Introduction
Limb ischemia is deleterious to human health, and
many patients are not suitable for surgical treatment due to
various factors, including anatomy. Therefore, the prognosis of
limb ischemia is poor, and causes great discomfort. In addition,
therapeutic strategies are rarely successful, leading to limb
amputation in many patients, specifically those who suffer from
severe pain. Increasing the levels of angiogenesis and improving
circulation in ischemia limbs may provide the most effective
treatment, and is thus the subject of numerous studies (1,2).
Stem cell transplantation in the treatment of ischemic diseases has
recently been investigated by numerous studies (3,4),
with animal model experiments and clinical studies being carried
out in order to elucidate the effects of stem cell transplantation
on ischemic diseases.
Stem cell transplantation has limitations,
predominantly associated with the quality and quantity of
endothelial progenitor cells (EPCs), which requires improvement in
order for treatment to be successful. Using genetic engineering to
improve the quality of EPCs, thus reducing the dosage of EPCs
required for transplantation, may provide a promising method of
limb ischemia treatment.
Vascular endothelial growth factor (VEGF) is an
endothelial cell-specific growth factor and a regulator of
physiological and pathological angiogenesis. In addition, VEGF is
able to increase the permeability of capillary vessels to different
macromolecules. VEGF consists of a family of polypeptide isoforms
generated from a single-copy gene by alternative splicing of the
primary transcript. VEGF is secreted by intact cells as a dimer of
the four isoforms of 121, 165, 189, and 206 amino acids and are
secreted from cells via a poorly characterized pathway (5). In the present study, in vitro
EPCs were obtained from the differentiation of bone marrow
mononuclear cells (BM-MNCs) extracted from New Zealand rabbits.
VEGF165 gene transfection was subsequently performed, and the cells
were transplanted into New Zealand rabbits with ischemic hind
limbs. The levels of neovascularization and the effects of
transplantation on limb ischemia were detected. The present study
aimed to provide an experimental basis for the clinical application
of stem cells and gene therapy in the treatment of ischemic
diseases.
Materials and methods
Animals
New Zealand rabbits (age, 3–4 months) were obtained
from the Animal Laboratory of Beijing Institute of Heart Lung and
Blood Vessel Diseases (Beijing, China). The animals were maintained
at a room temperature of 20–22°C and humidity of 40–70% under a
12-h light/dark cycle, with access to food and water ad
libitum. The study was approved by the ethics committee of
Beijing Anzhen Hospital, Capital Medical University (Beijing,
China).
EPC isolation, culture, and
identification
New Zealand rabbits were intravenously injected via
the marginal ear vein with 3% sodium pentobarbital (30 mg/kg;
Beijing Tianlai Biologic Products, Inc., Beijing, China), and
bilateral iliac crest multipoint punctures were performed prior to
marrow fluid aspiration. The quantity of bone marrow obtained from
a single puncture was ~10–15 ml. Gradient centrifugation was
performed in Ficoll (Sigma-Aldrich, St. Louis, MO, USA) in order to
separate the mononuclear cells. The cells were then plated onto
culture dishes in M199 culture medium (Gibco Life Technologies,
Carlsbad, CA, USA) supplemented with 15% fetal bovine serum (GE
Healthcare Life Sciences, Logan, UT, USA), 20 µg/ml VEGF
(PeproTech EC Ltd., London, UK), 2 µg/ml alkaline fiber cell
growth factor (bFGF; PeproTech EC Ltd.), 2 µg/ml
insulin-like growth factor-1 (IGF-1; PeproTech EC Ltd.), and half
of the medium was changed every 3–4 days.
Following 10 days of culture, the cells were
collected and fixed in sections, prior to being observed under a
transmission electron microscope (TEM; JEM-1010; JEOL., Ltd.,
Tokyo, Japan). The cells were fixed and mounted onto slides
following 14 days of culture, and indirect immunofluorescence was
carried out using the following primary antibodies: Rabbit-anti-von
Willebrand factor (vWF) polyclonal antibody (sc14014; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), and rabbit-anti-CD133
monoclonal antibody (ab19898; Abcam plc, Cambridge, UK), and the
following secondary antibodies: Fluorescein isothiocyanate
(FITC)-labeled goat-anti-rabbit antibody (sc3839; Santa Cruz
Biotechnology, Inc.), and tetramethylrhodamine (TRITC)-labeled
goat-anti-rabbit antibody (sc3841; Santa Cruz Biotechnology, Inc.).
All dilutions were 1:200.
In vitro gene transfer in EPCs
Following 14 days in culture, the EPCs were
transfected with a recombinant VEGF165-encoding gene (Ad-VEGF165)
using Lipofectamine 200 (Invitrogen Life Technologies, Carlsbad,
CA, USA), and post-transfection, the cells were incubated at 37°C
in an atmosphere containing 5% CO2 for 2 h. Three
multiplicities of infection (MOI; 10, 50, and 100) were used, and
cellular proliferation was assessed by MTT assay (Sigma-Aldrich). A
total of 20 ml MTT solution (5 mg/ml) was added to each well, and
colorimetric assays were conducted at λ=570 nm following incubation
for 4 h. Following experimentation, the EPCs were transfected with
50 MOI Ad/VEGF165, and the transfection efficiency was measured by
fluorescence using a bx51m fluorescence microscope (Olympus Optical
Co., Ltd., Tokyo, Japan). EPCs were transfected with either Ad or
Ad-VEGF165, and the proliferative activity of the non-transfected
cells (non/EPCs), Ad-transfected EPCs (Ad/EPCs), and
Ad-VEGF165-transfected EPCs (Ad-VEGF/EPCs) was evaluated.
Analysis of the protein expression levels
of VEGF by ELISA
On days 1, 4, 7, and 14 following gene transfer, the
Ad-VEGF165/EPCs, Ad/EPCs, and non/EPCs were reseeded
(5×103/200 µl/well), and the supernatant was
collected. The protein expression levels of VEGF were detected
using an ABC-ELISA (Beijing Jingmei Biologic Products, Inc.,
Beijing, China) with the supernatant as the sample.
