oligonucleotide pair for pre-miRNA of microRNA let-7, and
linker sequences with HpaI and XhoI sites were
chemically synthesized. The following oligonucleotides were used:
Bottom strand, 5′-CGgttaac
CGGGGTGAGGTAGTAGGTTGTGTGGTTT
CAGGGCAGTGATGTTGCCCCTCGGAAGATAACTATACAACCTACTGCCTTCCCTGctcgag
CG-3′. Sequences corresponding to microRNA let-7 seed
sequences are represented in capitalized and bold characters, and
restriction enzyme sites are represented in lower case and bold
characters. In order to produce the expression plasmid, the pairs
of oligonucleotides were annealed as follows: Denaturation at 95°C
for 5 min; annealing at 58°C for 45 min, then the oligonucleotides
were inserted into the multiple cloning sites between the
HpaI and XhoI sites in the pLL3.7 vector. The
negative control plasmid, pLL3.7-miR-Mut, was constructed in a
similar manner, with the exception that 22 nucleotides in sequences
corresponding to microRNA let-7 seed sequences were mutated
(TGAGGTAGTAGGTTGTGTGGTT was changed to TGTCCTAGAAGCATGAGAGCAT). The
pLL3.7-miR-let-7 and pLL3.7-miR-Mut vectors were then
recombined in the package cell lines, 293T, in order to create
lentiviruses. Recombinant viruses were propagated in 293T cells,
and purified, and titered using standard methods, as described
previously (17,20). The corresponding viruses were
termed Ldv-miR-let-7 or Ldv-miR-Mut. Cotransfection of EpSCs
was conducted with 4×107 PFU/ml Ldv-miR-let-7 or
Ldv-miR-Mut lentivirus, respectively, according to the
manufacturer's instructions. Cells were seeded in a 6-well plate in
DMEM (Sigma-Aldrich) supplemented with 10% fetal bovine serum
(Hyclone Laboratories, Inc.), 100 U/ml penicillin and 100
µg/ml streptomycin (Hyclone Laboratories, Inc.), at 37°C in
a humidified atmosphere of air with 5% CO2, until they
reached 80% confluence.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from was isolated using TRIzol Reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions. RNA samples were treated with Dnase I
(Sigma-Aldrich) and reverse-transcribed into cDNA using the
ReverTra Ace-α First Strand cDNA Synthesis kit (Toyobo, Co., Ltd.,
Osaka, Japan). RT-qPCR was conducted using a RealPlex4 real-time
PCR detection system, obtained from Eppendorf Co., Ltd. (Hamburg,
Germany), with SyBR Green RealTime PCR Master Mix (Toyobo, Co.,
Ltd.) used as the detection dye. RT-qPCR amplification was
performed over 40 cycles with denaturation at 95°C for 15 sec and
annealing at 58°C for 45 sec. Target cDNA was quantified using the
relative quantification method (20,25).
A comparative threshold cycle (Ct) was used to determine gene
expression relative to that of a control (calibrator), and
steady-state mRNA levels were reported as an n-fold difference
relative to the calibrator. For each sample, the maker genes Ct
values were normalized using the following formula: ΔCt = Ct(gene)
− Ct(18sRNA). In order to determine relative expression levels, the
following formula was used: ΔΔCt = ΔCt(Ldv − miR-let-7 group) −
ΔCt(Ldv-miR-Mut group). The values used to plot relative
expressions of markers, were calculated using the expression
2−ΔΔCt. The mRNA levels were calibrated based on levels
of 18sRNA. The cDNA of each stem cell markers was amplified using
the primers shown in Table I.
 | Table IPrimers used for RT-qPCR. |
Table I
Primers used for RT-qPCR.
| Gene | RT-qPCR primers
(5′→3′) |
|---|
| p53 | F:
GCTTTCCACGACGGTGAC |
| R:
GCTCGACGCTAGGATCTGAC |
| CCND1 | F:
TCCTCTCCAAAATGCCAGAG |
| R:
GGCGGATTGGAAATGAACTT |
| CDK4 | F:
TGCAGTCCACATATGCAACA |
| R:
GTCGGCTTCAGAGTTTCCAC |
| 18S rRNA | F:
CAGCCACCCGAGATTGAGCA |
| R:
TAGTAGCGACGGGCGGTGTG |
| Let-7b | F:
TGAGGTAGTAGGTTGTGTGGTT |
| R:
GCTGTCAACGATACGCTACCTA |
Western blotting analysis
Protein extracts were resolved using 12% SDS-PAGE
and transferred onto polyvinylidene difluoride (PVDF) membranes
(Merck Millipore, Bedford, MA, USA). The PVDF membranes were
blocked with WB blocking solution (Beyotime Biotechnology Co., Ltd,
Zhejiang, China), and subsequently washed four times for 15 min
with Tris-buffered saline with Tween-20 (TBST; Merck Millipore) at
room temperature and incubated with the following primary
antibodies: Rabbit anti-human p53 polyclonal antibody (cat. no.
12571), rabbit anti-human CCND1 polyclonal antibody (cat. no.
