Introduction
Spinal cord injury (SCI) usually leads to permanent
loss of function, resulting in marked economic burden to the family
of the individual, as well as society as a whole. There is
currently no effective therapeutic method for the treatment of SCI
(1,2). Since the development of tissue
engineering technology in the biomedical field, numerous studies
have investigated the possibility of using tissue engineering to
repair SCI (3,4). The underlying repair mechanism of
tissue engineering is the secretion of neurotrophins from seed
cells, and the eventual differentiation of the cells into neurons.
Therefore, the selection of appropriate seed cells, which stably
secrete neurotrophins and easily differentiate into neurons, is an
important topic of discussion (5,6).
Seed cells include non stem cells (SCs), such as fibroblasts; and
SCs, which may be either non-adult SCs, such as neural and
embryonic SCs, or adult SCs, such as adipose-derived SCs (ADSCs)
and muscle-derived SCs. Compared with non-SCs, SCs are regarded as
superior; whereas compared with non-adult SCs, adult SCs are
regarded as superior (7),
particularly ADSCs, which are advantageous due to convenience
sampling and easy expansion. ADSCs are currently the most popular
type of seed cells, and previous studies have demonstrated their
ability to secrete neurotrophins and differentiate into neurons;
however, these abilities are limited (8,9).
Gene therapy has been proposed as a method to overcome this problem
via transfection of the necessary genes into the cells resulting in
their consistent expression, which may prompt the differentiation
of ADSCs into neurons (10).
Brain-derived neurotrophic factor (BDNF) and neurotrophin (NT)-3
possess superior properties, as compared with other types of
neurotrophic factors, in the maintenance of neuronal survival and
the promotion of SC differentiation into neurons (11,12);
however, the synergistic effects of BDNF and NT-3 on the neuronal
differentiation of ADSCs have yet to be elucidated. The present
study constructed lentiviruses specifically expressing BDNF and
NT-3, and used these to co-transfect ADSCs. The effects of BDNF and
NT-3 on the neuronal differentiation of ADSCs were then determined.
The present study aimed to provide a foundation for future
experiments regarding the suitable selection of seed cells for use
in tissue engineering for the treatment of SCI.
Materials and methods
Experimental animals
Two female Sprague-Dawley rats, (age, 1 month;
weight, 150±30 g) were provided by the Centre of Experimental
Animals, School of Medicine, Xi'an Jiaotong University (Xi'an,
China). The rats were maintained in a controlled environment
(18–26°C; 40–70% relative humidity), and were given ad
libitum access to food and water. All the experiments of the
present study were approved and supervised by the Ethics Committee
of the Xi'an Jiaotong University.
Isolation, culture and identification of
ADSCs
Chloral hydrate (0.1 ml/100 g; The Second Affiliated
Hospital of Xi'an Jiaotong University) was used to anesthetize the
rats. Their adipose tissue (1×1 cm) was then harvested, sectioned
and incubated for 0.5 h with 0.1% collagenase type I
(Sigma-Aldrich, St. Louis, MO, USA) in a centrifuge tube containing
Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 10%
fetal bovine serum (FBS; Hyclone, GE Healthcare, Logan, UT, USA).
The tissue was subsequently centrifuged at 2,000 x g for 10 min,
the supernatant was discarded and phosphate-buffered saline (PBS)
was added, prior to further centrifugation at 1,000 x g for 10 min.
The sediment was then suspended in DMEM/F12 supplemented with 10%
FBS, the cell density was adjusted to 1×105/ml, and the
cells were seeded in a 25-cm2 cell culture flask. Once
the original generation of cells reached 90% confluence, the entire
medium was aspirated, and the cells were washed with PBS to remove
any tissue fragments and blood cells. Subsequently, 0.25%
trypsin/0.02% EDTA solution was added to the cells for 3 min for
digestion, and DMEM/F12 was added to terminate the digestion. The
cells were then centrifuged at 1,000 x g for 6 min. The supernatant
was aspirated and the cells were suspended in DMEM/F12 supplemented
with 10% FBS, the cells were passaged at a ratio of 1:3, adjusted
to a density of 1×105/ml and cultured until they had
reached 90% confluence, which usually takes ~72 h for
first-generation cells.
Third-generation cells were used in the present
study, and were seeded at a density of 1×105/well in
6-well plates. The cells were then divided into the experimental
and control groups. Once the cells had reached 80% confluence, the
experimental group was placed into osteogenic induction media,
consisting of 0.1 µmol/l dexamethasone (Sigma-Aldrich), 10
mmol/l β-glycerophosphate sodium (Sigma-Aldrich), 50 mg/l vitamin C
(Sigma-Aldrich), 0.01 µmol/l vitamin D3
(Sigma-Aldrich) and 10% FBS/low glucose-DMEM. The control group
continued to be cultured in DMEM/F12 supplemented with 10% FBS.
