Introduction
Ovarian cancer is one of the most lethal types of
gynecological malignancy. The response to platinum-based
chemotherapy is poor in recurrent patients, thus, the aim of
current research is to establish novel therapeutic methods
(1).
Cell lines and animal models are valuable research
tools for increasing understanding of the molecular mechanisms of
neoplasia and for developing potential therapeutic strategies
(2). In our previous studies, the
SKOV3 human ovarian carcinoma cell line was developed and served as
a reliable model of ovarian cancer disease progression (3).
Interleukin (IL)-2 is a cytokine from the
cytokine-receptor gamma c-chain family with numerous functions,
including stimulating the proliferation of T cells, inducing the
production of natural killer cells, inducing cytotoxic T lymphocyte
generation, and facilitating the proliferation and synthesis of
immunoglobulins produced by B cells (4). IL-2 is administered to boost immunity
in patients with the HIV infection (5) and cancer, including ovarian cancer,
metastatic melanoma and renal cell carcinoma (6). IL-2 treatment reliably results in a
16–20% objective clinical response rate in cancer patients,
demonstrating significant durability of responses in selected
patients (7).
However, when IL-2 is administered systemically at
high doses, the tumor regression is associated with transient
side-effects ranging from general malaise, fever, nausea, and
vomiting to the more severe reactions, including hepatic
dysfunction, increased capillary permeability and decreased
systemic vascular resistance (8,9).
Therefore, a therapeutic strategy that specifically delivers IL-2
to the tumor location may significantly reduce the required IL-2
dosage, thus alleviating many of the side-effects that are commonly
associated with its systemic administration (10).
Human amniotic fluid (AF)-derived stem cells are
becoming an important source of cells for cellular therapy. They
have the ability to differentiate into cells of all three embryonic
germ layers and possess a high proliferation rate (3). During our previous study, mesenchymal
stem cells (MSCs) were isolated from human second and third
trimester AF and their biological characteristics were demonstrated
(3), in addition, a series of
experiments were conducted to investigate the possible therapeutic
functions of these human AF-MSCs.
In the present study, IL-2 and the green fluorescent
protein (GFP) gene were fused to form a plasmid vector, enhanced
GFP-human interleukin-2 (pEGFP-hIL-2), which was stably transfected
into AF-MSCs. These stem cells were intravenously injected at
various doses into ovarian cancer nude mice models. Tumor
formation, and the expression and activity of hIL-2 were analyzed.
The pathological examination results were used to identify the
therapeutic action of hIL-2 on ovarian cancer. The aim of the
current study was to evaluate the migratory potential of AF-MSCs
into tumor cells in vivo, as well as determine their
function as delivery vehicles for anti-tumor molecules, such as
IL-2, to neoplasia sites (11,12).
Materials and methods
Materials and ethical approval
Unless otherwise stated, all chemicals used in the
present study were purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). The current study was conducted in accordance
with the Declaration of Helsinki and EU Directive 2010/63/EU.
Written and verbal informed consent was obtained from each
volunteer, and the study protocol was approved by the Ethics
Committee of Harbin Medical University (Harbin, China). All animal
experiments were conducted in accordance with the institutional
Policies and Guidelines for the Care and Use of Laboratory Animals
and all efforts were made to minimize animal suffering.
Isolation and culture of MSCs from human
second trimester AF
AF samples were harvested from 30 female volunteers
who underwent amniotic membrane puncturation. The mean ± standard
deviation of pregnancy duration (fetal age + 2 weeks) was 18±1
weeks. The AF samples were collected by puncture through the
amniotic membrane and MSCs were isolated from the AF of 22
samples.
The samples were centrifuged at 100 × g for 5 min
and all of the isolated cells were plated in six 35-mm petri dishes
containing low-glucose Dulbecco's modified Eagle's medium
supplemented with 100 U/ml penicillin, 0.1 mg/ml streptomycin, 10
ng/ml basic fibroblast growth factor, 10 ng/ml epidermal growth
factor (all obtained from PeproTech, Inc., Rocky Hill, NJ, USA) and
20% fetal bovine serum. The medium was renewed after the cells had
been incubated at 37°C in a humidified atmosphere of 5% carbon
dioxide for 7 days; any non-adhering cells were removed. After
expansion to 70% confluence, the cells were harvested by
trypsinization and subcultured. Markers of AF-MSCs were analyzed by
reverse-transcription polymerase chain reaction (RT-PCR), flow
cytometry and differentiation potential assays (4).
EGFP gene transfection into AF-MSCs
For transfection of the EGFP gene into the AF-MSCs,
40 µl Lipofectamine™ 2000 and 3 µg pEGFP-N1 (Clontech
Laboratories, Inc., Mountain View, CA, USA) were dissolved into 2
ml antibiotic-free AF-MSC growth medium. The AF-MSCs
(5×104 cells) were passaged four times and cultured in
the1-ml Lipofectamine™ 2000/pEGFP-N1 mixture for 20 h. The mixture
was removed and the cells were cultured for 1 week with an AF-MSC
culture medium containing G418 (350 µg/ml). The expression
of EGFP in the cells was monitored under an inverted UV microscope
(TE2000-U; Nikon, Inc., Tokyo, Japan). One week later, the stable
transfectants were selected and the transfected AF-MSCs were
passaged as a single-cell suspension. RNA was extracted from the
fifth passage of the EGFP gene-transfected AF-MSCs, and used for
RT-PCR analysis of the cell markers.
