Introduction
Osteosarcoma, a bone sarcoma, is the eighth most
common malignancy occurring in all age groups, the prevalence of
which is 20% in adolescents and 5% in children (1,2).
Bone sarcomas are generally derived from the mesenchymal or
non-epithelial tissues and predominantly occur as a high-grade
tumor with pulmonary metastases (3). Following diagnosis, the average life
span of patients with bone sarcoma at the metastasis stage is <5
years (1,2). Despite improvements in surgery and
multi-therapeutic agents, treatment failure and tumor recurrence
have major implications on the success of cancer treatment, with
failure in treatment strategies ultimately leading to poor survival
rates in patients with cancer (4).
Therefore, understanding the mechanism of tumorigenesis at the
molecular level is crucial to provide effective treatment
strategies to completely eradicate the tumor refractory. The
molecular mechanisms underlying the drug resistance and tumor
recurrence of bone tumors remains to be fully elucidated. Previous
investigations in several types of cancer have demonstrated that
the presence of a small population of cancer stem cells (CSCs) are
the major cause for drug resistance and tumor relapse. These CSCs
share the properties of stem cells, including self-renewal,
multi-drug resistance, and high proliferation rates and
differentiation potential (5–8). In
addition, these CSCs are highly tumorigenic and, therefore, have
been termed 'tumor initiating cells' (9).
Several studies have reported the existence of CSCs
in different types of cancer, based on Hoechst 33342 dye exclusion
assays, which is considered to be a valuable technique to isolate
CSCs (10–12). The CSCs which exclude this dye are
termed 'side population' (SP) cells, and exhibit enhanced
overexpression of the adenosine triphosphate binding cassette (ABC)
protein transporter, ABCG2 (13).
The SP cells have been isolated and characterized in several types
of solid tumor and well-established cell lines (12). The SP cells have been demonstrated
to possess the features of CSCs and are considered to be enriched
CSCs. Notably, previous findings have revealed that stem-like
properties of CSCs and their maintenance are regulated by the
Wnt/β-catenin pathway in different types of cancer, including
breast, liver and colon cancer (14–16).
Therefore, the isolation and characterization of SP cells may
assist in understanding the molecular mechanism underlying
CSC-mediated tumoringeneis. Previous reports concerning the
identification and characterization of CSCs in osteosarcoma stem
lines are limited, in which CSCs have been observed to be highly
tumorigenic in mouse models (8,17).
These data suggest that osteosarcoma may also contain a small
population of CSCs, which are responsible for treatment failure,
tumor recurrence and metastasis. Consequently, the present study
aimed to investigate the presence of cancer stem-like SP cells from
osteosarcoma samples using a fluorescence-activated cell sorting
(FACS-based Hoechst 33342 dye exclusion technique. In addition, the
sorted SP cells were analyzed for the activation of the
Wnt/β-catenin signaling pathways and expression of stemness
genes.
Materials and methods
Cell isolation and culture
Human osteosarcoma samples (n=10) were obtained from
10 patients, who had not undergone chemotherapy treatment, at the
Department of Traumatology, Linyi People's Hospital (Linyi, China).
This study was approved by the Linyi People's Hospital research
review and ethics committee (Linyi, China), and informed consent
was obtained from all participants. The patients and tumor details
were as follows: Patients were aged between 38 and 49 years and
included six males and four females. (18). The tumor samples were classified by
a pathologist at Linyi People's Hospital (Linyi, China) using the
three-tier grading scheme: Low grade (grade 1), intermediate grade
(grade 2) and high grade (grade 3). The tumor samples were
fibroblastic osteosarcoma in site and were classified as high
grade. Following isolation, the samples were washed extensively in
phosphate-buffered saline (PBS) and incubated overnight in
Dulbecco's modified Eagle's medium (DMEM/F12; Gibco-BRL, Invitrogen
Life Technologies, Carlsbad, CA, USA), containing penicillin (500
U/ml), streptomycin (500 µg/ml) and amphotericin B (1.25
µg/ml; Gibco-BRL). Enzymatic digestion was performed using
collagenase (1.5 mg/ml; (Gibco-BRL) and hyaluronidase (20
µg/ml) in PBS for 1 h. The cells were cultured in DMEM with
10% FBS, supplemented with antibiotics [penicillin (500 U/ml) and
streptomycin (500 µg/ml)] and maintained in T-75 flasks at
37°C in a humidified 5% CO2/95% air atmosphere. When the
cells reached a confluence of 90%, they were removed from the
culture flask using trypsin-EDTA (0.25% 53 mM EDTA; Sigma-Aldrich,
St. Louis, MO, USA) washed in PBS, and the cells were resuspended
in 10% DMEM. The number of cells were counted using a hemocytometer
(LW Scientific, Lawrenceville, GA, USA).