After 2 and 4 weeks, alkaline phosphate (ALP) and alizarin red
staining were performed, respectively (13).
Construction of lentivrius and
transfection of ADSCs
Total RNA was extracted from the U87 human
glioblastoma cell line (Sigma-Aldrich) using RNAiso (Takara Bio,
Inc., Otsu, Japan), and cDNA was synthesized by reverse
transcription (RT) using an RT kit (Promega Corporation, Madison,
WI, USA). Polymerase chain reaction (PCR) was conducted using a PCR
Amplifier (cat. no. 070-851; Biometra GmbH, Göttingen, Germany) to
amplify the gene expression of BDNF and NT-3. The primers used are
listed in Table I (Sangon Biotech
Co., Ltd., Shanghai, China). The PCR cycling conditions were as
follows: 94°C for 30 sec, followed by 30 cycles at 98°C for 10 sec,
55°C for 15 sec and 72°C for 45°C, and a final extension step at
72°C for 45 sec. Following PCR, BDNF and NT-3 were subcloned into
pLV-CMV-EF1a-green fluorescent protein (GFP) and pLV-CMV-EF1a-red
fluorescent protein (RFP) expression lentivectors, respectively.
Subsequently, 293T cells (cat. no. ab95494; Abcam, Cambridge, MA,
USA) were transfected with pLV/helper packaging plasmid mix,
containing either pLV-CMV-EF1a-BDNF-GFP or pLV-CMV-EF1a-NT-3-RFP
expression lentivector (40 µg), and pMD2.G (20 µg;
Invitrogen Life Technologies, Carlsbad, CA, USA) and psPAX2 (20
µg; Invitrogen Life Technologies) at a ratio of 2:1:1.
Finally, two types of lentivirus, Lenti-BDNF-GFP and Lenti-NT-3-GFP
were obtained.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer | Primer sequence
(5′-3′) |
|---|
|
Neurotrophin-3-ClaI | Forward,
AATCGATATGTCCATCTTGTTTTATGTGATA |
|
Neurotrophin-3-SpeI | Reverse,
AACTAGTTCATGTTCTTCCGATTTTTCTCGA |
| Brain-derived
neurotrophic factor-ClaI | Forward,
AATCGATATGACCATCCTTTTCCTTACTATG |
| Brain-derived
neurotrophic factor-SpeI | Reverse,
AACTAGTTTATCTTCCCCTTTTAATGGTCAA |
The third-generation ADSCs were inoculated at a
density of 75–80%, and then transfected with the lentiviruses at a
multiplicity of infection (MOI) of 1, 10 and 100, in order to
determine the best MOI value in each group. Three cell groups were
generated: i) The Lenti-BDNF-GFP transfected group; ii) the
Lenti-NT-3-RFP transfected group; and iii) the Lenti-BDNF-GFP and
Lenti-NT-3-RFP co-transfected group. The efficiency of lentiviral
gene transfer into ADSCs was determined according to the presence
of fluorescent cells, as detected by fluorescence microscopy
(DMI4000B; Leica Microsystems GmbH, Wetzlar, Germany) 72 h
post-transfection. After determining the suitable MOI, flow
cytometry (cat. no. 175487; Beckman Coulter, Inc., Brea, CA, USA)
was used to detect the expression of GFP in the Lenti-BDNF-GFP
transfected group, the expression of RFP in the Lenti-NT-3-RFP
transfected group, and the co-expression of GFP and RFP in the
Lenti-BDNF-GFP and Lenti-NT-3-RFP co-transfected group.
Induction of the transfected cells into
neural cells
Neural induction media containing 10% FBS/L-DMEM, 10
µmol/l forskolin, 5 mmol/l KCl, 2 mmol/l sodium valproate, 1
mol/l hydrocortisone and 5 mg/l insulin (all Sigma-Aldrich) was
used to induce the differentiation of transfected ADSCs into
neurons. The ADSCs were divided into four groups: i) The BDNF and
NT-3 co-overexpression group (BDNF and NT-3 co-transfected ADSCs);
ii) the BDNF overexpression group (BDNF transfected ADSCs); iii)
the NT-3 overexpression group (NT-3 transfected ADSCs); and iv) the
control group (untransfected ADSCs). After 10 days, the
identification of BDNF, NT-3 and neuron-specific enolase (NSE) was
conducted using immunofluorescent staining, RT-quantitative (q)PCR
and western blotting.