Establishment of a New Zealand rabbit
model with hind limb ischemia
Sodium pentobarbital was intravenously injected into
the marginal ear vein of male New Zealand rabbits, and a 2.5 cm
longitudinal incision was made at the groin. The incision was made
in the skin and subcutaneous tissue, and the soft tissues were
subsequently separated from the femoral artery, which was carefully
dissected from the femoral vein and nerve. Each branch of the
femoral artery was then dissected, and the deep femoral arteries,
superficial epigastric arteries, and inferior epigastric arteries
were sectioned and ligated. During the surgical procedure, the
femoral vein and nerve were protected.
EPC transplantation
Following culture for 10 days the EPCs were digested
with trypsin (Gibco Life Technologies) in order to obtain a single
cell suspension, and the cell concentration was adjusted to
5×105 cells/ml. Purebred male New Zealand rabbits were
randomly divided into three groups (n=20) two days following the
ischemic surgical procedure: (A) A control group that received
culture medium; a (B) Ad/EPCs-transplantation group; and a (C)
Ad-VEGF165/EPCs-transplantation group. A total of 10 points
(0.5×0.5 cm) were selected in each New Zealand rabbit ischemia limb
for injection of the cell suspension, and 0.5 ml cell suspension
(5×106) being injected into each point (5 ml/rabbit in
total). The group A rabbits were injected with 5 ml M199
medium.
Monitoring of transplanted cells
In order to monitor the fate of the transplanted
EPCs, the EPCs were labeled with 5-bromo-deoxyuridine (Brdu;
Sigma-Aldrich). Two New Zealand rabbits from each group were
transplanted with Brdu-EPCs, as previously described (6). Seven days post-transplantation, the
rabbits were sacrificed via the injection of 3% sodium
pentobarbital, and the gastrocnemius muscle of the ischemic limbs
was obtained and sectioned (6 µm) prior to being frozen and
assayed by Brdu immunohistochemical staining.
Histological assessment
On days 7, 14, 21, and 28 following the surgical
procedure, two rabbits from each group were randomly selected and
sacrificed via the injection of 3% sodium pentobarbital, and the
gastrocnemius muscle of the ischemia limbs was obtained and
sectioned prior to being fixed in paraffin. The tissue specimens
were examined by hematoxylin and eosin (HE) staining, and the
endothelial cells in the tissue samples were determined by
immunohistochemical staining using a vWF antibody. The capillary
density of the vWF-positive cells was determined by counting the
number of cells in five random high-power (magnification, ×200)
fields using a CKX-31 microscope (Olympus Optical Co., Ltd.).
Monitoring of the skin temperature
changes of New Zealand rabbits following transplantation
On days 2, 4, 7, 14, and 28 following
transplantation, changes in skin temperature were monitored by
scanning the lower limbs of the rabbits using an infrared scanner
(FLIR Systems, Wilsonville, OR, USA).
Analysis of the levels of recanalization
in the New Zealand rabbits by computer tomography ateriography
(CTA)
On the 14th day following transplantation, the New
Zealand rabbits of each group underwent CTA using a 64-slice spiral
computerized tomography scanner (Siemens, Munich, Germany). The
rabbits were anesthetized by intravenous injection of 3% sodium
pentobarbital into the marginal ear vein, and a scalp needle was
fixed following anesthesia to connect the syringes. The CT scans
were carried out, and the lower limb artery reconstruction of each
group was examined.
Statistical analysis
All data were analyzed by SPSS 11.5 (SPSS, Inc.,
Chicago, IL, USA). The results of the groups were compared using a
one-way analysis of variance, and R-E-G-W-Q and Tukey tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
EPC isolation, culture, and
identification
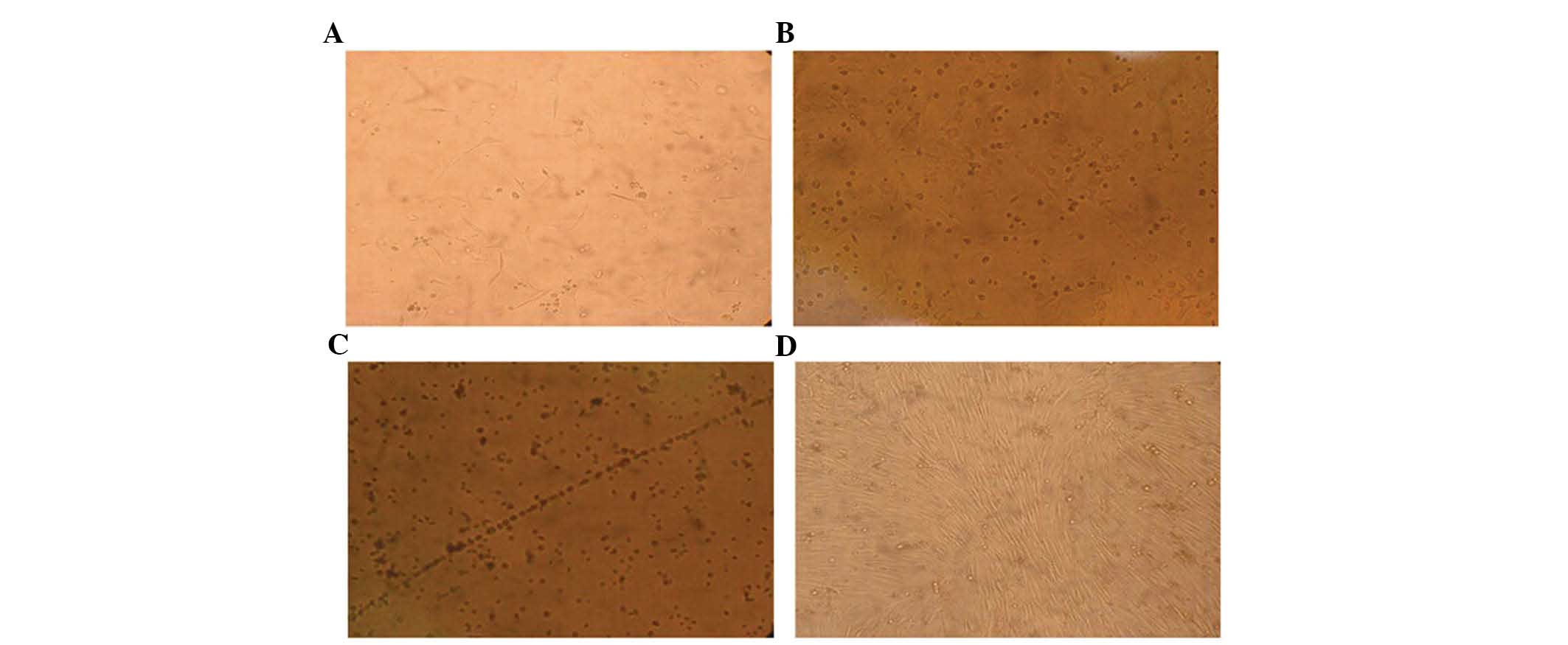
A small number of primary cells adhered to the
surface of the culture vessels following differentiation and 48–72
h culture. The number of adhering cells gradually increased, and
formed spindles (Fig. 1A and B).