FACS analysis
The cells were cultured in DMEM with 10% FBS,
supplemented with antibiotics and maintained in T-75 flasks at 37°C
in a humidified 5% CO2/95% air atmosphere. At a
confluence of 90%, the cells were removed from the culture flask
using trypsin-EDTA (0.25% 53 mM EDTA), washed in PBS and then
resuspended in 10% DMEM. The number of cells was counted using a
hemocytometer. The cells were divided into two groups as follows:
Control group, cells + Hoechst 33342 dye (n=7). Drug-treated group,
cells + verapamil + Hoechst 33342 dye (n=7). The cells
(~106 cells/ml 10% DMEM) were labeled with Hoechst 33342
stock-bis-benzimide (5 µl/ml; Sigma-Aldrich) either alone or
in combination with verapamil (0.8 µl/ml). The cells were
then resuspended in 500 µl Hank's balanced salt solution
(HBSS) containing 10 mM HEPES for FACS analysis. Finally, the cells
were counterstained using 2 µg/ml propidium iodide (PI) and
assessed using a flow cytometer (Attune NxT; Life
Technologies).
In vitro proliferation activity
The FACS-sorted SP and non-SP cells were seeded in a
96-well plate at 2×106 cells/well, and then cultured in
a CO2 incubator. Each group was set up in triplicate.
The proliferation activity of the cells was measured every day for
7 days. Each well was supplemented with Cell Counting Kit (CCK)-8
solution (10 µl; Dojindo Molecular Technologies, Inc.,
Rockville, MD, USA) and incubated in a CO2 incubator for
2–3 h. The optical density (OD) was then determined at 450 nm.
These data were used to calculate the cell growth and produce
graphs, based on the mean value of OD450 and standard
deviation values for each well.
Cell resistance assay
The determine cell resistance, 1×103
cells/plate were cultured in 96-well plates and treated with
chemotherapeutic drugs at the following concentrations: 10
µg/ml 5-fluorouracil (5-FU), 250 mM gemcitabine, 100 mM
oxaliplatin, 30 ng/ml paclitaxel, 5 mg/ml cisplatin, 10 mg/ml
etoposide and 2 µg/ml oxaliplatin. The mean OD450
values obtained were presented as a graph, and cell resistance in
the groups was calculated using the following formula: Cell
resistance rate (%) = (experimental group OD450 value /
control group OD450 value) × 100. The values presented
are the average of three independent experiments.
Immunofluorescent staining
The FACS-sorted SP cells were fixed onto glass
slides using ice-cold 4% paraformaldehyde at 4°C for 10 min, and
blocked with 1% bovine serum albumin for 30 min at room temperature
to inhibit nonspecific binding of immunoglobulin (Ig)G. Following
washing with PBS, the cells were incubated with mouse anti-human
ABCG2 antibody at 4̊C overnight. Following another wash with PBS,
the cells were incubated with goat anti-mouse
IgG-horesradish-peroxidase (HRP) for 30 min at room temperature.
The cells were coun-terstained using hematoxylin, and were mounted
with glycerol vinyl alcohol aqueous mounting solution (19). Under an optical microscope, the
ABCG2+cells were stained red. All images were processed
using Adobe Photoshop CS4.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted and complementary DNA was
prepared using a Reverse Transcriptase kit (Thermo Fisher
Scientific, Pittsburgh, PA, USA). RT-qPCR analysis was subsequently
performed using IQ Supermix with SYBR-Green (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) with 25 ng cDNA per sample. The sequences
of human specific primers used were as follows: ABCG2, forward
5′-TCAATCAAAGTGCTTCTTTTTTATG-3′ and reverse
5′-TTGTGGAAGAATCACGTGGC-3′; Sox2, forward
5′-CACACTGCCCCTCTCACACAT-3′ and reverse
5′-CATTTCCCTCGTTTTTCTTTGAA-3′; Nanog, forward
5′-CCAACATCCTGAACCTCAGCTAC-3′and reverse 5′-GCCTTCTGCGTCACACCATT);
CD133, forward 5′-TCTTGACCGACTGAGACCCAAC-3′and reverse
5′-ACTTGATGGATGCACCAAGCAC-3′; CCND1, forward
5′-TGATGCTGGGCACTTCATCTG-3′ and reverse
5′-TCCAATCATCCCGAATGAGAGTC-3′; OCT-4 forward
5′-GCAATTTGCCAAGCTCCTGAA-3′and reverse 5′-GCAGATGGTCGTTTGGCTGA-3′;
Nestin, forward 5′-GACGGAGGAGGTAGCCCGCA-3′ and reverse
5′-GCCTCCACAGCCAGCTGGAACT-3′; and GAPDH, forward
5′-TCTGCTCCTCCTGTTCGACA-3′ and reverse 5′-AAAAGCAGCCCTGGTGACC-3′.