Immunofluorescent staining
Medium was removed from the cells and they were
fixed with 4% paraformaldehyde for 30 min. A total of 30%
H2O2 was used to block endogenous peroxidase
activity, and the cells were subsequently incubated with 0.3%
Triton-X 100 (Sigma-Aldrich) for 20 min at room temperature. The
cells were then incubated with rabbit polyclonal anti-NSE (1:50
dilution; cat. no. ab53025; Abcam) at 4°C overnight. The cells were
incubated with a fluorescein isothiocyanate-conjugated goat
anti-rabbit immunoglobulin G secondary antibody (1:100 dilution;
cat. no. ab6717; Abcam) for 4 h at room temperature. Finally, the
cells were incubated with 100 μg/l DAPI for 15 min.
Fluorescence microscopy was used (magnification, ×200) to observe
the positively-stained cells in each group. Three wells were
selected, and five fields from each were used to calculate the
percentage of positive cells (stained red) from the number of total
cells (blue-stained nuclei). The experiment was repeated three
times.
RT-qPCR to detect BDNF, NT-3 and NSE mRNA
expression levels
Total RNA was extracted from the transfected ADSCs
using TRIzol® reagent (Invitrogen Life Technologies),
and was reverse transcribed using oligo (dT) primers, according to
the manufacturer's instructions (Takara Bio, Inc.). RT-qPCR was
conducted using a PCR Amplifier (cat. no. 070-851; Biometra GmbH)
and SYBR Green (cat. no. 4367659; Invitrogen Life Technologies).
The PCR cycling conditions were as follows: 94°C for 30 sec,
followed by 40 cycles at 98°C for 10 sec and 60°C for 20 sec, and a
final step at 72°C for 20 sec. The primer pairs for BDNF, NT-3, NSE
and GAPDH (which served as a housekeeping gene) are listed in
Table II (Sangon Biotech Co.,
Ltd.). The expression levels of the genes of interest were
determined according to the standard curve, using the
2-ΔΔCt quantification method (14).
 | Table IIPrimer sequences. |
Table II
Primer sequences.
| Primer | Primer sequence
(5′-3′) |
|---|
| NT-3 | Forward,
CGTGGTGGCGAACAGAACAT |
| NT-3 | Reverse,
GGCCGATGACTTGTCGGTC |
| BDNF | Forward,
CTACGAGACCAAGTGCAATCC |
| BDNF | Reverse,
AATCGCCAGCCAATTCTCTTT |
| NSE | Forward,
CGAGGACACGTTCATTGCAGA |
| NSE | Reverse,
GAGCTGGTTGTACTTCGCCAGAC |
| GAPDH | Forward,
GCACCGTCAAGGCTGAGAAC |
| GAPDH |
Reverse,TGGTGAAGACGCCAGTGGA |
Western blotting to detect BDNF, NT-3 and
NSE protein expression
The transfected ADSCs were washed four times with
PBS. Cells were lysed with 10X radioimmunoprecipitation assay
buffer (Abcam) and protein concentration was determined using the
Bicinchoninic Acid kit (Sigma-Aldrich). Protein samples (50
µg) were separated by 10% SDS-PAGE (Sigma-Aldrich) and
transferred to polyvinylidene fluoride membranes (Sigma-Aldrich).
Non-specific reactivity was blocked using 5% skim milk in
tris-buffered saline containing Tween-20 (Sigma-Aldrich) for 60 min
at room temperature. The membranes were then incubated with rabbit
anti-rat BDNF (cat. no. ab6201), NT-3 (cat. no. ab65804) and NSE
(cat. no. ab53025) antibodies (1:1,000 dilution; Abcam) overnight
at 4°C, followed by incubation with goat anti-rabbit secondary
antibodies (1:10,000 dilution; cat. no. ab6717; Abcam). β-actin
antibodies (cat. no. ab129348; Abcam) served as an internal
control. Image Lab 8.0 software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to measure the BDNF, NT-3, NSE and
β-actin grey values, and the grey values of the proteins of
interest were compared with the β-actin grey value, in order to
determine the relative protein expression levels of BDNF, NT-3 and
NSE. Western blotting was repeated five times.
Statistical analysis
Statistical analyses were conducted using SPSS 17.0
(SPSS Inc., Chicago, IL, USA). One-way analysis of variance was
used for group comparisons. All values are presented as the mean ±
standard error and P<0.05 was considered to indicate a
statistically significant difference.
Results
ADSC morphology
Few cells had adhered to the bottom of the culture
flask at 10 h following inoculation (Fig. 1A), however, at 72 h
post-inoculation a large number of adherent cells were observed
(Fig. 1B). At 8 days, the cells
had reached 95% confluence (Fig.
1C). There was no marked difference observed in the morphology
of the third-generation cells, when compared with the primary cells
(Fig. 1D).
