Introduction
Gastric cancer is prevalent worldwide and although
the incidence and mortality have markedly fallen, it remains the
fourth most common cancer and the second leading cause of
cancer-associated mortality (1).
In spite of early use of adjuvant chemotherapy after surgery, poor
outcomes following surgical resection and high recurrence rates
remain, making gastric cancer challenging to treat. Compared with
no adjuvant treatment, combination-chemotherapy has been reported
to significantly prolong the disease-free period and the patients'
survival rate five years subsequent to surgery (2,3). The
occurrence of resistance is commonly observed during the course of
chemotherapy treatment. The efficacy of chemotherapy is limited by
simultaneous resistance to multiple drugs that are neither
mechanistically nor structurally linked. In the resistance of
cancer cells to chemotherapeutic agents, DNA methylation, gene
mutation and histone modification serve important roles (2,3). In
2007, Climent et al (4)
observed that the deletion of chromosome 11q, which carries the
region containing the microRNA (miR)-125b gene, may contribute to
the sensitivity of patients with breast cancer to
anthracycline-based chemotherapy. This suggested a possible link
between miRNA dysregulation and chemotherapy resistance.
miRNAs regulate gene expression in multicellular
organisms by post-transcriptionally affecting the stability and
translation of mRNAs (5), which
are transcribed by RNA polymerase II or III in the nucleus
(6). The primary capped and
polyadenylated transcripts (pri-miRNA) are cleaved by the Drosha
ribonuclease III enzyme to produce a ~70-nucleotide stem-loop
precursor miRNA (pre-miRNA) (7–9).
Pre-miRNA is transported to the cytoplasm by exportin 5 and is then
processed into mature miRNAs by the RNase III enzyme Dicer
(10,11). Mature miRNA is incorporated into an
RNA-induced silencing complex (RISC), which imperfectly pairs with
the 3′-untranslated region (3′UTR) of the target gene mRNA. As a
result, the translation of the target gene mRNAs is inhibited or
destabilized (12–14). Previous studies indicated critical
functions of miRNAs in diverse biological processes, including
tumor angiogenesis, proliferation, cell differentiation, apoptosis,
adhesion and metastasis of tumor cells (15–19)
and cancer chemotherapy multidrug resistance (MDR) (20). Therefore, elucidation of the
regulatory role of miRNAs may provide a novel understanding of the
molecular events in various biological processes, and suggest that
abnormally expressed miRNAs in various types of human cancer serve
as oncogenes or tumor suppressor genes by targeting transcripts of
essential protein coding genes in tumorigenesis.
Previous studies (21,22)
have suggested that, in addition to oncogenesis, the different
expression levels of certain miRNAs are associated with the
response to chemotherapeutic agents. Chemotherapy is frequently
unsuccessful due to either intrinsic or acquired MDR of cancer
cells following an initial round of treatment (23). Zhu et al (24) demonstrated that the MDR cancer cell
lines A2780DX5 and KB-V1 exhibited higher expression levels of
miR-27a and miR-451 than their parental lines A2780 and KB-3-1.
Downregulation of miR-27a or miR-451 expression has been reported
to reduce the expression levels of P-glycoprotein (P-gp) and MDR1
mRNA. The intracellular accumulation of cytotoxic drugs due to
being transported by P-gp was enhanced by the treatment with the
anti-miR-27a or anti-miR-451 (24). Xia et al (22) analyzed the possible role of miRNAs
in the development of MDR in gastric cancer cells. They identified
that miR-15b and miR-16 were downregulated in the MDR gastric
cancer cell line SGC7901/VCR compared with that in the control
group. In addition, overexpression of miR-15b or miR-16 has been
reported to sensitize SGC7901/VCR cells to vincristine,
doxorubicin, etoposide and cisplatin in an in vitro drug
sensitivity assay. By contrast, inhibition of miR-15b or miR-16
expression may contribute to MDR in SGC7901 cells. Meng et
al (25) also indicated that
miR-21, miR-141 and miR-200b were dysregulated in malignant
cholangiocytes. Downregulation of miR-21 and miR-200b increased
sensitivity to gemcitabine, whereas inhibition of miR-141 reduced
cell growth.
As described above, miRNAs serve as regulators of
gene expression and may influence the response of cancer cells to
chemotherapy. Thus, in the present study, the expression levels of
miR-197 were investigated in the fluorouracil (5-FU)-resistant
human gastric cancer cell line SGC7901/5-FU and its parental cell
line SGC7901. The present study focused on the effects of miR-197
on 5-FU drug resistance in SGC7901 gastric cancer cells in addition
to the identification of its direct target gene. It was
hypothesized that miR-197 may present a novel therapeutic for
preventing resistance against 5-FU by targeting the expression of
resistance-associated genes in patients with gastric carcinoma.
Materials and methods
Cell culture and transfection
Cells of the human gastric cancer cell line SGC-7901
(American Type Culture Collection, Manassas, VA, USA) were cultured
in RPMI-1640 medium (Gibco-BRL, Invitrogen Life Technologies,
Carlsbad, CA, USA), which was supplemented with 10%
heat-inactivated fetal bovine serum, 100 IU penicillin/ml and 100
µg/ml streptomycin (Invitrogen Life Technologies). All of
the cells were maintained in a humidified 5% (v/v) atmosphere of
CO2 at 37°C. The 5-FU-resistant variant SGC7901/5-FU was
obtained from the parent cell line (SGC-7901) by step by step
exposure to increasing concentrations of 5-FU (5, 10, 20 and 40
mg/ml in three-day intervals; Sigma-Aldrich, St. Louis, MO, USA).