3300), rabbit anti-human cyclin-dependent kinase 4 (CDK4)
polyclonal antibody (cat. no. 12790), rabbit anti-human E-cadherin
polyclonal antibody (cat. no. 3195), rabbit anti-human claudin 1
(CLDN1) polyclonal antibody (cat. no. 4933), rabbit anti-human
GAPDH polyclonal antibody (cat. no. 5174), all at 1:1,000 (Cell
Signaling Technology, Inc., Danvers, MA USA). Following extensive
washing, membranes were incubated with a peroxidase-conjugated goat
anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology, Inc.,
Santa Cruz, Ca, USA) for 1 h. After washing four times for 15 min
with TBST at room temperature, the immunoreactivity was visualized
using an enhanced chemiluminescence (ECL kit, Pierce Biotechnology,
Inc., Rockford, IL, USA).
Immunofluorescence staining analysis
The cultured cells were washed three times with PBS
and fixed with 4% paraformaldehyde (Sigma-Aldrich) for 30 min.
Following blocking, the cells were incubated overnight at 4°C with
primary antibodies as follows: Rabbit anti-human p53 polyclonal
antibody (cat. no. 12571), rabbit anti-human CCND1 polyclonal
antibody (cat. no. 3300), rabbit anti-human CDK4 polyclonal
antibody (cat. no. 12790), rabbit anti-human E-cadherin polyclonal
antibody (cat. no. 3195), rabbit anti-human CLDN1 polyclonal
antibody (cat. no. 4933), rabbit anti-human GAPDH polyclonal
antibody (cat. no. 5174), all at 1:1,000 (Cell Signaling
Technology, Inc.) and then incubated with Cy3-conjugated goat
anti-rabbit IgG secondary antibody (1:200; Abcam, Cambridge, UK)
and 5 mg/ml DAPI (Sigma-Aldrich) at room temperature for 30 min.
The cells were then thoroughly washed with TBST and viewed through
a fluorescence microscope (DMI3000; Leica, Allendale, NJ, USA).
Northern blotting
Northern blotting was conducted as previously
described (17,20). For all cell treatment groups, 20 µg
of total RNA was analyzed on a 7.5 M urea, 12% PAA denaturing gel
and transferred to a Hybond N+nylon membrane (Amersham, Freiburg,
Germany). Membranes were cross-linked using ultraviolet light for
30 sec at 1,200mJ/cm2 and hybridized to the
let-7b antisense Starfireprobe, 5′-AACCACACAACCTACTACCTCA-3′
(Sangon Biotech Co., Ltd, Shanghai, China) for the detection of
22-nucleotide let-7b fragments, according to the
manufacturer's instructions. After washing, membranes were exposed
to a Kodak XAR-5 film (Sigma-Aldrich) for 20–40 h. A human U6 snRNA
probe (5′-GCAGGGGCCATGCTAATCTTCTCTGTATCG-3′) was used as a positive
control, with an exposure time of 15–30 min.
Flow cytometric (FCM) analysis of the
cell cycle by PI staining
Each group of cells were seeded at 3×105
per well in 6-well plates and cultured until they reached 85%
confluence. Each group of cells was washed three times with PBS,
then collected by centrifugation (Allegra X-22R, Beckman Coulter,
Brea, CA, USA) at 1,000 x g for 5 min. The cell pellets were the
resuspended in 1 ml of PBS, fixed in 70% ice-cold ethanol, and
maintained in a freezer for >48 h. Prior to FCM analysis, the
fixed cells were centrifuged, washed twice with PBS, and
resuspended in PI staining solution (Sigma-Aldrich) containing 50
µl/ml PI and 250 µg/ml RNase A (Sigma-Aldrich). The
cell suspension, which was maintained in darkness, was incubated
for 30 min at 4°C and analyzed using the FACS (FACSAria, BD
Biosciences, San Jose, CA, USA). A total of 20,000 events were
acquired for analysis using CellQuest software version 2.1.0.
Statistical analysis
Each experiment was performed as least three times,
and data are presented as the mean ± standard error where
applicable. Differences were evaluated using Student's t-test and
P<0.05 was considered to indicate a statistically significant
difference. GraphPad Prism version 5.0 was also used for
statistical analyses.
Results
α6+/β1+EpSCs are present at low levels in
hypertrophic anal papillae
The anal papillae examined in this study arose due
to chronic inflammation caused by fibrous connective tissue
hyperplasia. The anal papillae were cylindrical or walnut-shaped,
with a large upper part and smaller lower part. The smooth surface
was milky white, and no bleeding was observed (Fig. 1A and B). Pathological examination
demonstrated significant endothelial hyperplasia in the anal
papillae, accompanied by infiltration of inflammatory cells and
vascular proliferation, although no cell heterogeneity was observed
(Fig. 1C). A magnetic-activated
cell sorting system was used to isolate and enrich the α6+/β1+
subpopulation from the hypertrophied anal papillae. Following
isolation, cells were quantified using FCM. α6+/β1+EpSCs
represented 0.27±0.02% of the total population in five primary
samples, whereas α6-/β1-cells represented 59.51±8.31% of the total
population (Fig. 1D–F). These
results demonstrated that α6+/β1+epidermal stem cells (EpSCs),
although occurring at a low frequency, may be successfully enriched
using magnetic-activated cell sorting.