ALP staining was conducted 2 weeks after osteogenic
induction. In the experimental group, black particles were detected
in the cytoplasm (Fig. 2A),
whereas these particles were not observed in the control group
(Fig. 2B). A total of 4 weeks
after osteogenic induction alizarin red staining was conducted and
calcium nodules were observed in the experimental group (Fig. 2C), whereas these nodules were not
detected in the control group (Fig.
2D).
Selection of the optimum MOI value for
ADSC transfection
ADSCs were transfected with lentiviruses at an MOI
of 1, 10 and 100 and the number of fluorescent cells gradually
increased with the increasing MOI. When cells were transfected with
a MOI of 100, the fluorescent intensity was significantly increased
in the Lenti-BDNF-GFP transfected group (Fig. 3A), the Lenti-NT-3-RFP transfected
group (Fig. 3B), and the
Lenti-BDNF-GFP and Lenti-NT-3-GFP co-transfected group (Fig. 3C), concurrently a cell cytotoxic
effect was detected. The optimum MOI was determined to be 100, due
to the higher transfection efficiency and slightly reduced
cytopathogenicity. As determined by flow cytom-etry, the percentage
of GFP-positive cells was 91.3% in the Lenti-BDNF-GFP transfected
group (Fig. 3D), the percentage of
RFP-positive cells was 77.5% in the Lenti-NT-3-RFP transfected
group (Fig. 3E), and the
percentage of GFP- and RFP-positive cells was 82.2 and 67.0%,
respectively in the Lenti-BDNF-GFP and Lenti-NT-3-RFP
co-transfected group (Fig.
3F).
 | Figure 3Determination of lentiviral