Absence of tumor formation
The EGFP-transfected human AF-MSCs were injected
into the rear leg muscles of 4-week-old male SCID beige mice
(CB17.B6-PrkdcscidLystbg/CrlVr; Heze Better
Biotechnology co., Ltd., Shandong, China). Fifteen mice were
injected with 3×106 cells per animal. Three months after
the injection, the injected muscles were subjected to histological
examination and no tumor was observed.
RT-PCR
Total RNA was extracted from the transfected AF-MSCs
using Tri Reagent according to the manufacturer's instructions, and
RT-PCR was performed using a One Step RT-PCR kit with selective DNA
primers for the genes, octamer-binding protein 4 (OCT4) and β-actin
as follows: Sense, 5′-CGTGAAGCTGGAGAAGGAGAAGCTG-3′ and antisense,
5′-CAAGGGCCGCAGCTTACACATGTTC-3′ for OCT4 (length, 247 bp); and
sense, 5′-TGGCACCACACCTTCTACAATGAGC-3′ and antisense,
5′-GCACAGCTTCTCCTTAATGTCACGC-3′ for β-actin (length, 396 bp).
NTERA-2 cl. D1 cells from a pluripotent human testicular embryonic
carcinoma cell line (ATCCCRL-1973) and MRC-5 cells derived from
human diploid fibroblasts from fetal lung tissue (ATCCCCL-171)
served as positive and negative controls, respectively, for OCT4
expression analysis.
hIL-2 gene extraction
Peripheral blood samples (4 ml) were obtained from
the volunteers and mixed with Hanks' balanced salt solution (HBSS)
at a 1:1 volume. The mixture was added to 8 ml human lymphocyte
separating medium and the samples were centrifuged at 150 × g for
15 min. The cells were separated into four layers as follows: First
layer, plasma; second layer, lymphocytes and monocytes; third
layer, lymphocyte separating medium; and fourth layer, red blood
cells (Fig. 1).
The lymphocytes from layer two were washed with
HBSS, and broken down using TRIzol. The mixture was kept still for
5 min, then mixed with chloroform (volume, 1:0.2) and centrifuged
at 225 ×g for 15 min at 4°C. Isopropyl alcohol was added to the
mixture at a volume of 1:1.2, maintained at 4°C for 1 h and
centrifuged at 225 ×g for 10 min, then washed with alcohol and
mixed with 20 µl diethylpyrocarbonate. The mixture was then
stored at −80°C in a refrigerator. RT-PCR was performed to
synthesize cDNA with the above-mentioned sample.
The DNA primers were designed according to Genbank
(http://www.ncbi.nlm.nih.gov/genbank/). The primers
were as follows: Upstream, 5′GGAATTCATGTACAGGATG3′ for passage 1
(P1); and downstream, 5′GACTGAACTCAGCTGG3′ for P2 (synthesized by
Invitrogen Life Technologies). Following the PCR reaction and
agarose gel electrophoresis, a DNA product (length, 462 bp) was
obtained and recycled.
hIL-2 gene segment cloning and
identification
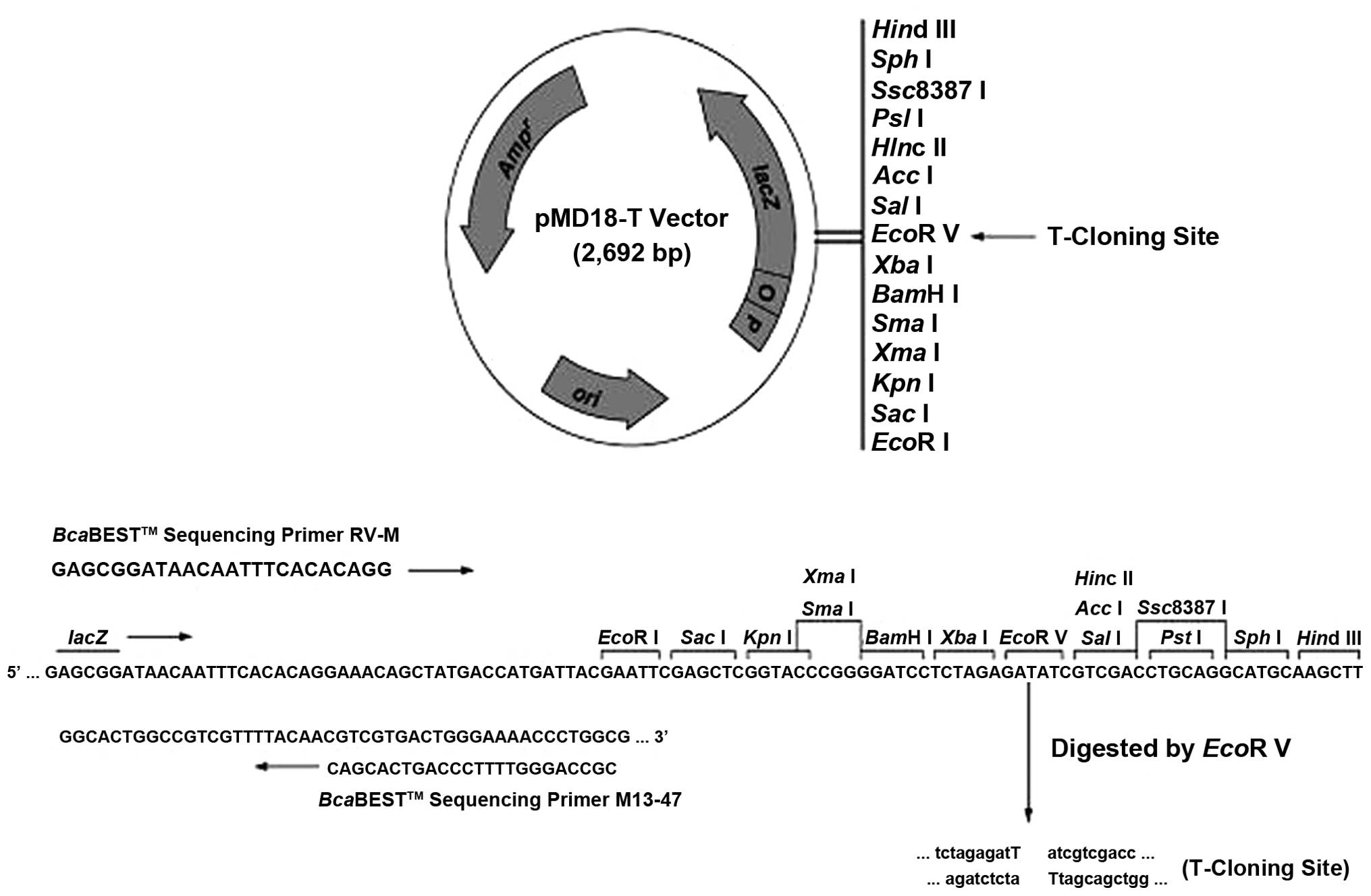
The purified product was combined with a pMD18-T
Simple Vector (Fig. 2) and 10
µl of the above-mentioned DNA product was mixed with 100
µl JM109 competent cells (provided by Dr Yin Zhe, Department
of Microbiology, China Agricultural University, Harbin, China) in
an ice bath. The mixture was placed in ice for 30 min, heated at