Following eight days of culture, numerous spindles were observed,
having formed by the connection of adherent cells growing in a
straight line (Fig. 1C). These
observations are characteristic of endothelial cells. Numerous cell
masses appeared following 7–10 days culture, and the spindle cells
formed a bud at the edge of the cell masses, this structure
resembled a blood island. Following culture for ~14 days, the cells
fused together forming large funicular structures (Fig. 1D).
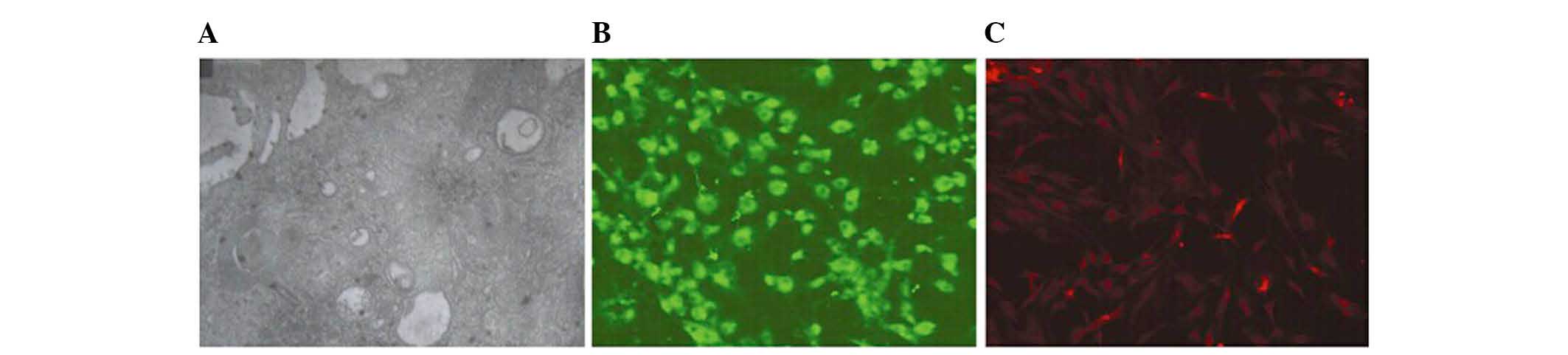
Nuclei, mitochondria, and vacuoles were observed in
the cells using TEM, along with a few pinocytotic vesicles, which
were characteristic of endothelial cell structure. Microvilli were
visible close to the cellular membrane (Fig. 2A). Using anti-vWF polyclonal
antibody and FITC-labeled antibody as primary and secondary
antibodies, respectively, the spindle cells emitted green
fluorescence under the fluorescence microscope (Fig. 2B). Using anti-CD133 monoclonal
antibody and TRITC-labeled antibody as primary and secondary
antibodies, respectively, the spindle cells emitted red
fluorescence under the fluorescence microscope (Fig. 2C). Since vWF and CD133 are
components of endothelial cells, these results indicate that the
BM-MNCs had successfully differentiated into EPCs.
Monitoring of transplanted cells
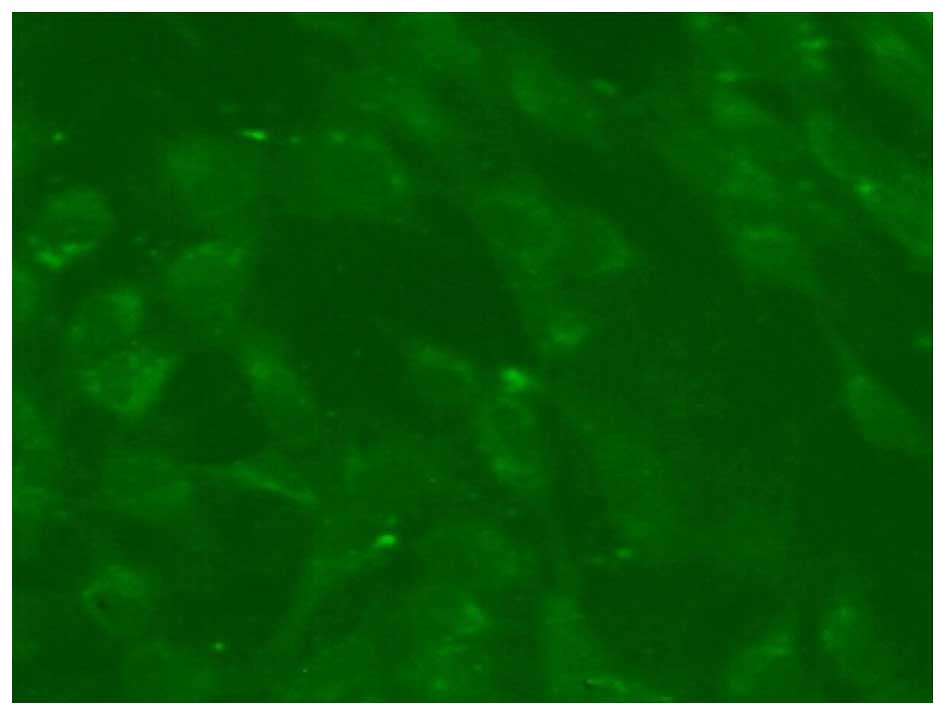
The Brdu-labeled EPCs emitted green fluorescence, as
determined by fluorescence microscopy (Fig. 3).