transfection efficiency. (A–C) Fluorescence intensity of
adipose-derived stem cells in the (A) Lenti-BDNF-GFP transfected
group (magnification, ×200), (B) Lenti-NT-3-RFP transfected group
(magnification, ×200) and the (C) Lenti-BDNF-GFP and Lenti-NT-3-RFP
co-transfected group (magnification, ×100). Number of (D)
GFP-positive cells in the Lenti-BDNF-GFP transfected group, (E)
RFP-positive cells in the Lenti-NT-3-RFP transfected group and (F)
GFP- and RFP-positive cells in the Lenti-BDNF-GFP and
Lenti-NT-3-RFP co-transfected group, as determined by flow
cytometry. RFP, red fluorescent protein; GFP, green fluorescent
protein; BDNF, brain-derived neurotophic factor; NT-3,
neurotrophin-3. |
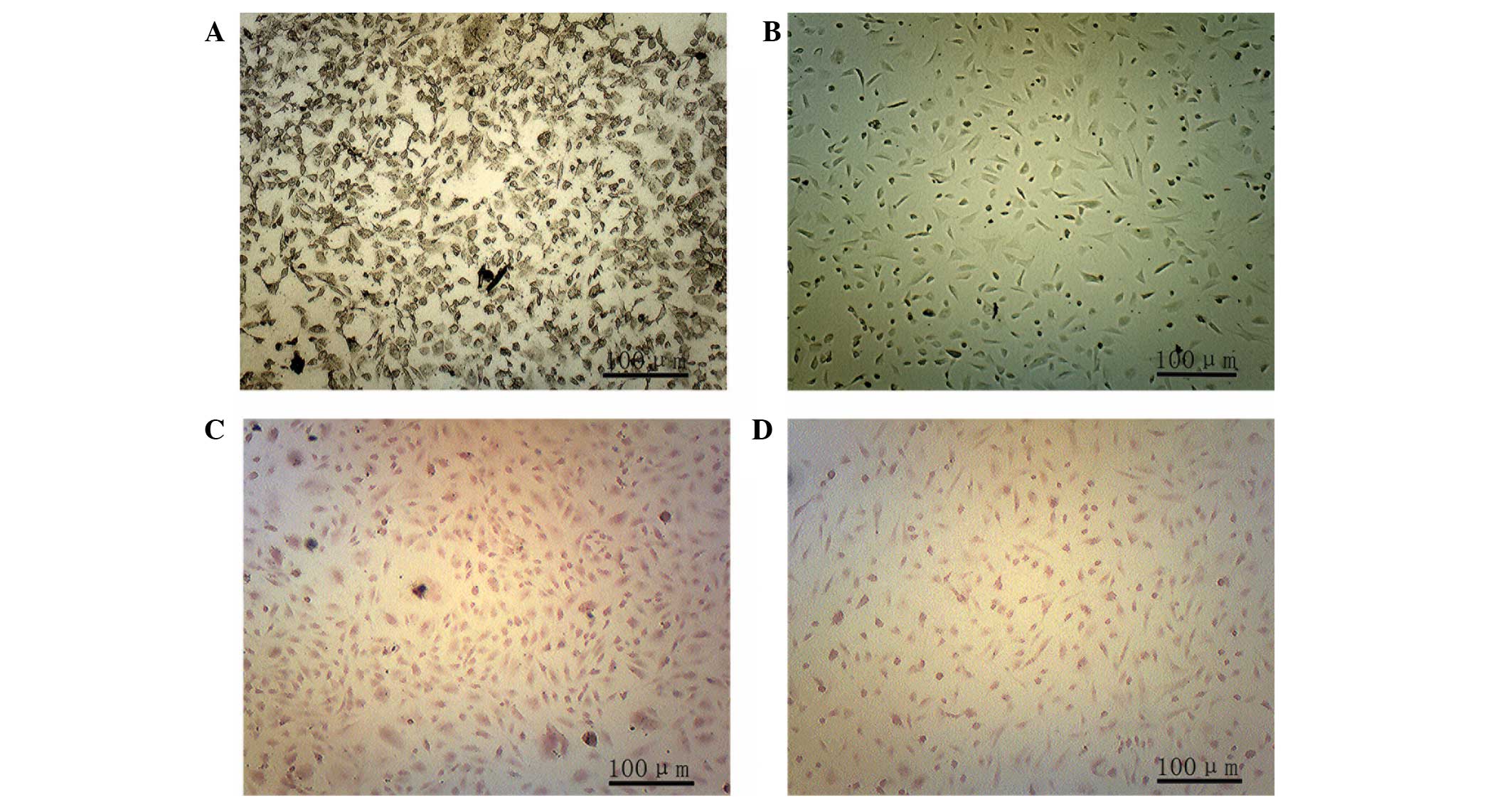
Detection of NSE by immunofluorescent
staining
ADSCs were induced to differentiate into neurons,
and after 10 days NSE was most highly expressed in the BDNF and
NT-3 co-transfected group (Fig.
4A), as compared with the other groups (P<0.05). The BDNF
transfected group expressed the next highest levels of NSE, which
were significantly lower than those in the co-transfected group
(P<0.05), whereas the expression levels of NSE were lower in the
NT-3 transfected group, as compared with the above groups; however,
were higher when compared with the control group (P<0.05;
Fig. 4B).
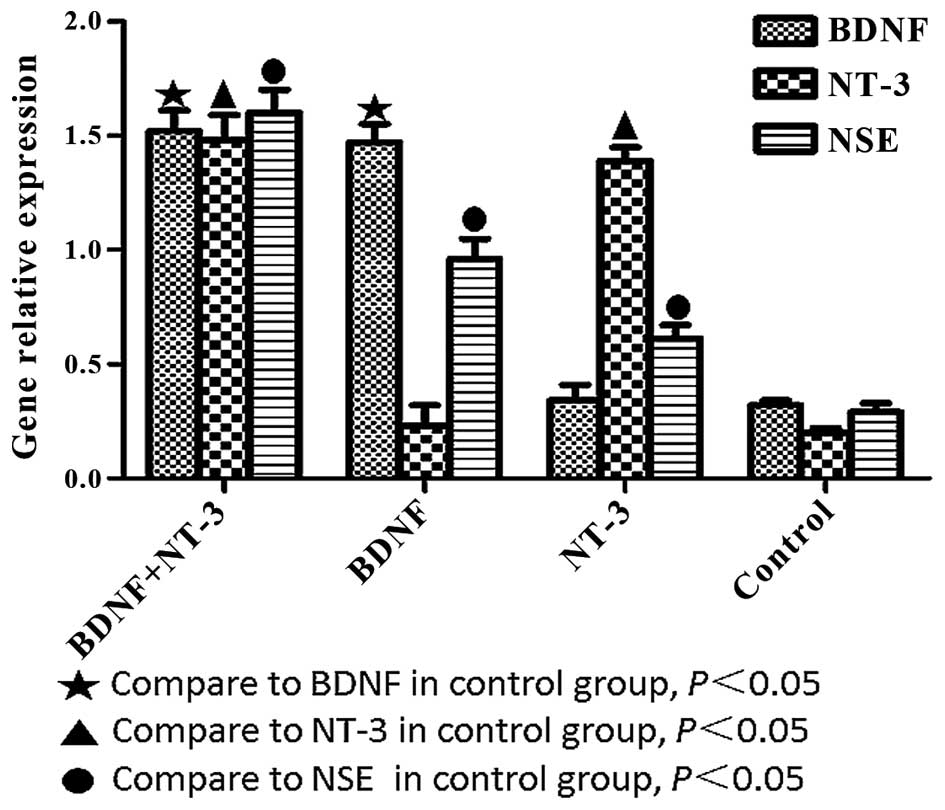
Detection of BDNF, NT-3 and NSE mRNA
expression levels by RT-Qpcr
BDNF, NT-3 and NSE mRNA expression levels were
significantly higher in the experimental groups, as compared with
the control group (P<0.05; Fig.