42°C for 90 sec and placed in an ice bath for a further 1–2 min.
Following this, 800 µl medium without antibiotics was added
into the above-mentioned sample and centrifuged at 22 × g for 40
min at 37°C. After centrifugation, ~100 µl of the
supernatant was kept and spread on Luria-Bertani (LB) agar plates
with Penbritin, X-gal and osopropyl β-D-1-thiogalactopyranoside
(100 µg/ml of each). Twenty-four hours later, the LB mixture
inoculated with Penbritin was agitated at 37°C for 8 h. The plasmid
was extracted according to the manufacturer's instructions.
Following PCR amplification, the sample was digested with
EcoRI and SalI, and agarose gel electrophoresis was
performed. The length of the DNA fragment obtained was 462 bp,
which was verified by Invitrogen Life Technologies, and the
recombinant plasmid was designated pMD-hIL-2.
Transient transfection of pMD-hIL-2 into
AF-MSCs
The AF-MSCs were inoculated during the logarithmic
growth phase and plated in 6-well petri dishes. When the cells
reached 80–90% confluence, the cells were washed with Opti-minimal
essential medium cell culture fluid (Invitrogen Life Technologies),
and the recombinant plasmid, pMD-hIL-2 was transfected into the
AF-MSCs using Lipofectamine™ 2000 reagent, according to the
manufacturer's instructions. After 48 h, the cells were selected
using 500 µg/ml G418; selection continued for 14 days, and
the monoclonal cells were isolated and cultured. hIL-2 gene
expression activity was detected in the cells using an hIL-2
detection kit. GFP gene expression was detected under a
fluorescence microscope (Eclipse 600; Nikon, Inc.).
Establishing an ovarian cancer animal
model
When the SKOV3 ovarian cancer cells (cultured in
vitro) reached 70% confluence, the cells were washed with PBS,
digested with trypsin and washed again with PBS. The cells were
then suspended in PBS and the concentration of the cells was
adjusted to 2×107 cells/ml. Each specific pathogen free
(SPF) grade nu/nu-BALB/c nude mouse (n=50; age, 4 weeks) was
administered with 0.2 ml (4×106 cells) of the suspended
cells, which were subcutaneously injected into the scapula region.
This was performed in particularly clean conditions to ensure the
procedure was aseptic.
Targeted ovarian cancer therapy using
AF-MSCs
When the subcutaneously transplanted tumor reached 1
cm in diameter, 0.2 ml of AF-MSCs transiently transfected with the
recombinant plasmid, pMD-hIL-2 were injected through the caudal
vein of each ovarian cancer mouse at 2×107 cells/ml. Two
control groups were established; a sodium chloride group and a
pEGFP-hIL-2 group, with 15 mice in each group. The littermates were
raised in an SPF animal center under identical conditions. The body
weight and tumor size of each mouse was noted daily and every seven
days, one mouse from each group was sacrificed with ether. The
fluorescence distribution was analyzed and pathological sections of
the tumor were obtained, which were used to detect ovarian cancer
cell apoptosis under an electron microscope (JEM-1200EX; JEOL,
Ltd., Tokyo, Japan).