Proliferative activity of the transfected
EPCs
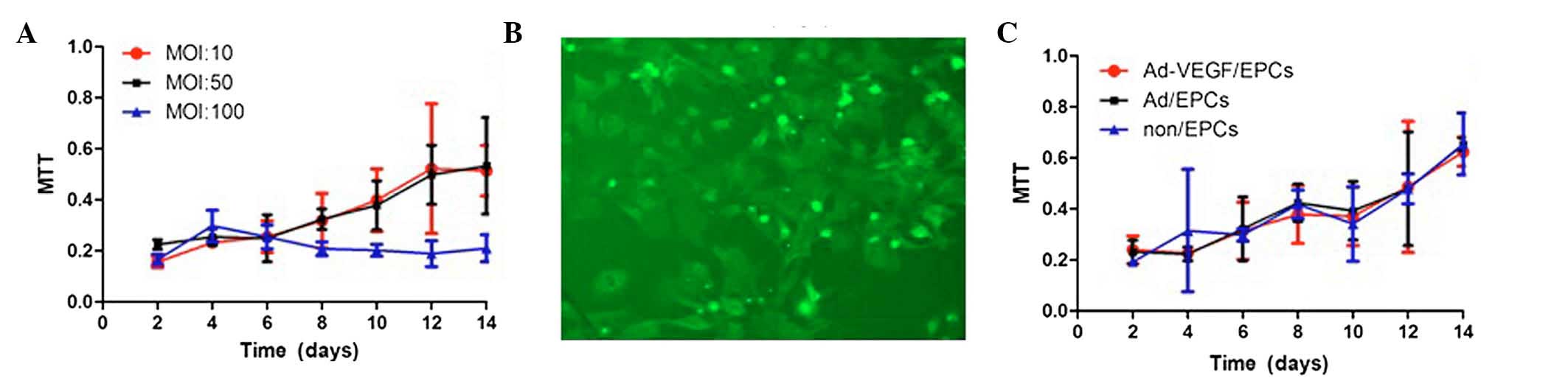
An MTT assay was used to detect the proliferative
activity of the transfected EPCs. Three multiplicities of infection
(MOI: 10, 50, and 100) were investigated (Fig. 4A). Following experimentation, the
EPCs were transfected with 50 MOI Ad/VEGF165, and the efficiency of
transfection was measured by fluorescence microscopy. The EPCs
transfected with Adv-GFP-VEGF165 contained a green fluorescent
protein gene, and 24 h post-transfection the majority of cells
emitted green fluorescence, as determined by fluorescence
microscopy (Fig. 4B). The EPCs
were transfected with either Ad or Ad-VEGF165, and the
proliferative activity of the non/EPCs, Ad/EPCs, and Ad-VEGF/EPCs
were evaluated. The proliferative activities of the non/EPCs,
Ad/EPCs, and Ad-VEGF/EPCs were similar (Fig. 4C).
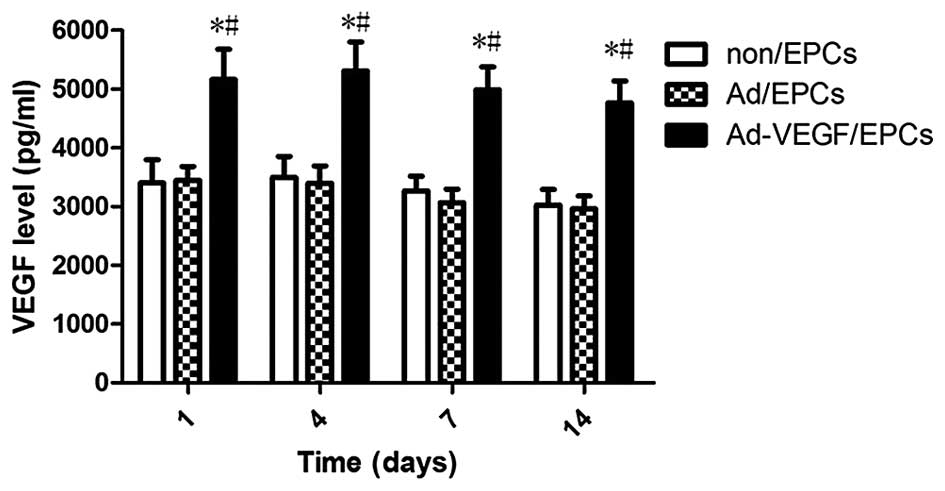
Protein expression levels of VEGF
post-transfection
On the 1, 4, 7, 14 days following gene transfer, the
Ad-VEGF165/EPCs, Ad/EPCs, and non/EPCs were reseeded, and the
supernatant was collected. The protein expression levels of VEGF
were detected in the supernatant using an ABC-ELISA. As shown in
Fig. 5, the protein expression
levels of VEGF in the Ad-VEGF165/EPCs were significantly higher, as
compared with those of the non/EPCs (P<0.01) and Ad/EPCs
(P<0.01) on the same day.