To maintain the 5-FU-resistant phenotype, 5-FU was added to the
culture media with a final drug concentration of 1 mg/ml 5-FU for
SGC7901/5-FU cells. Lipofectamine 2000 reagent (Invitrogen Life
Technologies) was used for the cell transfection in accordance with
the manufacturer's instructions.
Luciferase reporter assay
The gastric cancer cells were co-transfected with
miR-197 and MAPK1 3′UTR or MAPK1 3′UTR-mutant (Zhiyou Bio Company,
Guangzhou, China) in 48-well plates. The vector pDsRed2-N1
(Clontech Laboratories, Inc., Mountain View, CA, USA) expressing
red fluorescent protein (RFP) was transfected and used for
normalization. The intensities of enhanced green fluorescent
protein (EGFP) and RFP fluorescence were measured using a F-4500
Fluorescence Spectrophotometer (Hitachi, Ltd., Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed in order to detect the
relative levels of the transcripts. The large RNA and small RNA of
tissue samples were isolated using an mirVana™ miRNA Isolation kit
(Ambion Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions. The transcripts were generated from 2
µg RNA extracted from the cells through reverse
transcription using Moloney murine leukemia virus reverse
transcriptase (Promega Corporation, Madison, WI, USA). The β-actin
gene was used as an internal control standard for the PCR reaction.
PCR was performed under the following conditions: 94°C for 4 min,
followed by 40 cycles of 94°C for 1 min, 56°C for 1 min and 72°C
for 1 min. PCR was performed in a total volume of 50 µl with
AmpliTaq Gold 360 DNA Polymerase (Applied Biosystems, Foster City,
CA, USA). After CE on an ABI 3130 Genetic Analyzer (Applied
Biosystems), data were collected using GeneMapper v4.0 software for
fragment analysis (Applied Biosystems).
MTT assay
SGC-7901 and SGC-7901/5-FU cells were respectively
seeded into a 96-well plate at a denstiy of 8,000 cells/well one
day prior to transfection. The cells were transfected with
anti-miR-197, miR-197 or the control vector (0.15 µg/well;
Shanghai GenePharma Co., Ltd., Shanghai, China). 5-FU was then
added so that the final concentrations were 0.5, 1, 2, 4 and 8
µmol/l. Following 48 h of incubation, the MTT assay was used
to evaluate the cell viability. MTT solution (20 µl;
Sigma-Aldrich) was added to 100 µl culture media and cells
were incubated for a further 4 h at 37°C The growth inhibition rate
was calculated as: Growth inhibition (%) = (1-A of experimental
group/A of control group) ×100, where A represents the absorbance
at 570 nm, which was measured using a µQuant Universal
Microplate Spectrophotometer (BioTek Instruments, Inc., Winooski,
VT, USA).
Western blot analysis
Cultured cells were lysed using
radioimmunoprecipitation assay buffer (0.1% SDS, 1% Triton X-100, 1
mM MgCl2 and 10 mM Tris-HCl; pH 7.4; all from
Sigma-Aldrich) in 4°C for 30 min. The protein extracts (50
µg) were fractionated by 10% SDS-PAGE and then transferred
onto nitrocellulose membranes. The following antibodies were used:
Anti-MAPK1 (sc-136288; species, mouse; 1:500) and anti-GAPDH
(sc-365062; species, mouse; 1:1,000) (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). The bound antibodies were detected using
the ECL Plus Western Blotting Detection system (GE Healthcare,
Little Chalfont, UK) and the chemiluminescent signals were detected
with the using high-performance chemiluminescence film (GE
Healthcare).
Small interfering (si)RNA
transfection
According to the manufacturer's instructions, the
SignalSilence MAPK1 siRNA kit (Cell Signaling Technology, Inc.,
Danvers, MA, USA) was used to silence MAPK1 protein expression.
Subsequent to transfection for 48 h, siRNA transfection efficiency
was measured using western blot analysis.
Flow cytometric analysis
In order to synchronize the cell growth, the
transfected cells were maintained in serum-free culture medium for
starvation. After 24 h, one group of cells was harvested and the
other was cultured in complete medium for 24 h. The harvested cells
were fixed in 95% ethanol and stored at −60°C. The fixed cells were
washed with phosphate-buffered saline, re-suspended in propidium
iodine (PI) staining buffer (Bio-Light, Shanghai, China), which
contained 50 mg/ml PI, 50 mg/ml Annexin V, and 50mg/ml RNaseA, and
were then incubated at 4°C for 30 min in the dark. The samples were
analyzed using a FACScan flow cytometer and Cell Quest 2.0 analysis
software (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation. A two-tailed Student's t-test was used for comparison
and P<0.05 was considered to indicate a statistically
significant difference. GraphPad 5.0 (GraphPad, Inc., La Jolla, CA,
USA) was used for all statistical analyses.