Results
Characteristics of EGFP-transfected
AF-MSCs
Human AF-MSCs were successfully isolated from second
trimester AF (Fig. 3) and the EGFP
gene was transfected into the AF-MSCs via lipofection (Fig. 4; EGFP gene transfection efficiency,
30%). The EGFP-transfected AF-MSCs were found to express OCT4
(Fig. 5). Furthermore, EGFP was
expressed in all of the transgenic cells (following selection of
the stably transfected cell lines) and could be readily visualized;
this expression was maintained over ten passages. The growth curve
of the EGFP-transfected AF-MSCs exhibited almost the same
characteristics as untransfected AF-MSCs. From day three, the cells
entered the logarithmic growth stage and the growth peak was
observed on day seven. The doubling time was 30.5 h. No significant
difference was identified between the different passages (Fig. 6).
Ovarian cancer nude mice model
formation
SKOV3 cells were subcutaneously injected into the
right scapula region of each nude mouse. After one week, no
redness, swelling or ulcerations were observed in the injection
region and the diameter of the subcutaneously transplanted tumor
was 0.1–0.3 cm. The tumor grew at a rate of 1 cm per month.
hIL-2 gene extraction and
identification
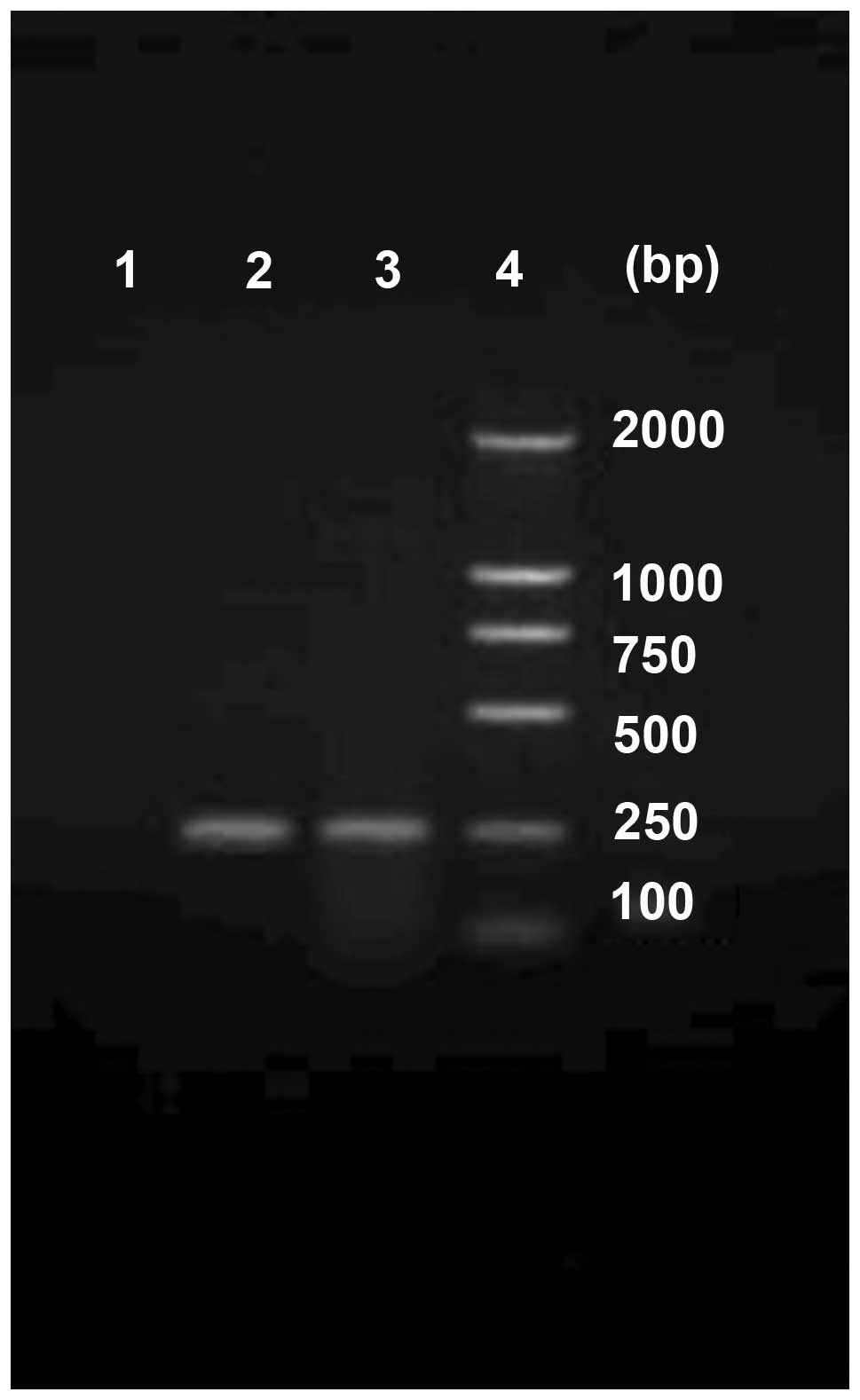
Human lymphocytes were separated from the peripheral
blood of healthy adults, the total RNA was extracted and following
RT-PCR, electrophoresis and DNA purification, the hIL-2 gene was
obtained (Fig. 7). After combining
with a pMD18-T Simple Vector, a sequencing test indicated that the
sequence was correct.