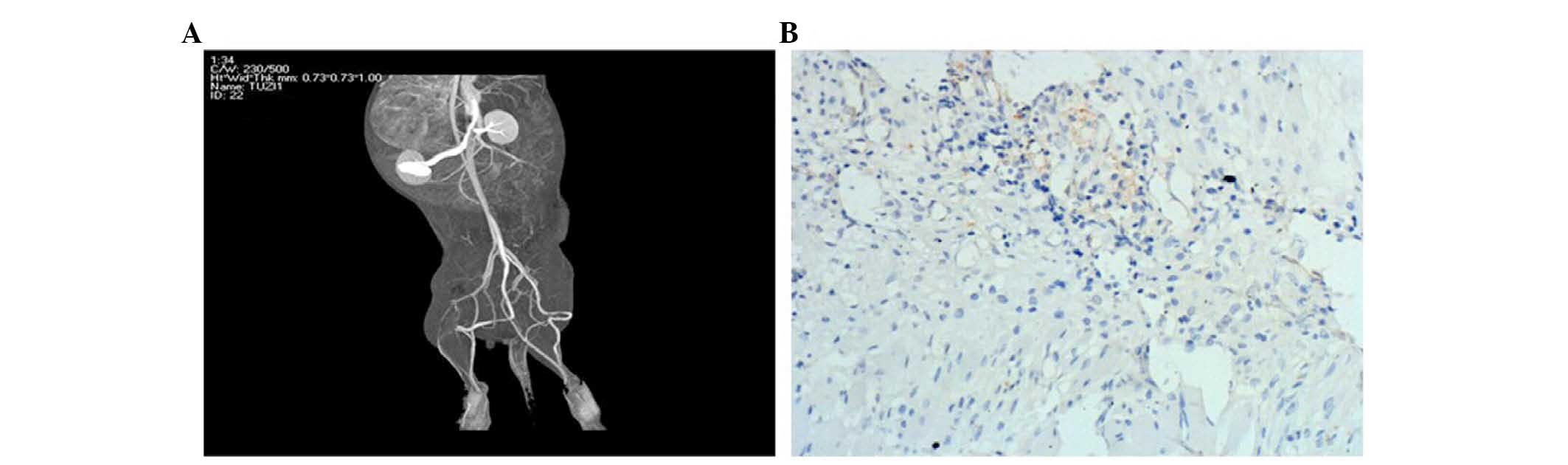
The limb ischemic model and survival of
the transplanted cells
A total of 24 h following ischemic experimentation,
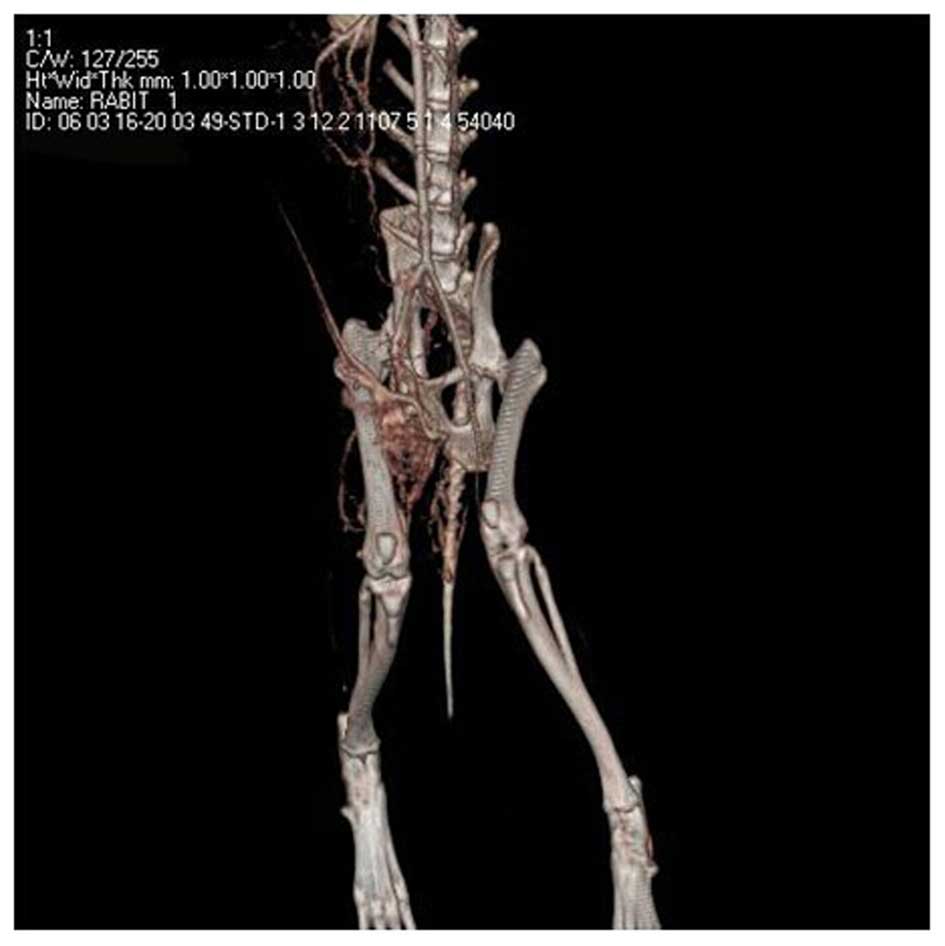
the effects of ischemia were examined by CTA. As shown in Fig. 6A, distal artery occlusion was
observed in the ischemic limb. Two New Zealand rabbits in each
group were transplanted with Brdu-labeled EPCs, as previously
described. On the 7th day following transplantation, the
gastrocnemius muscle of the ischemic limbs was sectioned and
frozen, prior to being assayed by Brdu-immunohistochemical
staining. Brdu-positive cells (brown) were observed (Fig. 6B), indicating the survival of
transplanted EPCs and directional integration of the cells into the
ischemic area, as well as EPC-induced angiogenesis.
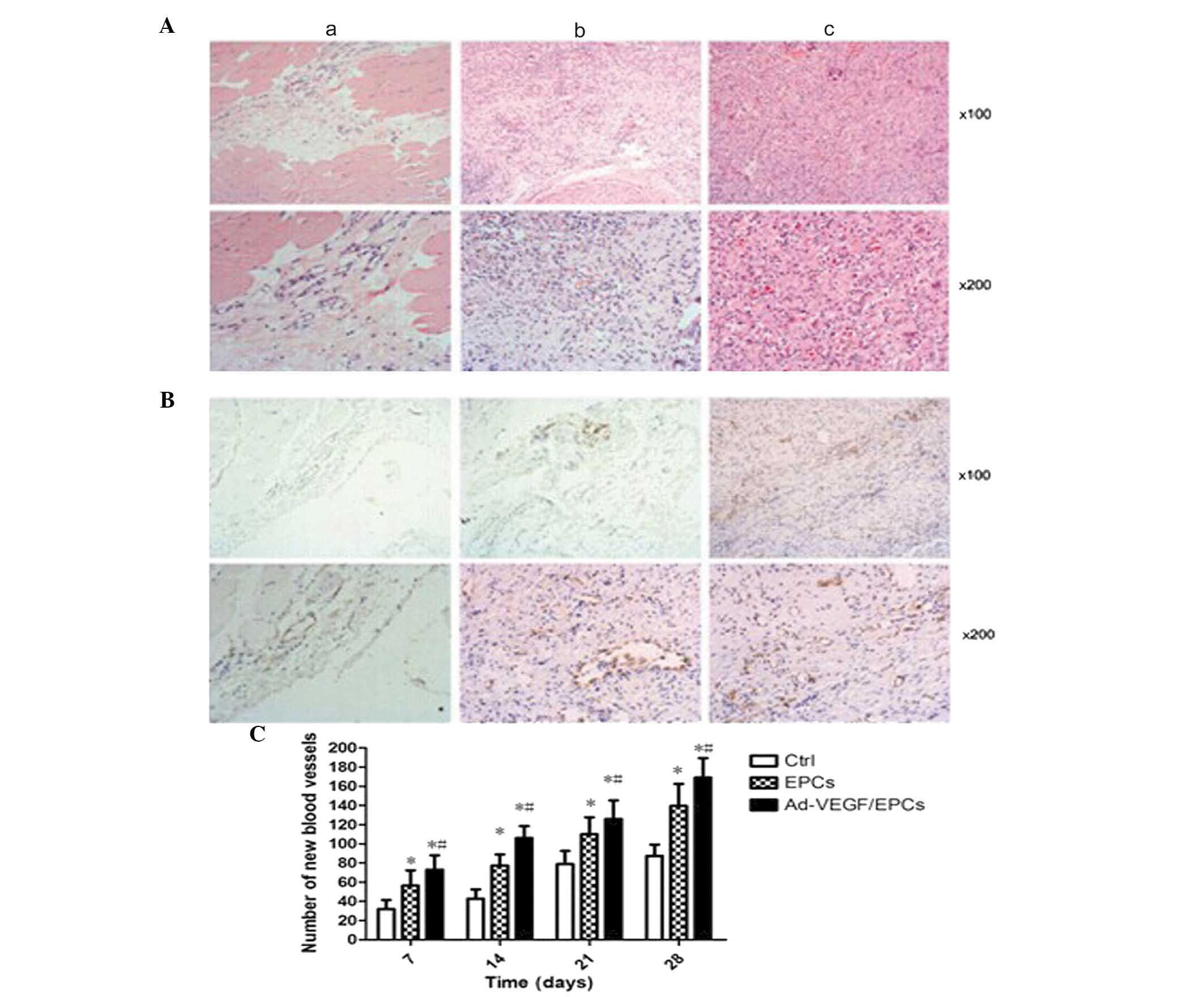
Histological examination of the rabbits
following transplantation
The tissue specimens were examined by HE staining,