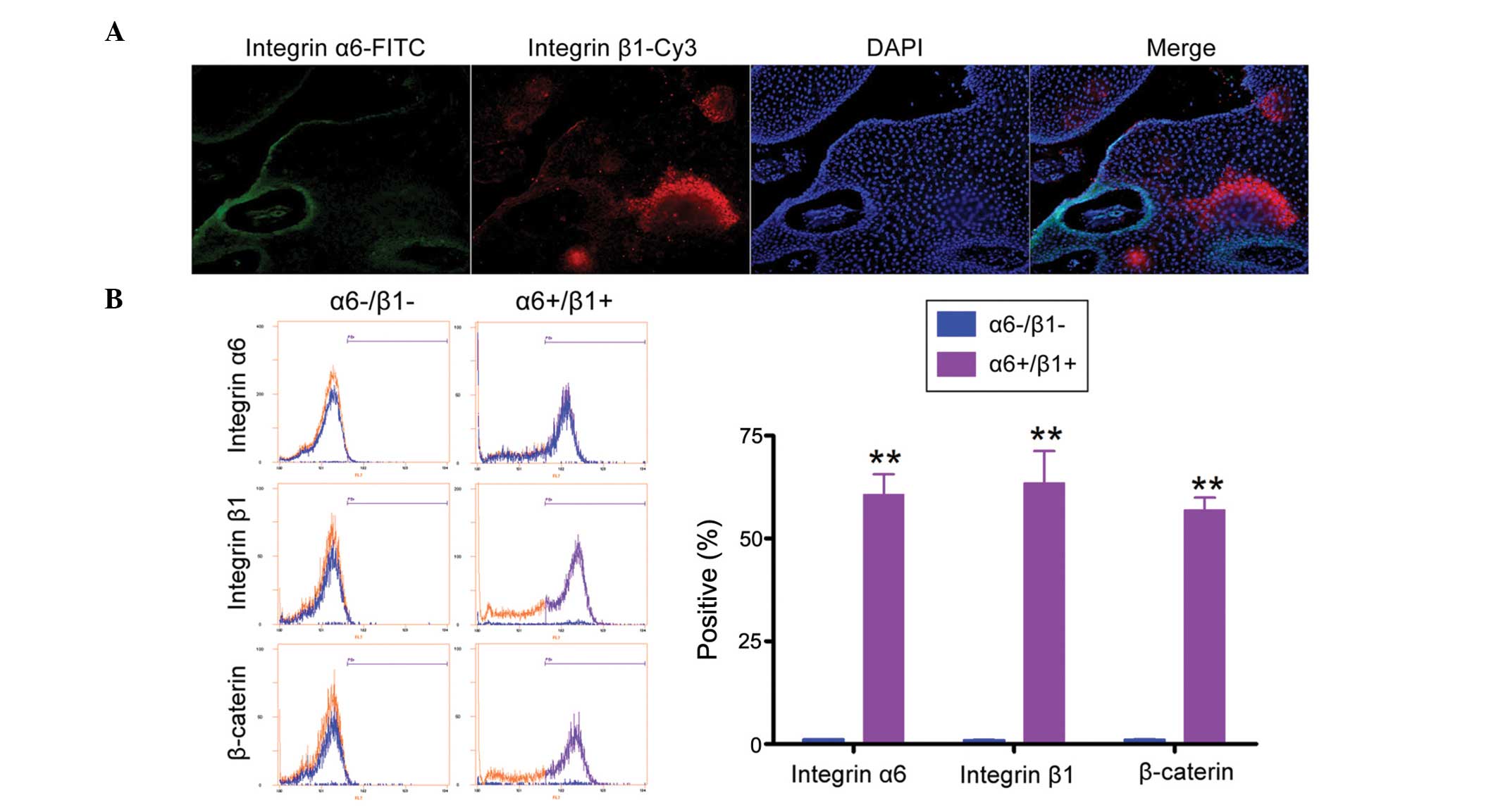
α6+/β1+EpSCs express epidermal stem cell
markers
FCM and immunofluorescent (IF) staining were used to
compare the relative expression levels of epidermal stem cell
markers in α6+/β1+EpSCs and α6-/β1-cells. The IF assay confirmed
that α6+/β1+EpSCs expressed higher levels of the epidermal stem
cell markers, integrin α6 and β1 (Fig.
2A). FCM also demonstrated that the expression of integrin α6,
β1 and β-caterin was significantly higher in α6+/β1+EpSCs than that
in α6-/β1-cells (Fig. 2B; Table II). These results demonstrate that
the α6+/β1+ subpopulation possesses epidermal stem cell
characteristics.
 | Table IIAssessment of EpSC marker expression
using FCM. |
Table II
Assessment of EpSC marker expression
using FCM.
| Biomarkers | α6-/β1-cells
(n=5) | α6+/β1+EpSCs
(n=5) |
|---|
| Integrin α6 | 1.155±0.015% | 60.645±4.955% |
| Integrin β1 | 1.025±0.035% | 63.450±7.789% |
| β-caterin | 1.080±0.079% | 56.905±2.965% |
α6+/β1+EpSCs proliferate rapidly and
express low levels of miRNA let-7
The proliferation rates of α6+/β1+EpSCs and
α6-/β1-cells were examined for up to 72 h following passaging.