Results
miR-197 is downregulated in
5-FU-resistant SGC7901/5-FU cells
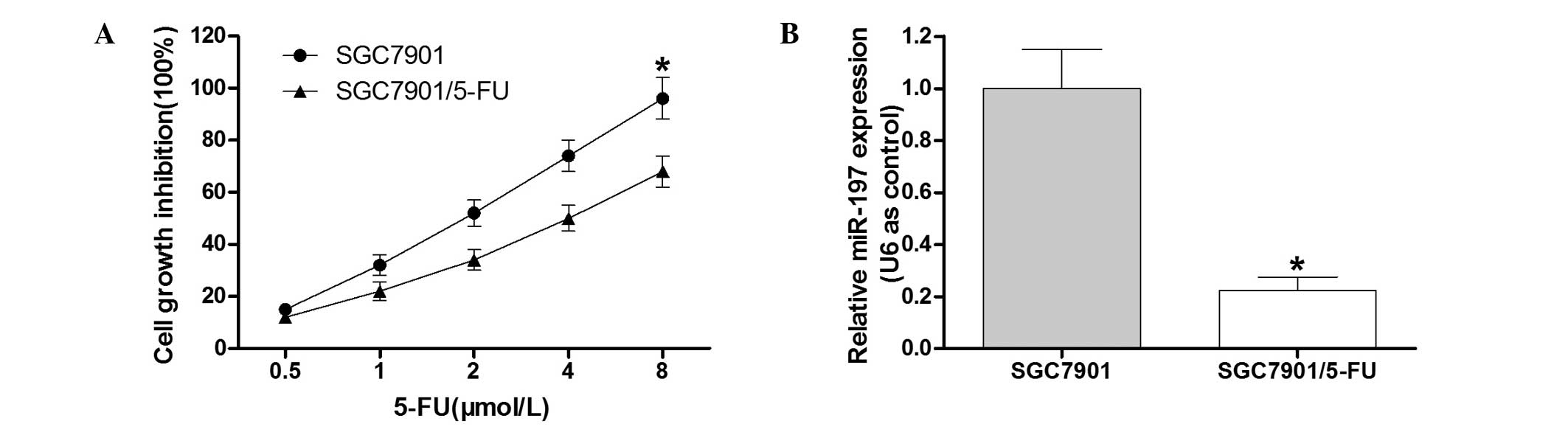
To detect the cell growth inhibition rate of the
SGC7901/5-FU cell line and the parental SGC7901 cell line, an MTT
assay was performed (Fig. 1A). The
results demonstrated that compared with the SGC7901 cell line, the
SGC7901/5-FU cells exhibited clear resistance to 5-FU. In order to
determine the role of miR-197 in the 5-FU-resistant gastric cancer
cell line SGC7901/5-FU, RT-qPCR analysis was conducted. The results
indicated that miR-197 was downregulated in SGC7901/5-FU cells
(Fig. 1B), which suggested that
miR-197 may contribute to 5-FU drug resistance in gastric cancer
cells.
Knockdown of miR-197 leads to resistance
to 5-FU in the SGC7901 cells
To investigate the association between miR-197 and
5-FU resistance in SGC7901 cells, the effect of downregulation of
miR-197 in 5-FU-sensitive SGC7901 cells was assessed. A
commercially synthesized miR-197 inhibitor (anti-miR-197) was used
to alter the miR-197 expression levels in SGC7901 cells. The
validity of miR-197 ectopic expression was confirmed by RT-qPCR
following transfection with anti-miR-197 or the control vector into
SGC7901 cells. The results indicated that the commercially
synthe-sized anti-miR-197 was able to reduce miR-197 expression in
SGC7901 cells compared with that in the control group (P<0.05;
Fig. 2A). The group transfected
with anti-miR-197 was observed to have a significantly higher
survival rate than the control group (Fig. 2B). These results suggested that
down-regulation of miR-197 led to 5-FU resistance of SGC7901
cells.
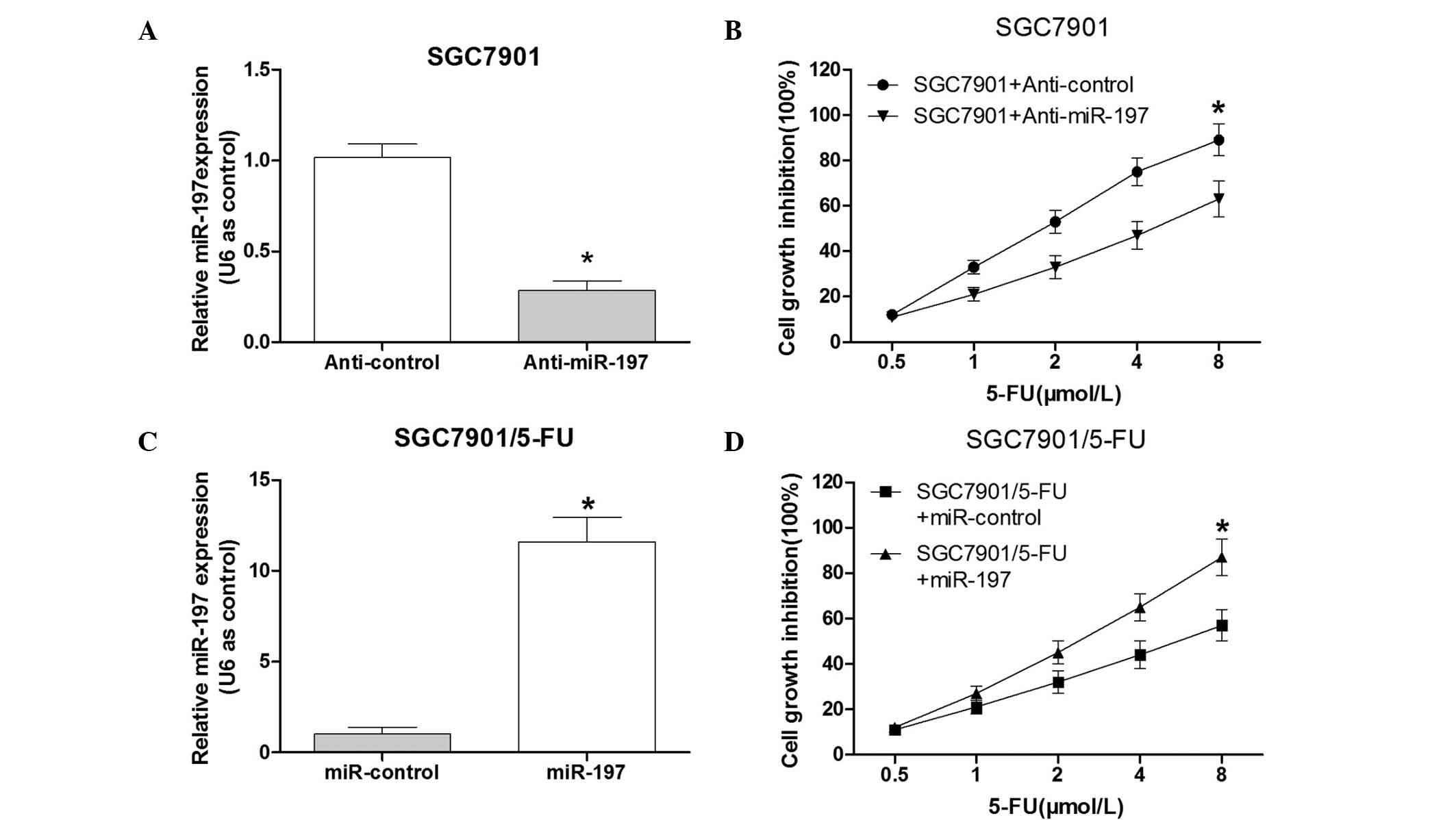 | Figure 2Expression levels of miR-197 affect
the sensitivity of SGC7901 cells to 5-FU. (A) Following
transfection with anti-miR-197, the expression levels of miR-197
were significantly reduced in SGC7901 cells. (B) Following
transfection with anti-miR-197 or control, SGC7901 cells were