and the endothelial cells in the tissues were examined by
immunohistochemical staining using the vWF antibody. The capillary
density of vWF-positive cells was determined by counting the number
of cells in five random high-power (magnification, ×200) fields.
Quantification of the histological analysis results revealed that
the density of blood vessels in group C was significantly higher,
as compared with groups A (P<0.01) and B (P<0.01). In
addition, the density of blood vessels in group B was significantly
higher, as compared with group A (P<0.01) (Fig. 7).
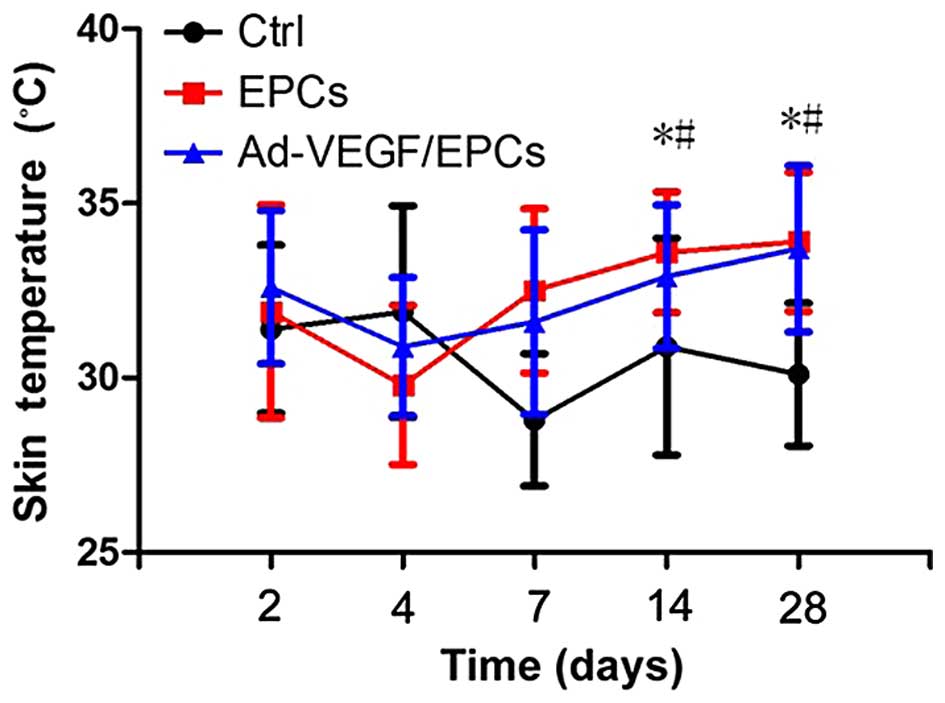
Skin temperature changes in New Zealand
rabbits following transplantation
On the 2nd, 4th, 7th, 14th, and 28th days following
transplantation, changes in skin temperature were monitored. The
skin temperatures of groups B and C were significantly higher, as
compared with group A at the 14th, and 28th days (P<0.01)
(Fig. 8).