Measurements were repeated in quintuplicate. No significant
differences were observed in the total number of cells between the
two groups at 0–12 h (Fig. 3A).
However, between 24–72 h, α6+/β1+EpSCs were found to divide
significantly more rapidly than α6-/β1-cells (Table III). The miRNA RT-qPCR assay
showed that the expression of miRNA let-7b was markedly
lower in α6+/β1+EpSCs at 72 h than that in α6-/β1-cells (Fig. 3B). Northern blot analysis
demonstrated strong let-7b pre-miRNA and mature miRNA
hybridization signals in α6-/β1-cells at 72 h, compared with levels
in α6+/β1+EpSCs (Fig. 3C). RT-qPCR
and western blotting were used to determine difference in the
expression of cell cycle-related proteins in the different groups
of cells. RT-qPCR showed that mRNA levels of p53, a protein
involved in apoptosis, were markedly lower in α6+/β1+EpSCs at 72 h
compared with levels in α6-/β1-cells (Fig. 3D and E). By contrast, mRNA levels
of the cell cycle-related proteins, CCND1 and CDK4, were markedly
higher in α6+/β1+EpSCs at 72 h than those in α6-/β1-cells (Fig. 3D and E). These findings were
confirmed by western blotting (Fig.
3E). These results indicate that miRNA let-7b expression
is reduced in α6+/β1+EpSCs, stimulating the expression of cell
cycle-related proteins and the proliferation of these cells.
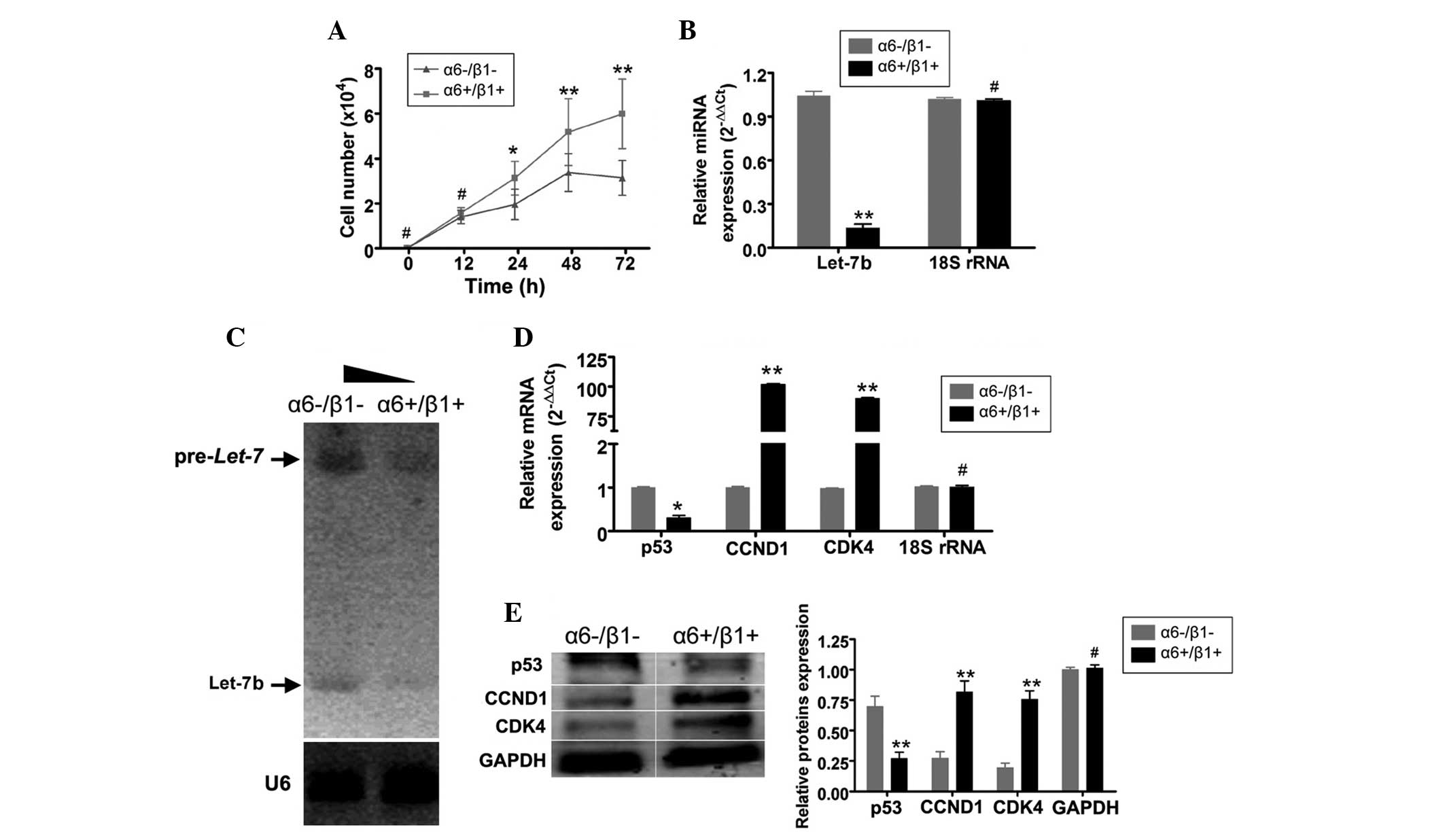 | Figure 3α6+/β1+EpSCs proliferated more
rapidly and expressed lower levels of microRNA let-7b than
α6-/β1-cells. (A) The proliferation rates of α6+/β1+EpSCs and
α6-/β1-cells were examined 12-72 h after passaging. Between 24-72
h, α6+/β1+EpSCs divided significantly more rapidly than
α6-/β1-cells. (B) An miRNA RT-qPCR assay demonstrated that
expression of miRNA let-7b was markedly lower in
α6+/β1+EpSCs at 72 h compared with that in α6-/β1-cells. (C)
Northern blot analysis revealed strong pre-miRNA let-7b and
mature microRNA let-7b hybridization signals in α6-/β1-cells
at 72 h compared with those α6+/β1+EpSCs. (D) RT-qPCR demonstrated
that the mRNA expression of p53 was markedly lower in α6+/β1+EpSCs
at 72 h compared with that in α6-/β1-cells. By contrast, mRNA
expression of the cell cycle-related factors, CCND1 and CDK4, was
significantly higher in α6+/β1+EpSCs at 72 h, compared with that in
α6-/β1-cells. (E) Western blotting confirmed that p53 protein
expression was significantly reduced in α6+/β1+EpSCs. The
expression of CCND1 and CDK4 proteins was significantly elevated in
α6+/β1+EpSCs. GAPDH served as a loading control
**P<0.01, vs. α6-/β1-cells and *P<0.05,
vs. α6-/β1-cells; n=3). EpSCs, Epidermal stem cells; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction;
CCND1, cyclin D1; CDK4, cyclin-dependent kinase 4. |
 | Table IIICell proliferation assay. |