Identification of the hIL-2 gene
recombinant plasmid, PEGFP-hIL-2
The recombinant plasmid, pEGFP-hIL-2 was digested by
the two enzymes, EcoRI and SalI, and following
agarose gel electrophoresis an exogenous fragment (length, 462 bp)
was identified (Fig. 8), the
result was indicated by Invitrogen Life Technologies.
Transient transfection of the recombinant
plasmid into AF-MSCs
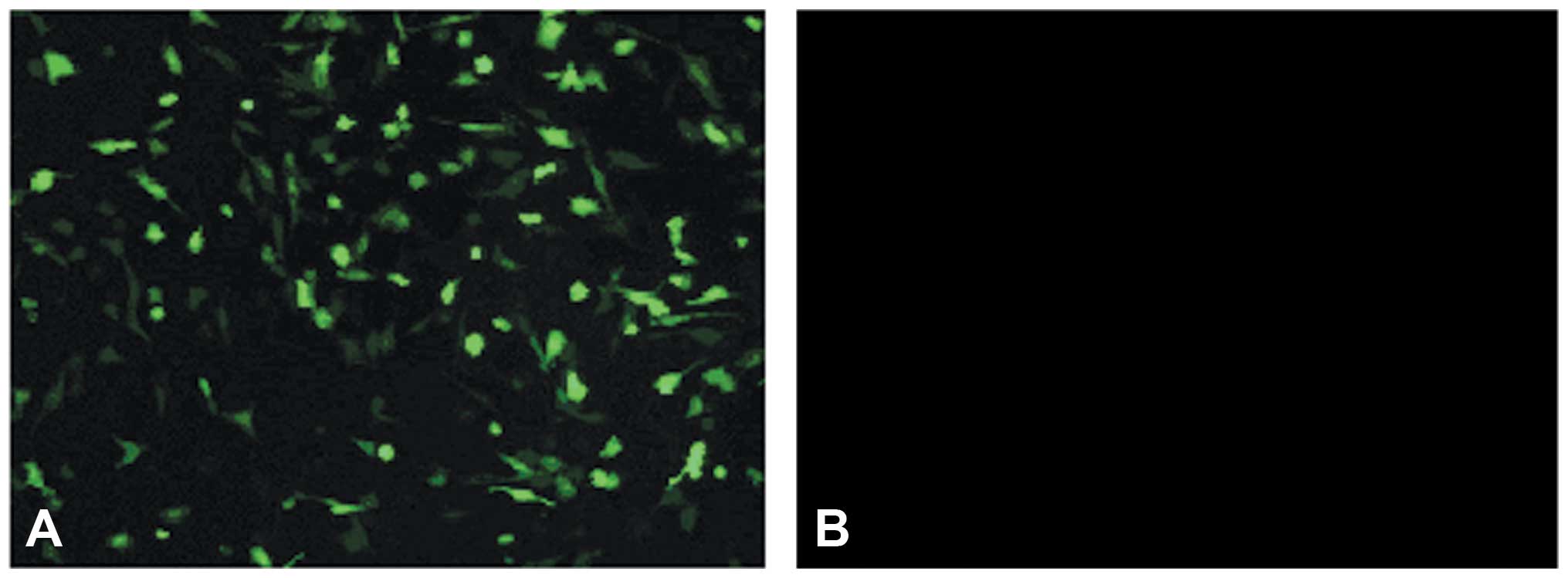
The recombinant plasmid-transfected AF-MSCs are
shown in Fig. 9A by green
fluorescence. Following continuous culture for two weeks, the green
fluorescence was stable; however, the untransfected AF-MSCs didn't
fluoresce (Fig. 9B).
hIL-2 gene expression detection
After the recombinant plasmid-transfected AF-MSCs
became stable, a hIL-2 testing kit was used to examine the clear
supernatant of these cells; the clear supernatant of the
untransfected AF-MSCs served as the negative control.
A standard substance dilution was used and the
optical density (OD) value was measured at 450 nm. The standard
curve is shown in Fig. 10. The OD
value of the clear supernatant of the recombinant
plasmid-transfected AF-MSCs at 450 nm was 1.103, while that of the
untransfected cells was 0.052. The OD value of the tested sample
was more than five times higher than that of the negative control,
therefore the result was positive according to the kit
instructions. The following formula was used: y=ax2+bx+c
as well as the standard curve, and the hIL-2 concentration in the
treated sample was found to be 17.258 pg/ml.