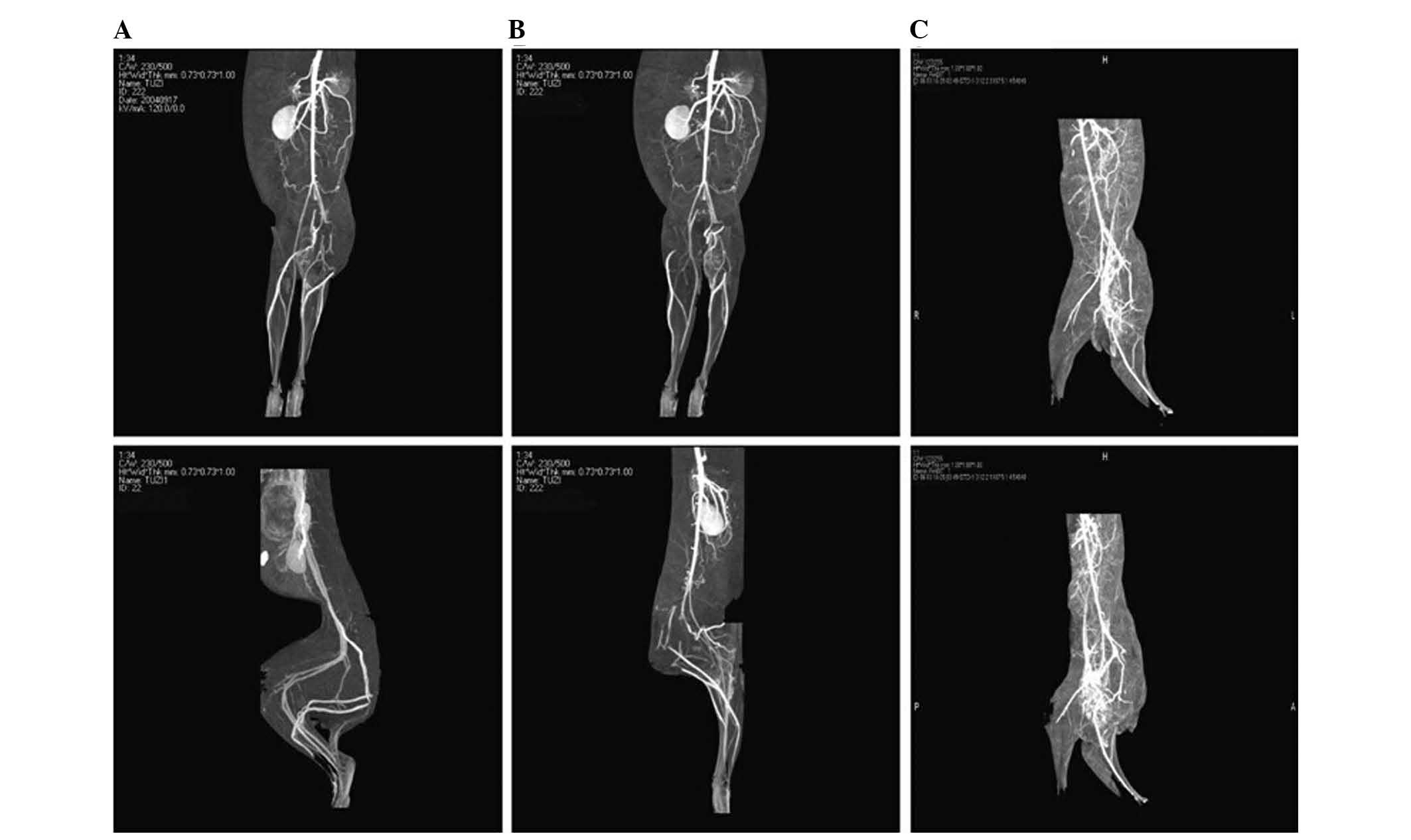
Analysis of recanalization of the New
Zealand rabbits, as determined by CTA
On the 14th day following transplantation, New
Zealand rabbits from each group underwent CTA using a Siemens
64-slice spiral CT. The lower limb artery reconstruction of each
group was examined. A higher number of small blood vessels in the
ischemic area and greater collateral circulation was observed in
group A, as compared with group B. The recanalization of group B
was markedly higher, as compared with group A (Fig. 9). The establishment of collateral
circulation of group C was observed following soft tissue removal
(Fig. 10).
Discussion
Stem cells are non-specialized cells that have the
ability to self-renew and differentiate into numerous cell lines.
Stem cells are able to differentiate into various tissues and
organs when stimulated by differentiation factors. EPCs have been
the subject of numerous studies since their discovery, and their
surface markers, mobilization, directional differentiation, and
effects on angiogenesis in the treatment of cardiovascular diseases
and cancers have been widely researched (7,8). As
compared with adult tissues, one of the advantages of stem cell
transplantation is that they are easily modified, and may serve as
gene therapy targets. Genetic modification of EPCs may improve
their function, thus enhancing the effects of EPC
transplantation.
Previous studies have reported that embryonic
hematopoietic stem cells (HSCs) and EPCs originate from a common
precursor cell, the blood-vascular stem cell (9–11).
HSCs and EPCs also share common antigens, including CD34, VEGF
receptor 2, Tie-2, CD117, and stem cell antigen-1 (9,11).
Since these initial reports on EPCs, this cell group has been
further studied, and numerous methods of EPC differentiation have
been reported (11–13).
The present study used a simple method to induce EPC
differentiation. BM-MNCs were obtained via centrifugation using
Ficoll centrifugal liquid, at a density of 1.0860±0.001. BM-MNCs
consist predominantly of lymphocytes and a small number of
mononuclear cells and platelets, as well as other cells. The
differentiation of BM-MNCs into EPCs was induced using VEGF, IGF-1,
and bFGF.
During culture, the adherent cells formed numerous
strips characteristic of EPCs, observations that have been reported
in a previous study (14).
The presence of EPCs was verified by examining the
presence of vWF, a specific surface maker of EPCs, and CD133, which
is expressed on the surface of immature EPCs.
In the present study, EPCs were transfected with 50
MOI Ad/VEGF165, since low MOI resulted in inefficient transfection,
and a high MOI resulted in cellular damage. The results of the MTT
assay demonstrated that cell proliferation was similar in the 10
and 50 MOI groups, however, in the 100 MOI group, cell growth
arrest and fragments of necrotic cells were observed. Therefore,
MOI 50 was used in the present study to ensure the transfection
efficiency was as high as possible, and that the cells did not
suffer damage. Satisfactory transfection efficiencies were achieved
and the proliferative activities of Ad-GFP-VEGF165/EPCs,
Ad-GFP/EPCs, and EPCs non-Ad/EPCs were similar, which suggested
that gene transfection with adenovirus had no effect on cell
proliferation.
An ELISA assay was used to detect the concentrations
levels of VEGF in the supernatant, demonstrating that the
concentration levels of VEGF in the supernatant of
Ad-GFP-VEGF165/EPCs was markedly higher, as compared with those of
the Ad-GFP/EPCs and non-Ad/EPCs. EPCs possess a VEGF secretory
function, and elevated concentration levels of VEGF in the
supernatants of the cultured cells confirmed the presence of EPCs.
The concentration levels of VEGF increased following gene
transfection, which demonstrated the success of the transfection,
and also provided evidence that VEGF has an important role
following cell transplantation.
In the present study, the Brdu-labeled EPCs were
transplanted into New Zealand rabbits, and seven days later muscle
tissue specimens of the ischemic limbs were obtained. The results
of immunohistochemical staining demonstrated that the labeled cells
had incorporated into the blood vessel wall.