treated with various doses of 5-FU (0.5, 1.0, 2.0, 4.0 or 8.0
µmol/l) for 48 h. An MTT assay was performed to determine
the cell growth inhibition. (C) Following transfection of the
miR-197 mimics, the expression levels of miR-197 were significantly
increased in SGC7901/5-FU cells. (D) Following transfection with
the miR-197 mimics or control, the SGC7901/5-FU cells were treated
with various doses of 5-FU (0.5, 1.0, 2.0, 4.0 and 8.0
µmol/l) for 48 h. The MTT assay was performed to determine
the cell growth inhibition. Values are expressed as the mean ±
standard deviation (n=3). *P<0.05 vs. control.
miR-197, microRNA-197; 5-FU, fluorouracil; SGC7901/5-FU, SGC7901
cell line resistant to 5-FU. |
Overexpression of miR-197 may partially
enhance 5-FU sensitivity in SGC7901/5-FU cells
To further determine the effects of miR-197
overexpression in SGC7901/5-FU cells, miR-197 mimics or the control
vector were transfected into SGC7901/5-FU cells. RT-qPCR analysis
confirmed that the miR-197 mimics were able to increase miR-197
expression in SGC7901/5-FU cells compared with that in the control
group (P<0.05; Fig. 2C). The
SGC7901/5-FU cells which were transfected with the miR-197 mimics
exhibited a significantly lower survival rate than the cells in the
negative control group following 5-FU exposure (Fig. 2D). These results suggested that the
overexpression of miR-197 may sensitize the SGC7901/5-FU cells to
5-FU.
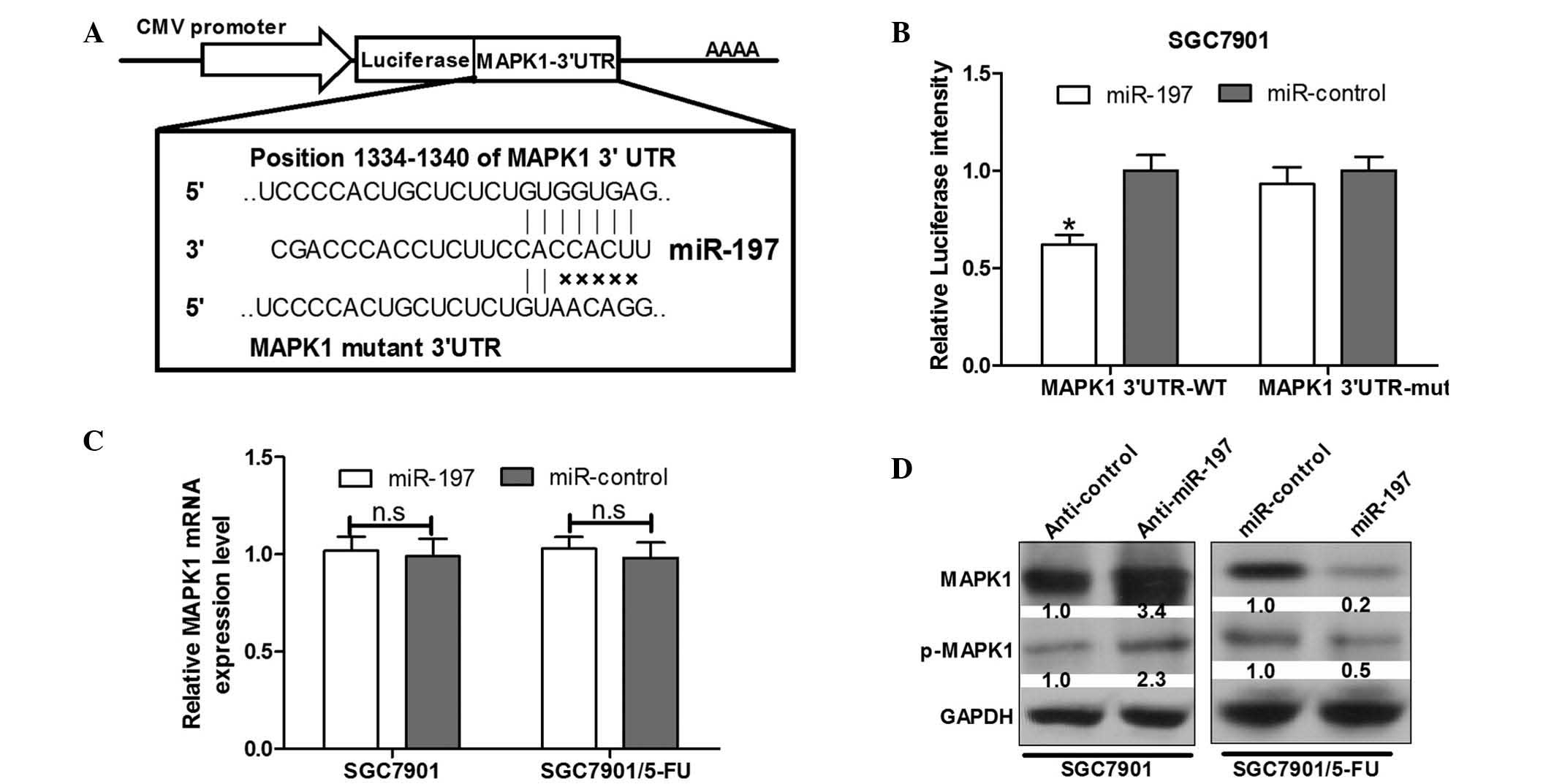
miR-197 directly targets the MAPK1 3′UTR
in SGC7901 gastric cancer cells
To determine the candidate target gene of miR-197, a
bioinformatics analysis using Targetscan 6.2 (http://www.targetscan.org/) was conducted to predict
potential target genes. It was observed that the miR-197
complementary binding sites were contained in the 3′UTR of MAPK1
mRNA (Fig. 3A). A luciferase
reporter assay was performed to validate that MAPK1 can be directly