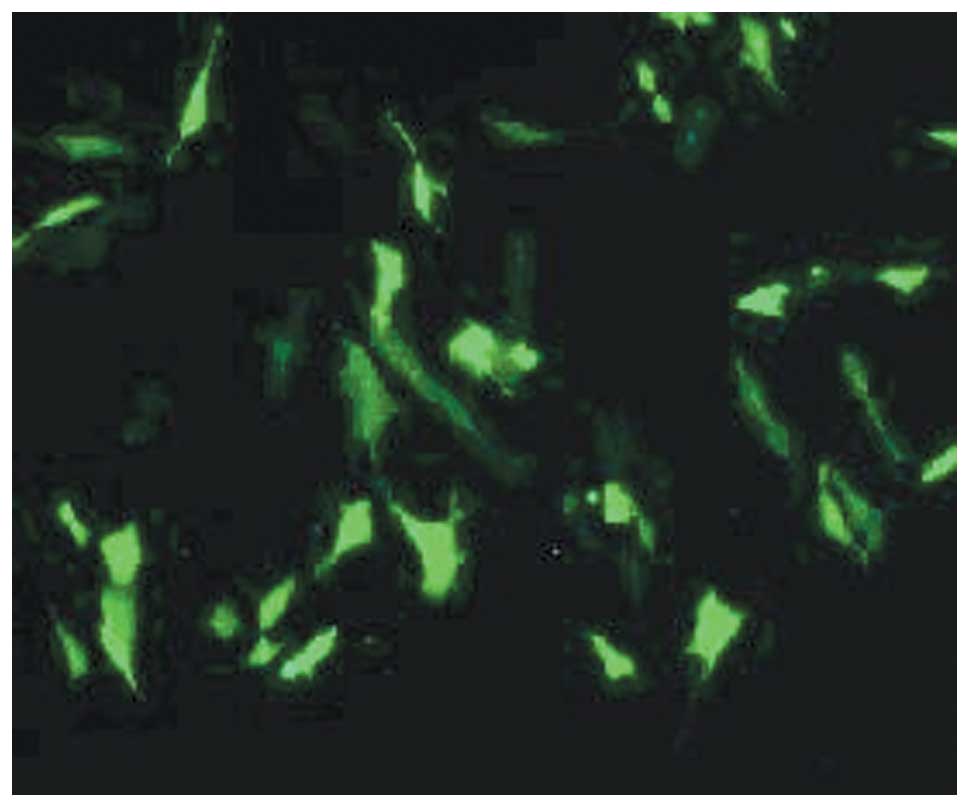
GFP gene expression detection
The recombinant plasmid-transfected AF-MSCs emitted
stable green fluorescence under a fluorescence microscope, which
demonstrated the expression of the GFP gene (Fig. 11A), while the untransfected cells
did not fluoresce (Fig. 11B).
Pathological examination of ovarian
cancer tissue and surrounding tissue
Six weeks after the recombinant plasmid-transfected
AF-MSCs were injected into the ovarian cancer mouse model, green
fluorescence was apparent around the tumor tissue, whereas the
other issue emitted only slight fluorescence. No other tumors
formed in the animal body. After the animals were sacrificed, the
tumor tissue and the surrounding tissue were immediately excised
and frozen sections were made. Green fluorescence was observed from
the AF-MSCs around the tumor tissue, as shown in Fig. 12. Acetone was used to fix the
sections and they were stained with hematoxylin and eosin
(H&E). Fusiform shaped AF-MSCs were demonstrated by the
H&E, as well as anachromasis (Fig. 13).
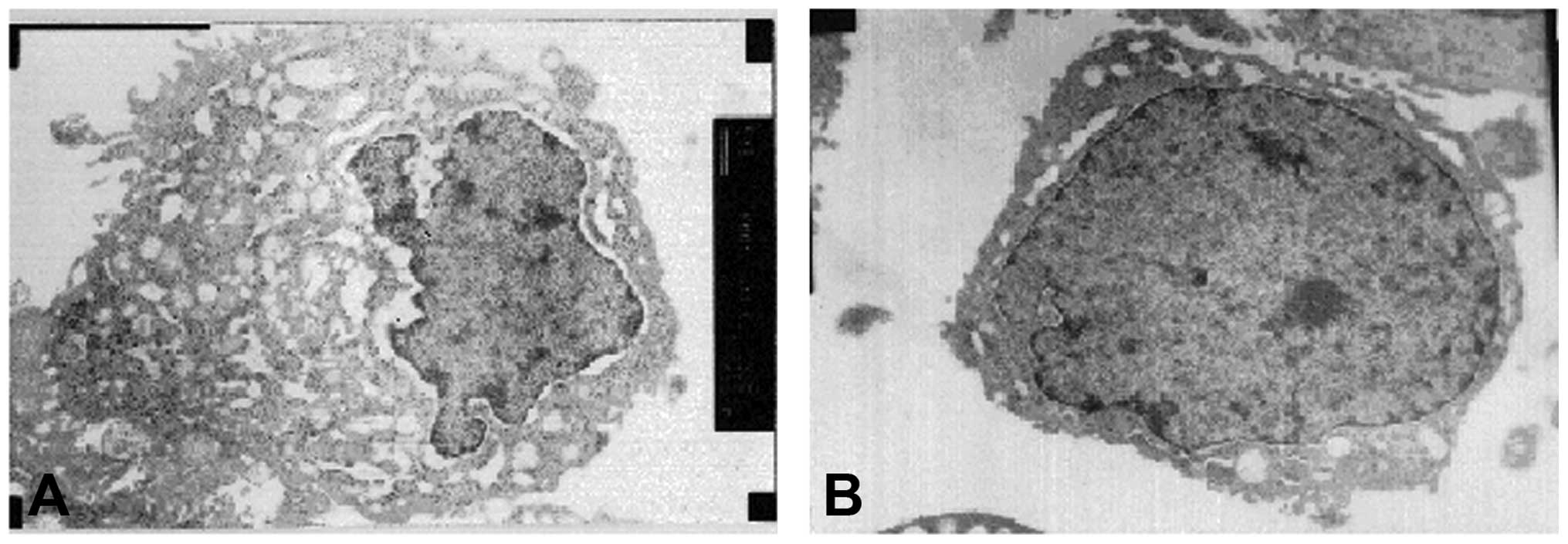
Ultrastructure examination of ovarian
cancer cells
The ovarian cancer tissue of the treatment group
(nude mice that were injected with the pEGFP-hIL-2 recombined
plasmid) was fixed using glutaraldehyde, and the ultrastructure of
SKOV3 ovarian cancer cells was observed under a transmission
electron microscope. The reductus of the nuclear membrane was
observed to be depressed, chromatin was condensed, more compact,
concentrated and parted, and there were more layers of endoplasmic
reticulum surrounding the nucleus. Furthermore, the endoplasmic
reticulum had become swollen and expanded, as shown in Fig. 14A. The ultrastructure of the
ovarian cancer cells from the untreated group (observed by
transmission electron microscope) are presented in Fig. 14B.