5). The mRNA expression levels of BDNF were significantly
higher in the BDNF and NT-3 co-transfected group, as compared with
the NT-3 transfected group (P<0.05). The mRNA expression levels
of NT-3 were significantly higher in the BDNF and NT-3
co-transfected group, as compared with the BDNF transfected group
(P<0.05). NSE mRNA expression levels were highest in the
co-transfected group, followed by the BDNF transfected group,
followed by the NT-3 transfected group (P<0.05).
Detection of BDNF, NT-3 and NSE protein
expression levels by western blotting
BDNF, NT-3 and NSE protein expression levels were
significantly higher in the experimental groups, as compared with
those in the control group (P<0.05; Fig. 6A). The protein expression levels of
BDNF were significantly higher in the BDNF and NT-3 co-transfected
group, as compared with the NT-3 transfected group (P<0.05). The
protein expression levels of NT-3 protein were significantly higher
in the BDNF and NT-3 co-transfected group, as compared with the
BDNF transfect group (P<0.05). NSE protein expression levels
were highest in the co-transfected group, followed by the BDNF
transfected group, followed by the NT-3 transfected group
(P<0.05; Fig. 6B). The results
of western blot analysis were consistent with the results of the
immunofluorescent staining and RT-qPCR.
Discussion
SCI usually leads to permanent loss of function.
Tissue engineering is a promising method that may be used to treat
SCI, the underlying repair mechanisms of which are the secretion of
neurotrophins from seed cells, and ultimately the differentiation
of seed cells into neurons (15,16).
Therefore, the identification of a suitable seed cell that secretes
neurotrophins and easily differentiates into neurons is important
for the treatment of SCI.
ADSCs, which are adult SCs, have the advantage of
convenience sampling, easy expansion, and possess the ability to
differentiate into neurons; therefore, ADSCs are considered to be a
promising seed cell in tissue engineering for the treatment of SCI
(17). There are no specific
surface antigens detected on ADSCs, therefore identification of
ADSCs is usually based on organizational sources and their multiple
differentiation potential (18).
The present study obtained ADSCs by combining zymogen digestion
with the differential velocity adherent method, and assumed that
the separated cells were SCs, which therefore possess multilineage
differentiation potential. The third-generation cells initially
underwent osteogenic induction; after 2 weeks, calcium cobalt
staining was positive and after 4 weeks, alizarin red staining was
positive. These results indicated that the ADSCs had successfully
differentiated into osteo-blasts and possessed osteoblastic
characteristics. In addition, the cells were isolated from adipose
tissue, therefore it was reasonable to assume that the separated
cells were ADSCs. Previous studies have demonstrated that ADSCs
secrete neurotrophins, resulting in neuronal differentiation,
however, this ability is limited. BDNF and NT-3 are important
neurotrophins, which possess superior properties, when compared
with other types of neurotrophic factors, in the maintenance of
neuronal survival, inhibition of cell apoptosis, and promotion of
SC differentiation into neurons (19,20).
In order to improve the neurotrophin secretory ability of ADSCs,
two types of lentivirus were constructed in the present study:
Lenti-BDNF-GFP and Lenti-NT-3-RFP. The lentiviruses were
transfected into third-generation ADSCs at various MOIs.
Transfection with an MOI of 100 for 72 h was associated with
improved transfection efficiency and reduced cytopathogenicity.
Furthermore, flow cytometry was used to determine transfection
efficiency; the percentage of GFP-positive cells was 91.3% in the
Lenti-BDNF-GFP transfected group, the percentage of RFP-positive
cells was 77.5% in the Lenti-NT-3-RFP transfected group, and the
percentage of GFP- and RFP-positive cells was 82.2 and 67.0%,
respectively, in the Lenti-BDNF-GFP and Lenti-NT-3-RFP
co-transfected group. The transfected cells were subsequently
induced to differentiate into neurons, and after 10 days the
expression levels of BDNF and NT-3 were detected. The BDNF and NT-3
co-transfected group expressed BDNF and NT-3 mRNA and protein at
high levels. The BDNF transfected group expressed high levels of
BDNF and low levels of NT-3, the NT-3 transfected group expressed
high levels of NT-3 and low levels of BNDF, and the control group
expressed BDNF and NT-3, however at significantly lower levels than
the transfected groups. Furthermore, NSE expression levels were
determined using immunofluorescent staining, RT-qPCR and western
blotting, the results of which demonstrated that NSE was most
highly expressed in the BDNF and NT-3 co-transfected group,
followed by the BDNF transfected group, followed by the NT-3
transfected group.