targeted by miR-197 using engineered EGFP reporter vectors that had
either the wild-type 3′UTR of MAPK1 or the mutant UTR with a 5-base
mutation in the complementary seed sequence (Fig. 3A). pDsRed2-NI was also
co-transfected for normalization. SGC7901 cells were co-transfected
with MAPK1 3′UTR and miR-197 or the control vector. Compared with
the control vector, over-expression of miR-197 was able to
significantly inhibit EGFP expression (P<0.05) (Fig. 3B). By contrast, EGFP expression
levels of cells transfected with a mutant of the MAPK1 3′UTR
binding site were not influenced by overexpression of miR-197
(Fig. 3B), indicating that miR-197
was able to complementarily bind to the specific sites of the MAPK1
mRNA 3′UTR and negatively regulate the expression of the MAPK1
gene.
miR-197 negatively regulates MAPK1 at the
post-transcriptional level
Downregulation of gene expression is the main
function of miRNAs, which occurs through translational repression,
cleavage of mRNA or in a variety of other processes by binding to
3′UTRs of target genes. To examine whether miR-197 suppresses
endogenous MAPK1 expression, SGC7901 and SGC791/5-FU cells were
transfected with anti-miR-197 and miR-197 mimics, and MAPK1 protein
expression was assessed via western blot analysis.
The results demonstrated that overexpression of
miR-197 reduced the expression levels of MAPK1 and the
phosphorylation of MAPK1 protein by 80 and 50%, respectively, in
SGC7901/5-FU cells (Fig. 3D).
However, upregulation of miR-197 in SGC7901/5-FU cells was not able
to reduce endogenous MAPK1 mRNA according to RT-qPCR analysis
(Fig. 3C). In addition, following
transfection with anti-miR-197, significant increases were observed
in the endogenous MAPK1 protein expression levels and the
phosphorylation of MAPK1 in SGC7901 cells (Fig. 3D); however, MAPK1 mRNA levels
remained unchanged (Fig. 3C).
These results suggested an inverse correlation between miR-197 and
MAPK1 protein expression levels.