Table III
Cell proliferation assay.
| Time (hours) | Cell numbers
(x104/ml; n=5)
|
|---|
| α6-/β1-cells | α6+/β1+EpSCs | miR-Let-7b
transcription group | miR-Mut
transcription group |
|---|
| 0 | 0 | 0 | 0 | 0 |
| 12 | 1.39±0.29 | 1.58±0.23 | 1.29±0.27 | 1.12±0.14 |
| 24 | 1.95±0.68 | 3.12±0.75 | 1.76±0.69 | 2.02±0.91 |
| 48 | 3.38±0.84 | 5.17±1.48 | 3.19±0.63 | 5.49±1.29 |
| 72 | 3.137±0.77 | 5.98±1.55 | 4.62±0.39 | 6.54±1.32 |
Overexpression of exogenous miRNA let-7b
decreases the proliferation rate of α6+/β1+EpSCs by interfering
with CCND1 expression
In order to determine whether the addition of
exogenous miRNA let-7b influences α6+/β1+EpSC proliferation,
recombinant lentiviruses expressing miR-let-7b or miR-Mut
were transfected into α6+/β1+EpSCs. Following expression of miRNA
let-7b, no significant differences in the number of cells
were detected between the two groups from 0-24 h (Fig. 4A). However, between 48–72 h,
α6+/β1+EpSCs transfected with miR-let-7b divided
significantly less rapidly than α6+/β1+EpSCs transfected with
miR-Mut (Table III). The effects
of the transfected miRNA on mRNA and protein expression were
detected by RT-qPCR and northern blotting, and western blotting,
respectively. The RT-qPCR results demonstrated that mRNA expression
of p53 was markedly higher in α6+/β1+EpSCs transfected with
miR-let-7b than that in α6+/β1+EpSCs transfected with
miR-Mut (Fig. 4B). By contrast,
mRNA expression of the cell cycle-related proteins, CCND1 and CDK4,
was lower in α6+/β1+EpSCs transfected with miR-let-7b than
that in α6+/β1+EpSCs transfected with miR-Mut (Fig. 4B). The western blotting results
confirmed that p53 protein expression was significantly increased
in α6+/β1+EpSCs transfected with miR-let-7b compared with
that in α6+/β1+EpSCs transfected with miR-Mut (Fig. 4C). The expression of the CCND1 and
CDK4 proteins was significantly decreased in α6+/β1+EpSCs
transfected with miR-let-7b compared with that in
α6+/β1+EpSCs transfected with miR-Mut (Fig. 4E). Northern blotting demonstrated a
strong let-7b hybridization signal in α6+/β1+EpSCs
transfected with miR-let-7 compared with the signal in
α6+/β1+EpSCs transfected with miR-Mut (Fig. 4D). Furthermore, FCM demonstrated
significant cell cycle arrest in α6+/β1+EpSCs transfected with
miR-let-7b. Compared with α6+/β1+EpSCs transfected with
miR-Mut, α6+/β1+EpSCs transfected with miR-let-7b were
arrested in the G2/M phase, and the percentage of S-phase cells in
this group was significantly decreased (Fig. 4E). These results indicate that
proliferation of the α6+/β1+EpSC subpopulation decreased when the
expression of cell cycle-related proteins was suppressed by the
addition of exogenous let-7b miRNA.