A previous study demonstrated that BDNF and NT-3
belong to a group of neurotrophins that are targeted by the Trk
receptor, which is divided into three types: TrkA, TrkB and TrkC
(21). BDNF is targeted by TrkB,
and NT-3 is targeted by TrkC, however NT-3 may occasionally bind to
TrkA and TrkB (22,23). Based on these previous findings and
the results of the present study, it may be hypothesized that BDNF
and NT-3 co-transfected cells easily differentiate into neurons due
to the increased secretion of BDNF and NT-3, which bind TrkB and
TrkC resulting in the formation of an active dimer. Via the process
of Trk receptor phosphorylation cell signals may be transmitted
that promote the differentiation of ADSCs into neurons. Another
hypothesis reported by Hapner et al (24) is that BDNF and NT-3 may prompt the
expression of the Trk receptor, which may promote SC neuronal
differentiation. An additional hypothesis is that BDNF and NT-3
exert a synergistic effect that may promote the differentiation of
ADSCs into neurons. In the present study, the BDNF and NT-3
transfected groups were able to secrete BDNF and NT-3, and the
number of NSE-positive cells in these groups was higher, as
compared with the control group, thus suggesting that ADSCs
modified by BDNF and NT-3 may easily differentiate into neurons. In
addition, when compared with the NT-3 transfected group, ADSCs
modified by BDNF more easily differentiated into neurons; however,
the mechanism behind this finding is unclear. The present study
hypothesized that BDNF and NT-3 may affect the differentiation of
ADSCs into neurons, with BDNF exerting a key role and NT-3 exerting
a secondary role, which may be important in axon growth, as
demonstrated by Francis et al (25). The results of the present study
demonstrate that ADSCs modified by BDNF and NT-3 differentiate into
neurons more easily.
In conclusion, the present study examined the
effects of BDNF and NT-3 overexpression on neuronal differentiation
of rat ADSCs. The results indicate that BDNF and NT-3 exert a
synergistic regulatory effect on ADSC neuronal differentiation, and
that ADSCs may easily differentiate into neurons after being
modified by BDNF and NT-3. ADSCs may therefore be suitable seed
cells for use in tissue engineering for the treatment of SCI. Our
future research will be focused on developing an in-depth
understanding regarding cell signaling following binding of BDNF
and NT-3 to the Trk receptor. Thus, the present study provides
insight into the use of tissue engineering technology for future
treatment of SCI.
Acknowledgments
The authors of the present study would like to thank
Professor Ying Mao (Xi'an Jiaotong University) who provided
guidance during the experiment.
References
|
1
|
Hayashi T, Kawano O, Sakai H, Ideta R,
Ueta T, Maeda T, Mori E, Yugue I, Takao T, Masuda M, et al: The
potential for functional recovery of upper extremity function
following cervical spinal cord injury without major bone injury.
Spinal Cord. 51:819–822. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cadotte DW and Fehlings MG: Spinal cord
injury: A systematic review of current treatment options. Clin
Orthop Relat Res. 469:732–741. 2011. View Article : Google Scholar :
|
|
3
|
Mortazavi MM, Harmon OA, Adeeb N, Deep A
and Tubbs RS: Treatment of spinal cord injury: A review of
engineering using neural and mesenchymal stem cells. Clin Anat.
28:37–44. 2015. View
Article : Google Scholar
|
|
4
|
Ribeiro-Samy S, Silva NA, Correlo VM, et
al: Development and characterization of a PHB-HV-based 3D scaffold
for a tissue engineering and cell-therapy combinatorial approach
for spinal cord injury regeneration. Macromol Biosci. 13:1576–1592.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanno H, Pressman Y, Moody A, Berg R, Muir
EM, Rogers JH, Ozawa H, Itoi E, Pearse DD and Bunge MB: Combination
of engineered Schwann cell grafts to secrete neurotrophin and
chondroitinase promotes axonal regeneration and locomotion after
spinal cord injury. J Neurosci. 34:1838–1855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abbasi M, Salehi M, Pasbakhsh P and
Sobhani A: Repair of spinal cord injury by co-transplantation of
embryonic stem cell-derived motor neuron and olfactory ensheathing
cell. J Stem Cells Regen Med. 6:812010.PubMed/NCBI
|
|
7
|
Ji W, Hu S, Zhou J, Wang G, Wang K and
Zhang Y: Tissue engineering is a promising method for the repair of
spinal cord injuries (Review). Exp Ther Med. 7:523–528.