Kocher et al (14) also observed that transplanted EPCs
were able to directionally integrate into the new blood vessels of
ischemic limbs. In addition, previous studies demonstrated that the
mRNA expression levels of VEGF in ischemic muscles were
significantly higher, as compared with non-ischemic muscles
(15,16). Therefore, these results suggest
that the predominant cause of EPC integration into the most severe
ischemic muscle area were the elevated expression levels of VEGF in
the ischemic area.
EPCs were transplanted into New Zealand rabbits
following limb ischemic experimentation, and the muscle tissue
specimens of ischemic limbs were obtained and analyzed by
immunohistochemical staining and CTA. As compared with the control
and Ad-GFP/EPCs groups, there was a greater density of new blood
vessels in the ischemic area, and collateral circulation was
greater in the Ad-GFP-VEGF165/EPCs group.
EPCs are easy to acquire, non-immunogenic so that
they do not cause immune rejection, and are able to migrate to the
area of new blood vessels. In addition, the proteins released by
EPCs readily enter the bloodstream and migrate throughout the body
where they have extensive roles. Therefore, EPCs may prove to be a
successful target for gene therapy.
Numerous studies have investigated the effects of
VEGF on angiogenesis (17,18). In the present study, concentration
levels of VEGF in the supernatant of the Ad-GFP-VEGF165/EPCs were
markedly higher, as compared with those in the Ad-GFP/EPCs and
non-Ad/EPCs. The results of the present study suggest that
transplantation with VEGF-transduced EPCs may improve the function
of EPCs, and may also have a role in angiogenesis via the action of
exocrine proteins. The present study also provides the basis for
the use of EPC transplantation in the treatment of limb
ischemia.
References
|
1
|
Suzuki H, Shibata R, Kito T, Ishii M, Li
P, Yoshikai T, Nishio N, Ito S, Numaguchi, Yamashita JK, et al:
Therapeutic angiogenesis by transplantation of induced pluripotent
stem cell-derived Flk-1 positive cells. BMC Cell Biol. 11:722010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenes RA, Jadlowiec CC, Bear M, Hashim P,
Protack CD, Li X, Lv W, Collins MJ and Dardik A: Toward a mouse
model of hind limb ischemia to test therapeutic angiogenesis. J
Vasc Surg. 56:1669–1679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang HC, Kim DS, Kim JY, Kim HS, Lim BY,
Kim HD, Lee JS, Eun BL and Kim DW: Behavioral improvement after
transplantation of neural precursors derived from embryonic stem
cells into the globally ischemic brain of adolescent rats. Brain
Dev. 32:658–668. 2010. View Article : Google Scholar
|
|
4
|
Zhou Y, Singh AK, Hoyt RF Jr, Wang S, Yu
Z, Hunt T, Kindzelski B, Corcoran PC, Mohiuddin MM and Horvath KA:
Regulatory T cells enhance mesenchymal stem cell survival and
proliferation following autologous co-transplantation in ischemic
myocardium. J Thorac Cardiovasc Surg. 148:1131–1137. 2014.
View Article : Google Scholar :
|
|
5
|
Guzmán-Hernández ML, Potter G, Egervári K,
Kiss JZ and Balla T: Secretion of VEGF-165 has unique
characteristics, including shedding from the plasma membrane. Mol
Biol Cell. 25:1061–1072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang J, Chen J, Wang W, et al: Birthdate
study of GABAergic neurons in the lumbar spinal cord of the
glutamic acid decarboxylase 67-green fluorescent protein knock-in
mouse. Front Neuroanat. 7:422013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fu SS, Li FJ, Wang YY, You AB, Qie YL,
Meng X, Li JR, Li BC, Xhang Y and Da Li Q: Kallikrein gene-modified
EPCs induce angiogenesis in rats with ischemic hindlimb and
correlate with integrin αvβ3 expression. PLoS One. 8:e730352013.
View Article : Google Scholar
|
|
8
|
Ahn GO and Brown M: Role of endothelial
progenitors and other bone marrow-derived cells in the development
of the tumor vasculature. Angiogenesis. 12:159–164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otani A, Kinder K, Ewalt K, Otero FJ,
Schimmel P and Friedlander M: Bone marrow-derived stem cells target
retinal astrocytes and can promote or inhibit retinal angiogenesis.
Nat Med. 8:1004–1010. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith C and Storms B: Hematopoietic stem
cells. Clin Orthop Relat Res. 379(Suppl): S91–S97. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khakoo AY and Finkel T: Endothelial
progenitor cells. Annu Rev Med. 56:79–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hristov M, Erl W and Weber PC: Endothelial
progenitor cells: Isolation and characterization. Trends Cardiovasc
Med. 13:201–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kocher AA, Schuster MD, Szabolcs MJ,
Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM and Itescu S:
Neovascularization of ischemic myocardium by human
bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis,
reduces remodeling and improves cardiac function. Nat Med.
7:430–436. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sellke FW, Wang SY, Stamler A, Lopez JJ,
Li J, Li J and Simons M: Enhanced microvascular relaxations to VEGF
and bFGF in chronically ischemic porcine myocardium. Am J Physiol.
271:H713–H720. 1996.PubMed/NCBI
|
|
16
|
Nakano M, Satoh K, Fukumoto Y, Ito Y,
Kagaya Y, Ishii N, Sugamura K and Shimokawa H: Important role of
erythropoietin receptor to promote VEGF expression and angiogenesis
in peripheral ischemia in mice. Circ Res. 100:662–669. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolff T, Mujagic E, Gianni-Barrera R,
Fueglistaler P, Helmrich U, Misteli H, Gurke L, Heberer M and Banfi
A: FACS- purified myoblasts producing controlled VEGF levels induce
safe and stable angiogenesis in chronic hind limb ischemia. J Cell
Mol Med. 16:107–117. 2012. View Article : Google Scholar
|
|
18
|
Mujagic E, Gianni-Barrera R, Trani M,
Patel A, Gürke L, Heberer M, Wolff T and Banfi A: Induction of
aberrant vascular growth, but not of normal angiogenesis, by
cell-based expression of different doses of human and mouse VEGF is
species-dependent. Hum Gene Ther Methods. 24:28–37. 2013.
View Article : Google Scholar : PubMed/NCBI
|