Discussion
Ovarian cancer is a common type of malignant tumor,
and the rate of case fatalities is the highest among the
gynecological malignant tumors. Ovarian cancer threatens the
physical and mental health of females, as well as their quality of
life. As the majority of patients have reached an advanced stage
when they are diagnosed with ovarian cancer, abdominal implantation
and metastasis occurs, resulting in a rapid decrease of the quality
of life. The five-year survival rate is ~30%. Over the past 30
years, gynecological oncological scientists worldwide have
attempted chemotherapy, radiotherapy and gene therapy on the basis
of cytoreductive surgery, and this has been the predominant
therapeutic method for ovarian cancer. However, relapse and
metastasis continue to be observed following surgery in most
advanced-stage ovarian cancer patients (13).
IL-2 administration stimulates existent forkhead box
P3 and T cell proliferation, and may promote regulatory T cell
tumor trafficking in patients with ovarian cancer (14). Certain physicians have attempted to
use IL-2 to cure patients suffering with metastasized ovarian
cancer; however, high concentrations of IL-2 are required to obtain
a therapeutic response. Although the treatment has a curative
effect, there are too many side-effects; therefore, targeted gene
therapy may act as a substitute for the traditional treatment
methods. Verification of the principle and efficiency of this
approach, to act locally at the tumor microenvironment and inhibit
malignant cell growth in vivo, was provided in other types
of cancer using MSCs derived from adult sources, such as bone
marrow (BM) (15). Specifically,
homing of MSCs following systemic or local administration has been
investigated in a variety of tumor animal models, including models
of lung metastasis (16), melanoma
(17), and brain glioma (18), amongst others. Collectively, these
studies supported the hypothesis that exogenously administrated
BM-MSCs preferentially engrafted at the tumor site by contributing
to the population of the stromal fibroblasts, thus forming the
tumor's microenvironment (19).
This important characteristic determined stem cells to be of
importance within targeted tumor therapy, particularly in patients
that have relapsed or are exhibiting a metastatic tumor, as
targeting the tumor tissue avoids undesirable side-effects in other
tissues, including liver, kidney, spleen and lung. AF-MSCs may be
considered as a powerful tool for gene therapy and tissue repair in
the clinical setting, due to their high proliferative activity and
chromosomal stability. AF-MSCs exhibited chromosomal stability with
no karyotypic abnormalities, even following high numbers of
passages, and possessed long telomeres and exhibited no tumorigenic
effect in vivo (20). In
the present study, AF-MSCs transduced with GFP were analyzed in a
mouse ovarian cancer model to evaluate the migratory properties
in vivo. GFP was expressed in all of the transgenic cells
(following selection of the stably transfected cell lines), and
notably, this expression was maintained over numerous passages. The
localization of AF-MSCs at the tumor site indicated that these
cells, similarly to BM-MSCs (21),
are able to reach the extravascular space and contribute to the
development of tumor connective stroma. It was found in the present
study that intravenously injected AF-MSCs, that stably express
hIL-2, are able to trace the subcutaneously transplanted ovarian
tumor cells, and secrete IL-2 locally, resulting in the apoptosis
of the tumor cells. The current study provides an important method
for targeted gene therapy to treat ovarian cancer. However, the
migratory properties of A-MSCs in an ovarian cancer mouse model
in vivo requires further evaluation for the potential
signaling mechanisms that may be involved.
Acknowledgments
The present study was supported by the post-doctoral
financial assistance of the Heilongjiang province (Heilongjiang,
China; grant no. LBH-Z11082), the post-doctoral financial
assistance of China (grant no. 2013M531073), the Opening Project of
the Key Laboratory of Medical Genetics (Harbin Medical University,
Heilongjiang Higher Education Institutions, Harbin 150081, China)
and the Special Prophase Project (the 973 Project; no. 2012
CB526700).
References
|
1
|
Smolle E, Taucher V, Pichler M, Petru E,
Lax S and Haybaeck J: Targeting signaling pathways in epithelial
ovarian cancer. Int J Mol Sci. 14:9536–9555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crallan RA, Georgopoulos NT and Southgate
J: Experimental models of human bladder carcinogenesis.