2014.PubMed/NCBI
|
|
8
|
Kapur SK and Katz AJ: Review of the
adipose derived stem cell secretome. Biochimie. 95:2222–2228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liqing Y, Jia G, Jiqing C, Ran G, Fei C,
Jie K, Yanyun W and Cheng Z: Directed differentiation of motor
neuron cell-like cells from human adipose-derived stem cells in
vitro. Neuroreport. 22:370–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Chu Y and Lou G: Fiber-modified
adenovirus can mediate human adipose tissue-derived mesenchymal
stem cell-based anti-angiogenic gene therapy. Biotechnol Lett.
32:1181–1188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu W, Lu G, Wang B, Ma Z and Li Y:
Transfection of BDNF gene promotes bone mesenchymal stem cells to
differentiate into neuron-like cells. Zhong Nan Da Xue Xue Bao Yi
Xue Ban. 37:441–446. 2012.In Chinese. PubMed/NCBI
|
|
12
|
Gao H, Wei M, Wang Y, Wu X and Zhu T:
Differentiation of GDNF and NT-3 dual gene-modified rat bone marrow
mesenchymal stem cells into enteric neuron-like cells. J Huazhong
Univ Sci Technolog Med Sci. 32:87–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji W, Zhang Y and Hu S: Biocompatibility
study of a silk fibroin-chitosan scaffold with adipose
tissue-derived stem cells in vitro. Exp Ther Med. 6:513–518.
2013.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen KD: Analysis of
relative gene expression data using real-time quantiative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Madigan NN, McMahon S, O'Brien T,
Yaszemski MJ and Windebank AJ: Current tissue engineering and novel
therapeutic approaches to axonal regeneration following spinal cord
injury using polymer scaffolds. Respir Physiol Neurobiol.
169:183–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Silva NA, Salgado AJ, Sousa RA, Oliveira
JT, Pedro AJ, Leite-Almeida H, Cerqueira R, Almeida A, Mastronardi
F, Mano JF, et al: Development and characterization of a novel
hybrid tissue engineering-based scaffold for spinal cord injury
repair. Tissue Eng Part A. 16:45–54. 2010. View Article : Google Scholar
|
|
17
|
Zhou J, Lu P, Ren H, Zheng Z, Ji J, Liu H,
Jiang F, Ling S, Heng BC, Hu X and Ouyang H: 17β-estradiol protects
human eyelid-derived adipose stem cells against cytotoxicity and
increases transplanted cell survival in spinal cord injury. J Cell
Mol Med. 18:326–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren Y, Wu H, Ma Y, Cang M, Wang R and Liu
D: Isolation, cultivation and identification of adipose-derived
stem cell in bovines. Sheng Wu Gong Cheng Xue Bao. 26:1645–1651.
2010.In Chinese.
|
|
19
|
Gibbons A, Wreford N, Pankhurst J and
Bailey K: Continuous supply of the neurotrophins BDNF and NT-3
improve chick motor neuron survival in vivo. Int J Dev Neurosci.
23:389–396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Gu J, Wang J, Feng X, Tao Y, Jiang
B, He J, Wang Q, Yang J, Zhang S, et al: BDNF and NT-3 expression
by using glucocorticoid-induced bicistronic expression vector
pGC-BDNF-IRES-NT3 protects apoptotic cells in a cellular injury
model. Brain Res. 1448:137–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sobue G, Yamamoto M, Doyu M, Li M, Yasuda
T and Mitsuma T: Expression of mRNAs for neurotrophins (NGF, BDNF,
and NT-3) and their receptors (p75NGFR, trk, trkB, and trkC) in
human peripheral neuropathies. Neurochem Res. 23:821–829. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Islam O, Loo TX and Heese K: Brain-derived
neurotrophic factor (BDNF) has proliferative effects on neural stem
cells through the truncated TRK-B receptor, MAP kinase, AKT and
STAT-3 signaling pathways. Curr Neurovasc Res. 6:42–53. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang YQ, Zeng X, He LM, Ding Y, Li Y and
Zeng YS: NT-3 gene modified Schwann cells promote TrkC gene
modified mesenchymal stem cells to differentiate into neuron-like
cells in vitro. Anat Sci Int. 85:61–67. 2010. View Article : Google Scholar
|
|
24
|
Hapner SJ, Nielsen KM, Chaverra M, Esper
RM, Loeb JA and Lefcort F: NT-3 and CNTF exert dose-dependent,
pleio-tropic effects on cells in the immature dorsal root ganglion:
Neuregulin-mediated proliferation of progenitor cells and neuronal
differentiation. Dev Biol. 297:182–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Francis N, Farinas I, Brennan C,
Rivas-Plata K, Backus C, Reichardt L and Landis S: NT-3, like NGF,
is required for survival of sympathetic neurons, but not their
precursors. Dev Biol. 210:411–427. 1999. View Article : Google Scholar : PubMed/NCBI
|