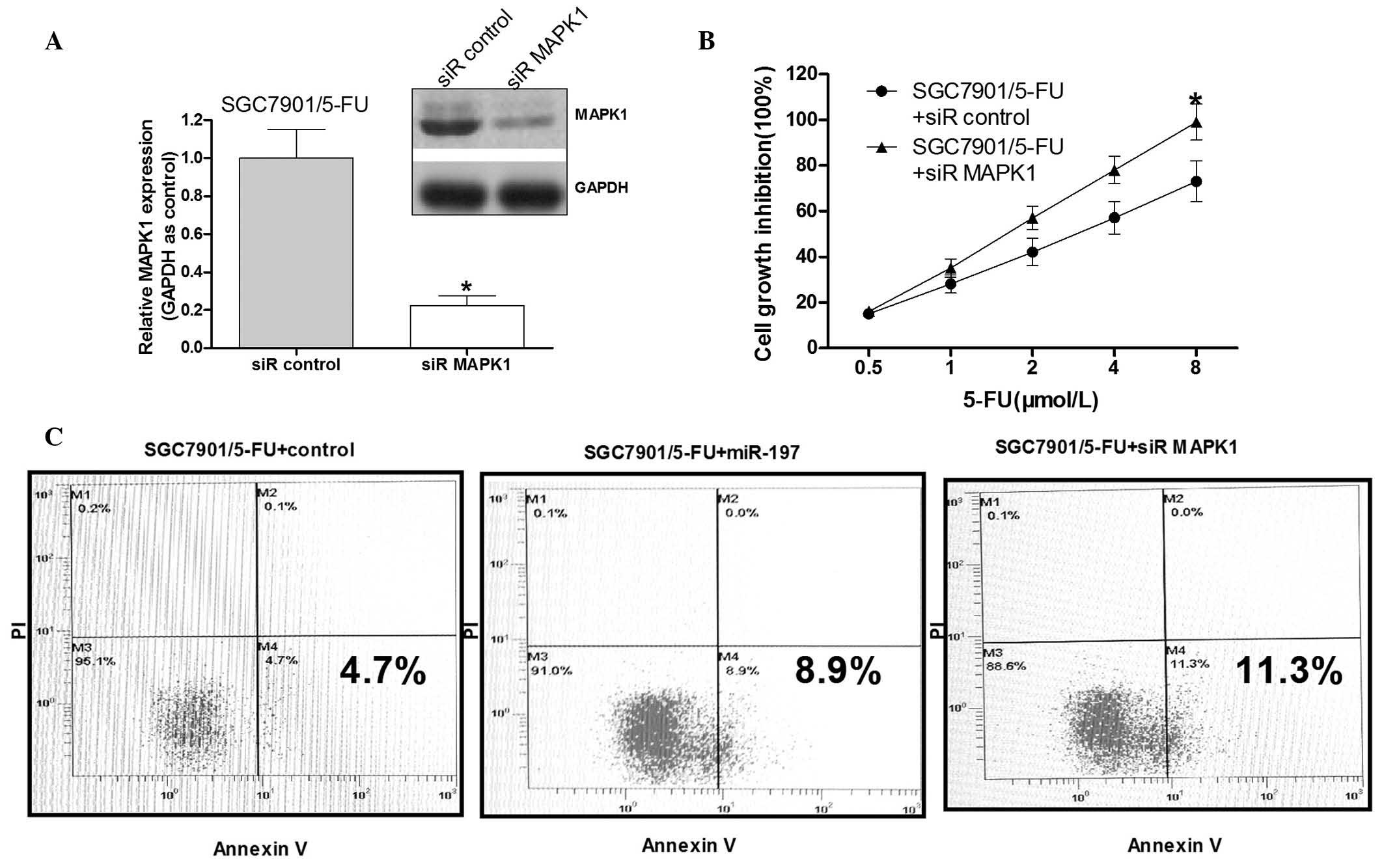
MAPK1 is a crucial signaling factor in
5-FU resistance in SGC7901/5-FU cells
To validate whether MAPK1 serves a role in
miR-197-induced 5-FU resistance, the MAPK1 gene was silenced to a
certain degree in the SGC7901/5-FU cells. Western blot analysis
demonstrated that MAPK1 siRNA effectively reduced the MAPK1 protein
levels (Fig. 4A). Following MAPK1
siRNA transfection, the cell growth inhibition rate by was then
detected following exposure to various concentrations of 5-FU
(0.5–8 µM). Knockdown of MAPK1 significantly increased the
growth inhibition rate of the SGC7901/5-FU cells compared with that
in the control group (Fig. 4B).
The SGC7901/5-FU cells transfected with miR-197 mimics in addition
to those transfected with siR-MAPK1 demonstrated a significantly
increased apoptotic rate compared with that in the negative control
group (P<0.05). These results suggested that miR-197 may improve
the 5-FU sensitivity of the SGC7901/5-FU cells by down-regulating
MAPK1.
Discussion
MDR is a multifactorial process, which is
responsible for the lack of chemosensitivity in primary and
secondary tumors (20). 5-FU is
the most commonly used chemotherapeutic drug in patients with
gastric cancer, and one of the main causes of chemotherapeutic
treatment failure in advanced gastric cancer is 5-FU resistance
(2,26). Therefore, the development of novel
strategies enhancing the efficacy of 5-FU is essential for
effective therapy. It is widely accepted that abnormal genetic
expression and the alteration of the dynamic balance between
oncogenes and tumor suppressor genes commonly leads to the
formation of tumors (27). miRNAs,
in addition to other genes, participate in the regulation of
oncogenes and tumor suppressor genes (5). Increasing numbers of studies have
identified that abnormal miRNA expression is also responsible for
the absence of a chemotherapeutic response. However, the role of
miRNAs in drug-resistance of gastric cancer remains to be fully
elucidated.
The present study aimed to investigate a novel
miRNA, which was able to regulate MAPK1 protein expression levels
through binding to specific sites of the MAPK1 3′UTR, and to
validate its effects on 5-FU resistance using SGC7901 cells. The
results showed that the suppression of MAPK1 in addition to miR-197
upregulation acted together to achieve sensitivity to the
chemotherapeutic 5-FU. It was therefore suggested that altering
miR-197 expression may improve the sensitivity of SGC7901 cells to
5-FU treatment.
Using RT-qPCR, it was identified that miR-197
exhibited lower expression levels in human 5-FU-resistant
SGC7901/5-FU gastric cancer cells than that in the parental cell
line SGC7901. The results suggested that miR-197 may be critical in
the development of gastric cancer chemotherapy resistance.
Therefore, it was hypothesized that miR-197 is an inhibitory factor
of 5-FU resistance in gastric cancer cells due to the low
expression levels of miR-197 in SGC7901/5-FU. The cell growth
inhibition rate was assessed using the MTT assay in order to
investigate the association between miR-197 and the growth
inhibition capacity of gastric cancer cell lines with or without
5-FU resistance. Following transfection with anti-miR-197, the
SGC-7901 cell growth inhibition rate was significantly reduced. In
addition, compared with the control group, upregulation of miR-197
enhanced cell growth inhibition following 5-FU treatment. miR-197
is therefore closely associated with 5-FU resistance.