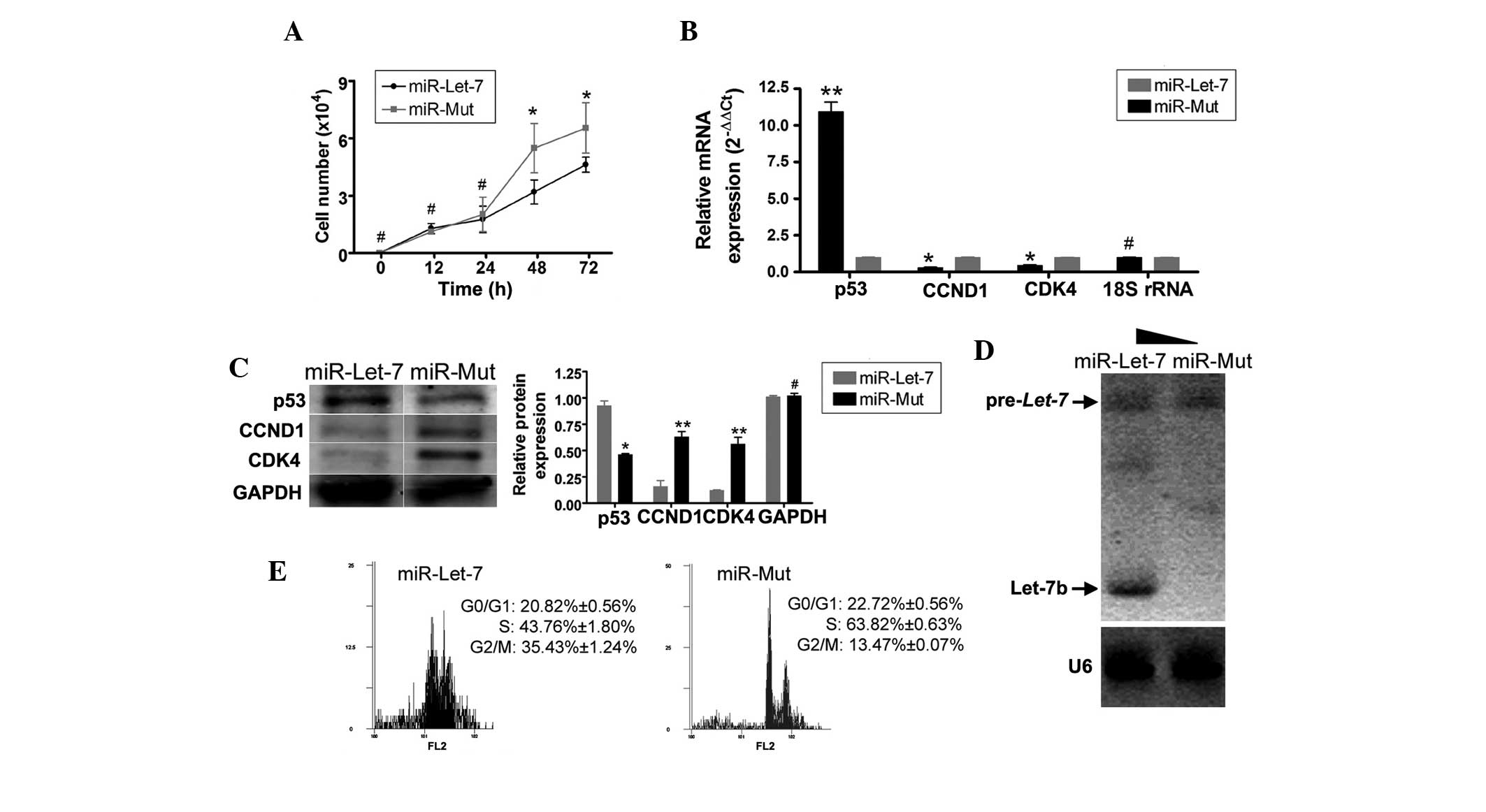 | Figure 4Overexpressed exogenous miRNA
let-7b decreased proliferation of α6+/β1+EpSCs by
interfering with CCND1 expression. (A) Between 48–72 h,
α6+/β1+EpSCs transfected with miR let-7b divided
significantly more slowly than α6+/β1+EpSCs transfected with
miR-Mut (*P<0.05, vs. miR-Mut; n=3). (B) RT-qPCR
demonstrated that p53 mRNA expression was markedly higher in
α6+/β1+EpSCs transfected with miR let-7b than that in α6+/β1+EpSCs
transfected with miR-Mut. By contrast, mRNA expression levels of
the cell cycle-related factors, CCND1 and CDK4, were markedly lower
in α6+/β1+EpSCs transfected with miR let-7b than in α6+/β1+EpSCs
transfected with miR-Mut (**P<0.01, vs. miR-Mut and
*P<0.05, vs. miR-Mut; n=3). (C) Western blotting
confirmed that p53 protein expression was significantly increased
in α6+/β1+EpSCs transfected with miR let-7b. The expression
of CCND1 and CDK4 proteins was significantly decreased in
α6+/β1+EpSCs transfected with miR let-7b
(**P<0.01, vs. miR-Mut and *P<0.05, vs.