Carcinogenesis. 27:374–381. 2006. View Article : Google Scholar
|
|
3
|
You Q, Cai L, Zheng J, Tong X, Zhang D and
Zhang Y: Isolation of human mesenchymal stem cells from
third-trimester amniotic fluid. Int J Gynaecol Obstet. 103:149–152.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waldmann TA: The biology of interleukin-2
and interleukin-15: Implications for cancer therapy and vaccine
design. Nat Rev Immunol. 6:595–601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sereti I, Anthony KB, Martinez-Wilson H,
Lempicki R, Adelsberger J, Metcalf JA, Hallahan CW, Follmann D,
Davey RT, Kovacs JA, et al: IL-2-induced CD4+ T-cell expansion in
HIV-infected patients is associated with long-term decreases in
T-cell proliferation. Blood. 104:775–780. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tourani JM, Pfister C, Tubiana N, Ouldkaci
M, Prevot G, Lucas V, Oudard S, Malet M, Cottu P, Ferrero JM, et
al: Subcutaneous Administration Propeukin Program Cooperative
Group: Subcutaneous interleukin-2 and interferon alfa
administration in patients with metastatic renal cell carcinoma:
Final results of SCAPP III, a large, multicenter, phase II,
nonrandomized study with sequential analysis design - the
Subcutaneous Administration Propeukin Program Cooperative Group. J
Clin Oncol. 21:3987–3994. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang T, Wall EM, Milne K, Theiss P, Watson
P and Nelson BH: CD8+ T cells induce complete regression of
advanced ovarian cancers by an interleukin (IL)-2/IL-15 dependent
mechanism. Clin Cancer Res. 13:7172–7180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vlad AM, Budiu RA, Lenzner DE, Wang Y,
Thaller JA, Colonello K, Crowley-Nowick PA, Kelley JL, Price FV and
Edwards RP: A phase II trial of intraperitoneal interleukin-2 in
patients with platinum-resistant or platinum-refractory ovarian
cancer. Cancer Immunol Immunother. 59:293–301. 2010. View Article : Google Scholar
|
|
9
|
Lee JM, Yoon SH, Kim HS, Kim SY, Sohn HJ,
Oh ST, Oh IH and Kim TG: Direct and indirect antitumor effects by
human peripheral blood lymphocytes expressing both chimeric immune
receptor and interleukin-2 in ovarian cancer xenograft model.
Cancer Gene Ther. 17:742–750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang TH, Mao CP, He L, Tsai YC, Liu K, La
V, Wu TC and Hung CF: Tumor-targeted delivery of IL-2 by NKG2D
leads to accumulation of antigen-specific CD8+ T cells in the tumor
loci and enhanced anti-tumor effects. PLoS One. 7:e351412012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Page C, Génin P, Baines MG and Hiscott
J: Interferon activation and innate immunity. Rev Immunogenet.
2:374–386. 2000.
|
|
12
|
Gao Y, Yao A, Zhang W, Lu S, Yu Y, Deng L,
Yin A, Xia Y, Sun B and Wang X: Human mesenchymal stem cells
overexpressing pigment epithelium-derived factor inhibit
hepatocellular carcinoma in nude mice. Oncogene. 29:2784–2794.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lynch HT, Casey MJ, Snyder CL, Bewtra C,
Lynch JF, Butts M and Godwin AK: Hereditary ovarian cancer:
Heterogeneity, molecular genetics, pathology, and management. Mol
Oncol. 3:97–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei S, Kryczek I, Edwards RP, Zou L,
Szeliga W, Banerjee M, Cost M, Cheng P, Chang A, Redman B, et al:
Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and
tumor trafficking in patients with ovarian carcinoma. Cancer Res.
67:7487–7494. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yong RL, Shinojima N, Fueyo J, Gumin J,
Vecil GG, Marini FC, Bogler O, Andreeff M and Lang FF: Human bone
marrow-derived mesenchymal stem cells for intravascular delivery of
oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res.
69:8932–8940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Studeny M, Marini FC, Champlin RE,
Zompetta C, Fidler IJ and Andreeff M: Bone marrow-derived
mesenchymal stem cells as vehicles for interferon-beta delivery
into tumors. Cancer Res. 62:3603–3608. 2002.PubMed/NCBI
|
|
17
|
Ren C, Kumar S, Chanda D, Chen J, Mountz
JD and Ponnazhagan S: Therapeutic potential of mesenchymal stem
cells producing interferon-alpha in a mouse melanoma lung
metastasis model. Stem Cells. 26:2332–2338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bexell D, Scheding S and Bengzon J: Toward
brain tumor gene therapy using multipotent mesenchymal stromal cell
vectors. Mol Ther. 18:1067–1075. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spaeth E, Klopp A, Dembinski J, Andreeff M
and Marini F: Inflammation and tumor microenvironments: Defining
the migratory itinerary of mesenchymal stem cells. Gene Ther.
15:730–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Coppi P, Bartsch G Jr, Siddiqui MM, Xu
T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ,
et al: Isolation of amniotic stem cell lines with potential for
therapy. Nat Biotechnol. 25:100–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren C, Kumar S, Chanda D, Chen J, Mountz
JD and Ponnazhagan S: Therapeutic potential of mesenchymal stem
cells producing interferon-alpha in a mouse melanoma lung
metastasis model. Stem Cells. 26:2332–2338. 2008. View Article : Google Scholar : PubMed/NCBI
|