Bioinformatics analysis was conducted to predict MAPK1 as one of
the candidate target genes of miR-197, which was then confirmed by
experimental evidence in the present study. Through complementarily
binding to the 3′UTR of MAPK1 seed region, miR-197 was able to
regulate MAPK1 protein expression directly. Overexpression of
miR-197 resulted in a reduction in the fluorescence intensity of
MAPK1-3′UTR reporter vector-transfected cells in the luciferase
reporter assay (Fig. 3B). However,
the 5-base mutation of the miR-197 binding sites in MAPK1-3′UTR
abolished the effect of miR-197 on the regulation of EGFP
fluorescence intensity (Fig. 3B).
In addition, endogenous MAPK1 expression levels and the
phosphorylation of MAPK1 protein were reduced in SGC7901/5-FU cells
with the overexpression of miR-197 and in SGC7901 cells transfected
with anti-miR-197 (Fig. 3D). The
alteration of miR-197 expression levels was observed to not effect
the MAPK1 mRNA levels. These results suggested that miR-197
regulated MAPK1 protein expression.
The MAPK cascade is one of the intracellular signal
transduction pathways specific to the amino acids serine, threonine
and tyrosine. MAPKs are present exclusively in eukaryotic cells,
which are involved in direct cellular responses to various stimuli,
including mitogens, hormones, cytokines and osmotic stress
(28). Extracellular
signal-regulated kinases (ERKs), c-Jun-N-terminal protein kinases
and p38 are all involved in the MAPK pathway (29). MAPKs participate in gene
expression, cell proliferation, differentiation, cell mitosis and
apoptosis (28). Due to the
integral role in the regulation of cancer cell proliferation,
invasion and survival, the MAPK pathway is considered to be a
potential target for therapeutic intervention in cancer. MAPK1 is
also known as ERK2; ERK was the first MAPK family member to be
identified, and it also serves as the key molecule in the MAPK
signaling pathway. ERKs are responsible for transporting
extracellular stimuli from the cell surface to the nucleus
(30). In 2007, Katayama et
al (31) demonstrated that the
ERK pathway was able to positively regulate P-gp expression in the
MDA-MB-231/MDR cell line, and that inhibition of the MAPK pathway
can suppress cell surface P-gp expression by promoting its
degradation. Furthermore, various studies have demonstrated that
modulation of ERK activation may reverse MDR in prostatic, gastric
and hematopoietic cancer (32–35).
Previous studies have suggested that modulation of
ERK activation may provide a novel method to reverse MDR in cancer
cells (36). In the present study,
the association between miR-197 and MAPK1 in gastric cancer cells
was investigated, and it was confirmed that miR-197 reduced cell
survival and 5-FU resistance in human gastric cancer by directly
targeting MAPK1. It was also identified that knockdown of MAPK1
improved the 5-FU sensitivity of SGC7901/5-FU cells, which is
consistent with the results of miR-197 overexpression. These
results indicated that miR-197 mimics may be used as a therapeutic
approach in 5-FU-resistant gastric cancer.
References
|
1
|
Murray CJ and Lopez AD: Alternative
projections of mortality and disability by cause 1990–2020: Global
Burden of Disease Study. Lancet. 349:1498–1504. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham D, Allum WH, Stenning SP, et
al: MAGIC Trial Participants: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rivera F, Vega-Villegas ME and López-Brea
MF: Chemotherapy of advanced gastric cancer. Cancer Treat Rev.
33:315–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Climent J, Dimitrow P, Fridlyand J,
Palacios J, Siebert R, Albertson DG, Gray JW, Pinkel D, Lluch A and
Martinez-Climent JA: Deletion of chromosome 11q predicts response
to anthracycline-based chemotherapy in early breast cancer. Cancer
Res. 67:818–826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek
SH and Kim VN: MicroRNA genes are transcribed by RNA polymerase II.
EMBO J. 23:4051–4060. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han J, Lee Y, Yeom KH, Kim YK, Jin H and
Kim VN: The Drosha-DGCR8 complex in primary microRNA processing.
Genes Dev. 18:3016–3027. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee Y, Ahn C, Han J, et al: The nuclear
RNase III Drosha initiates microRNA processing. Nature.
425:415–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee Y, Jeon K, Lee JT, Kim S and Kim VN:
MicroRNA maturation: Stepwise processing and subcellular
localization. EMBO J. 21:4663–4670. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lund E, Güttinger S, Calado A, Dahlberg JE
and Kutay U: Nuclear export of microRNA precursors. Science.
303:95–98. 2004. View Article : Google Scholar
|
|
11
|
Hutvágner G, McLachlan J, Pasquinelli AE,
Bálint E, Tuschl T and Zamore PD: A cellular function for the
RNA-interference enzyme Dicer in the maturation of the let-7 small
temporal RNA. Science. 293:834–838. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parker JS and Barford D: Argonaute: A
scaffold for the function of short regulatory RNAs. Trends Biochem
Sci. 31:622–630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peters L and Meister G: Argonaute
proteins: Mediators of RNA silencing. Mol Cell. 26:611–623. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pillai RS, Bhattacharyya SN and Filipowicz
W: Repression of protein synthesis by miRNAs: How many mechanisms?