miR-Mut; n=3). (D) Northern blotting demonstrated a strong
let-7b hybridization signal in α6+/β1+EpSCs transfected with
miR let-7 compared with that in α6+/β1+EpSCs transfected with
miR-Mut. (E) FCM demonstrated that α6+/β1+EpSCs transfected with
miR let-7b were arrested in the G2/M phase, and that the
percentage of cells in the S phase was significantly reduced.
EpSCs, epidermal stem cells; CCND1, cyclin D1; CDK4,
cyclin-dependent kinase 4; RT-qPCR, quantitative reverse
transcription-polymerase chain reaction; FCM, flow cytometry. |
Discussion
Clinically, anal papillae may result in increases in
local secretions, blood in the feces and anal itching (1–3).
However, the pathogenesis of hypertrophic anal papillae remains
unclear. The present study examined the mechanism underlying the
development of hypertrophic anal papillae, with respect to the
presence of EpSCs and miRNA-mediated epigenetics. In clinical
practice, the growth of anal papilla in certain patients is rapid,
and regrowth of new anal papilla tissue occurs in <1 year
following surgical removal (1–3). It
was hypothesized that the presence of cellular growth factors or
stem cells, promoted cell proliferation and frequent regeneration.
Due to the anatomical sites of anal papilla, it was hypothesized
that EpSCs may be involved. There are various types of stem cells
in the EpSC family, including follicular stem cells, hair follicle
stem cells and sebaceous isthmus precursor stem cells (5,6).
EpSCs exhibit three basic stem cell characteristics, which are
proliferation, self-renewal, as well as the ability to
differentiate into keratinocytes (5,6). In
mammals, EpSCs produce a large number of mature skin cells in order
to replace the epidermal and hair loss that occurs on a daily
basis, and they exhibit a high metabolism. The skin is one of the
most important regenerative organs in the body, and requires the
rapid proliferation and differentiation potential of EpSCs. The
present study found that α6+/β1+EpSCs cells were present in
patients with hypertrophic anal papillae. Following isolation and
enrichment of these α6+/β1+EpSCs, it was found that they exhibited
a rapid rate of proliferation in vitro, and expressed
elevated levels of cell cycle-related proteins. These results
suggest that EpSCs are present in human hypertrophied anal papillae
and that an increased rate of proliferation of these cells may be
one of the causes of anal papilla hyperplasia.
There are a variety of mechanisms involved in the
regulation of cell proliferation. Epigenetic regulation of
transcription and of the expression of cell cycle-related genes is
an area of current research. The miRNA, let-7b, is involved
in the regulation of cell proliferation. The present study explored
whether miRNA let-7b is involved in hypertrophic anal
papilla hyperplasia. let-7b is a well-studied miRNA that is
known to be involved in regulation of the cell cycle and
development, and is underexpressed in various types of cancer
(21). Restoration of normal
let-7b expression has been shown to inhibit cancer growth by
targeting various oncogenes and inhibiting the key regulators of a
number of mitogenic pathways (21–24).
The present study found that α6+/β1+EpSC expression of endogenous
microRNA let-7b was significantly lower than that in
α6-/β1-cells. Furthermore, target genes of microRNA let-7b,
including the cell cycle regulatory factors, CCND1 and CDK4, were
upregulated in α6+/β1+EpSCs compared with α6-/β1-cells. When the
exogenous precursor microRNA let-7 was overexpressed in
α6+/β1+EpSCs, it was demonstrated that the proliferation of
α6+/β1+EpSCs was significantly less rapid than EpSCs transfected
with miR-Mut. The expression of mature microRNA let-7b was
significantly higher, while that of cyclin CCND1 and CDK4 was
reduced. Compared with α6+/β1+EpSCs transfected with miR-Mut,
α6+/β1+EpSCs transfected with miR-let-7b were arrested in
the G2/M phase, and the percentage of S-phase cells in this group
was significantly reduced. These data indicate that proliferation
of the α6+/β1+EpSCs subpopulation was decreased when the level of
endogenous cell cycle-related proteins was suppressed by
overexpression of exogenous miRNA let-7b.
In conclusion, the present data suggest that two
important mechanisms contribute to the development and recurrence
of hypertrophic anal papillae. The first is hyperproliferation of
EpSCs. The second is the expression of miRNA let-7b, which
modulates expression of the cell cycle-related proteins, CCND1 and
CDK4, and contributes to excessive cellular proliferation.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81202811 and
81403401), the Project funded by China Postdoctoral Science
Foundation (grant no. 2014M550250), Shanghai Municipal Health
Bureau Fund (grant no. 20124320), Shanghai Municipal Health Bureau
Fund (grant no. 20124Y013) and Shanghai 'Xing Lin Xin Xing' Fund
(grant no. ZYSNXD011-RC-XLXX-20130025).
References
|
1
|
Gupta PJ: Hypertrophied anal papillae and
fibrous anal polyps, should they be removed during anal fissure
surgery? World J Gastroenterol. 10:2412–2414. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta PJ and Kalaskar S: Removal of
hypertrophied anal papillae and fibrous anal polyps increases
patient satisfaction after anal fissure surgery. Tech Coloproctol.
7:155–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta PJ: A study of the symptomatology of
hypertrophied anal papillae and fibrous anal polyps. Bratisl Lek
Listy. 106:30–33. 2005.PubMed/NCBI
|
|
4
|
Reiisi S, Esmaeili F and Shirazi A:
Isolation, culture and identification of epidermal stem cells from
newborn mouse skin. In vitro Cell Dev Biol Anim. 46:54–59. 2010.
View Article : Google Scholar
|
|
5
|
Janes SM, Lowell S and Hutter C: Epidermal
stem cells. J Pathol. 197:479–491. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barthel R and Aberdam D: Epidermal stem
cells. J Eur Acad Dermatol Venereol. 19:405–413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen S, Takahara M, Kido M, et al:
Increased expression of an epidermal stem cell marker, cytokeratin
19, in cutaneous squamous cell carcinoma. Br J Dermatol.
159:952–955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luis NM, Morey L, Mejetta S, et al:
Regulation of human epidermal stem cell proliferation and
senescence requires polycomb-dependent and-independent functions of
Cbx4. Cell Stem Cell. 9:233–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watt FM: Role of integrins in regulating
epidermal adhesion, growth and differentiation. EMBO J.
21:3919–3926. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nanba D, Toki F, Matsushita N, Matsushita
S, Higashiyama S and Barrandon Y: Actin flament dynamics impacts
keratinocyte stem cell maintenance. EMBO Mol Med. 5:640–653. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sumazin P, Yang X, Chiu HS, et al: An
extensive microRNA-mediated network of RNA-RNA interactions
regulates established oncogenic pathways in glioblastoma. Cell.
147:370–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poulton JS, Huang YC, Smith L, et al: The
microRNA pathway regulates the temporal pattern of Notch signaling
in Drosophila follicle cells. Development. 138:1737–1745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lei P, Li Y, Chen X, Yang S and Zhang J:
Microarray based analysis of microRNA expression in rat cerebral
cortex after traumatic brain injury. Brain Res. 1284:191–201. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoo AS, Sun AX, Li L, et al:
MicroRNA-mediated conversion of human fibroblasts to neurons.
Nature. 476:228–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai Y, Qiu Z, Diao Z, et al: MicroRNA-155
inhibits proliferation and migration of human extravillous
trophoblast derived HTR-8/SVneo cells via down-regulating cyclin
D1. Placenta. 33:824–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu T, Shen D, Xing S, et al: Attenuation
of exogenous angiotensin II stress-induced damage and apoptosis in
human vascular endothelial cells via microRNA-155 expression. Int J
Mol Med. 31:188–196. 2012.PubMed/NCBI
|
|
18
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El Ouaamari A, Baroukh N, Martens GA,
Lebrun P, Pipeleers D and van Obberghen E: miR-375 targets
3′-phosphoinositide-dependent protein kinase-1 and regulates
glucose-induced biological responses in pancreatic beta-cells.
Diabetes. 57:2708–2717. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu T, Cheng W, Huang Y, Huang Q, Jiang L
and Guo L: Human amniotic epithelial cell feeder layers maintain
human iPS cell pluripotency via inhibited endogenous microRNA-145
and increased Sox2 expression. Exp Cell Res. 318:424–434. 2012.
View Article : Google Scholar
|
|
21
|
Barh D, Malhotra R, Ravi B and Sindhurani
P: MicroRNA let-7: An emerging next-generation cancer therapeutic.
Curr Oncol. 17:70–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schultz J, Lorenz P, Gross G, Ibrahim S
and Kunz M: MicroRNA let-7b targets important cell cycle molecules
in malignant melanoma cells and interferes with
anchorage-independent growth. Cell Res. 18:549–557. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dangi-Garimella S, Yun J, Eves EM, et al:
Raf kinase inhibitory protein suppresses a metastasis signalling
cascade involving LIN28 and let-7. EMBO J. 28:347–358. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu F, Yao H, Zhu P, et al: Let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu T, Huang Y, Guo L, Cheng W and Zou G:
CD44+/CD105+ human amniotic fluid mesenchymal stem cells survive
and proliferate in the ovary long-term in a mouse model of
chemotherapy-induced premature ovarian failure. Int J Med Sci.
9:592–602. 2012. View Article : Google Scholar : PubMed/NCBI
|