Trends Cell Biol. 17:118–126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Felli N, Fontana L, Pelosi E, et al:
MicroRNAs 221 and 222 inhibit normal erythropoiesis and
erythroleukemic cell growth via kit receptor down-modulation. Proc
Natl Acad Sci USA. 102:18081–18086. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Huang H, Sun L, et al: MiR-21
indicates poor prognosis in tongue squamous cell carcinomas as an
apoptosis inhibitor. Clin Cancer Res. 15:3998–4008. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Aurora AB, Johnson BA, Qi X,
McAnally J, Hill JA, Richardson JA, Bassel-Duby R and Olson EN: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu N, Papagiannakopoulos T, Pan G, Thomson
JA and Kosik KS: MicroRNA-145 regulates OCT4, SOX2, and KLF4 and
represses pluripotency in human embryonic stem cells. Cell.
137:647–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma L, Young J, Prabhala H, et al: miR-9, a
MYC/MYCN-activated microRNA, regulates E-cadherin and cancer
metastasis. Nat Cell Biol. 12:247–256. 2010.PubMed/NCBI
|
|
20
|
Huh JH, Kim TH, Kim K, Song JA, Jung YJ,
Jeong JY, Lee MJ, Kim YK, Lee DH and An HJ: Dysregulation of
miR-106a and miR-591 confers paclitaxel resistance to ovarian
cancer. Br J Cancer. 109:452–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang Y, Shen H, Li H, Cao Y, Qin R, Long
L, Zhu X, Xie C and Xu W: miR-106a confers cisplatin resistance by
regulating PTEN/Akt pathway in gastric cancer cells. Acta Biochim
Biophys Sin (Shanghai). 45:963–972. 2013. View Article : Google Scholar
|
|
22
|
Xia L, Zhang D, Du R, et al: miR-15b and
miR-16 modulate multidrug resistance by targeting BCL2 in human
gastric cancer cells. Int J Cancer. 123:372–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan D, Zhang X, Chen X, Mou Z, Hu J, Zhou
S, Ding J and Wu K: Bird's-eye view on gastric cancer research of
the past 25 years. J Gastroenterol Hepatol. 20:360–365. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu H, Wu H, Liu X, Evans BR, Medina DJ,
Liu CG and Yang JM: Role of MicroRNA miR-27a and miR-451 in the
regulation of MDR1/P-glycoprotein expression in human cancer cells.
Biochem Pharmacol. 76:582–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng F, Henson R, Lang M, Wehbe H,
Maheshwari S, Mendell JT, Jiang J, Schmittgen TD and Patel T:
Involvement of human micro-RNA in growth and response to
chemotherapy in human cholangiocarcinoma cell lines.
Gastroenterology. 130:2113–2129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang CX, Huang S, Xu N, Fang JW, Shen P,
Bao YH, Mou BH, Shi MG, Zhong XL and Xiong PJ: Phase II study of
epirubicin plus oxaliplatin and infusional 5-fluorouracil as
first-line combination therapy in patients with metastatic or
advanced gastric cancer. Anticancer Drugs. 18:581–586. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kopnin BP: Targets of oncogenes and tumor
suppressors: Key for understanding basic mechanisms of
carcinogenesis. Biochemistry Biokhimiia. 65:2–27. 2000.PubMed/NCBI
|
|
28
|
Whitmarsh AJ and Davis RJ: Transcription
factor AP-1 regulation by mitogen-activated protein kinase signal
transduction pathways. J Mol Med Berl. 74:589–607. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HG, Lee CK, Cho SM, Whang K, Cha BH,
Shin JH, Song KH and Jeong SW: Neuregulin 1 up-regulates the
expression of nicotinic acetylcholine receptors through the
ErbB2/ErbB3-PI3K-MAPK signaling cascade in adult autonomic ganglion
neurons. J Neurochem. 124:502–513. 2013. View Article : Google Scholar
|
|
30
|
Lewis TS, Shapiro PS and Ahn NG: Signal
transduction through MAP kinase cascades. Adv Cancer Res.
74:49–139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Katayama K, Yoshioka S, Tsukahara S,
Mitsuhashi J and Sugimoto Y: Inhibition of the mitogen-activated
protein kinase pathway results in the down-regulation of
P-glycoprotein. Mol Cancer Ther. 6:2092–2102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kisucká J, Barancík M, Bohácová V and
Breier A: Reversal effect of specific inhibitors of
extracellular-signal regulated protein kinase pathway on
P-glycoprotein mediated vincristine resistance of L1210 cells. Gen
Physiol Biophys. 20:439–444. 2001.
|
|
33
|
Lin JC, Chang SY, Hsieh DS, Lee CF and Yu
DS: Modulation of mitogen-activated protein kinase cascades by
differentiation-1 protein: Acquired drug resistance of hormone
independent prostate cancer cells. J Urol. 174:2022–2026. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Li S, Han Y, Liu J, Zhang J, Li F,
Wang Y, Liu X and Yao L: Calebin-A induces apoptosis and modulates
MAPK family activity in drug resistant human gastric cancer cells.
Eur J Pharmacol. 591:252–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McCubrey JA, Steelman LS, Abrams SL, Lee
JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA,
D'Assoro AB, et al: Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT
pathways in malignant transformation and drug resistance. Adv
Enzyme Regul. 46:249–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu Y, Bally M, Dragowska WH and Mayer L:
Inhibition of mitogen-activated protein kinase/extracellular
signal-regulated kinase kinase enhances chemotherapeutic effects on
H460 human non-small cell lung cancer cells through activation of
apoptosis. Mol Cancer Ther. 2:641–649. 2003.PubMed/NCBI
|