GAPDH was used as a housekeeping gene. The parameters used to set
the qPCR reactions were as follows: Initial denaturation−95°C for
15 sec; annealing−58°C for 45 sec; extension−60°C for 30–45 sec;
cycles-35. The qPCR products were electrophoresed on a 1.2% agarose
gel and stained with ethidium bromide (0.5 mg/ml). The data
represented are the average values of three independent
experiments.
Western blot analysis
The proteins were extracted from the SP and non-SP
cells, and protein concentration was determined using a Bradford
assay. Following 10% SDS-PAGE and transfer onto a nitrocellulose
membrane (Sigma-Aldrich), the membranes were incubated with primary
antibodies overnight at 4°C. The following primary antibodies were
used: β-catenin (rabbit polyclonal anti-human, 1:1,000 dilution,
sc-7199); ABCG2 (mouse monoclonal anti-human, 1:1,000 dilution,
sc-18841); GAPDH (mouse monoclonal anti-human, 1:1,000 dilution,
sc-47724). The secondary immunoglobulin (Ig)G antibodies with
alkaline phosphatase markers were used with specificity for the
appropriate species (goat anti-rabbit, 1:5,000 dilution, sc-2034
and goat anti-mouse, 1:5,000 dilution, sc-2047) and incubated for 2
h at room temperature. All antibodies were purchased from Santa
Cruz Biotechnology Inc, (Dallas, TX, USA). Immuno-reacted proteins
were detected with using a chemiluminescence reagent kit (ab79907;
Abcam, Cambridge, MA, USA). Blots were detected and scanned using a
densitometer (Biorad GS-710; Bio-Rad Laboratories, Inc.).
Tumor cell implantation
For tumor cell implantation, 4–6 week-old NOD/SCID
mice were obtained from the Shanghai SLAC Laboratory Animal Co. Ltd
(Shanghai, China). All mice were fed a standard chow, and food and
water were available ad libitum. Animals were housed at the
appropriate temperature and with a standard 12-h light-dark cycle.
The FACS-purified SP- and non-SP H460 cells containing Matrigel
(1:1) at a concentration of 1×105 per 100 µl,
were administered to NOD/SCID mice by sub-cutaneous injection. The
density of the cells injected and growth of the mice were
monitored, according to the previously described protocol (20). The tumor volumes were measured
according to the following formula: V = 1/2 ab2 (where a
represents the long diameter and b represents the short diameter of
the tumor. After 4–5 weeks, the mice were sacrificed by
asphyxiation with CO2; tumors were harvested and
measured, and images were captured.
Sarcosphere formation assay
A sphere formation assay was performed, according to
a previously described procedure (5). The cells were plated at a density of
60,000 cells/well in ultra-low attachment six-well plates
containing serum-free DMEM/F12 medium, supplemented with N2,
epidermal growth factor (10 ng/ml) and human basic fibroblast
growth factor (10 ng/ml). The culture was analyzed for sphere
formation each day for 7 days and images were captured using
inverted phase contrast microscopy (Eclipse Ti-S; Nikon, Tokyo,
Japan). Spheres with a diameter of >150 µm were counted.
After 7 days of culture, the total number of sarcospheres that were
generated by the FACS-sorted SP and non-SP cells were
quantified.
Martigel invasion assay
The cellular invasiveness of the SP and non-SP cells
was determined using six-well Matrigel invasion chambers (BD
Biosciences, Franklin Lakes, CA, USA). The cells were seeded in
serum-free medium at a density of 2×105 per well. The
outer wells were filled with DMEM containing 5% FBS as a
chemoattractant, and incubated at 37°C for 48 h. Subsequently, the
non-invading cells were removed by swabbing the top layer of
Matrigel with a Q-tip (21). The
membrane containing the invaded cells was stained with hematoxylin
for 3 min, washed with PBS and mounted on slides. The entire
membrane containing the invading cells was visualized under a light
microscope at 40x objective and the number of cells counted, with
the values presented as the average value of three independent
experiments.
Statistical analysis
One-way analysis of variance and Student's t-test
were performed to determine significant differences between the
treatment and control groups. SPSS 11.5 software (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. Values are
expressed as the mean ± standard deviation. P<0.01 was
considered to indicate a statistically significant difference.
Results
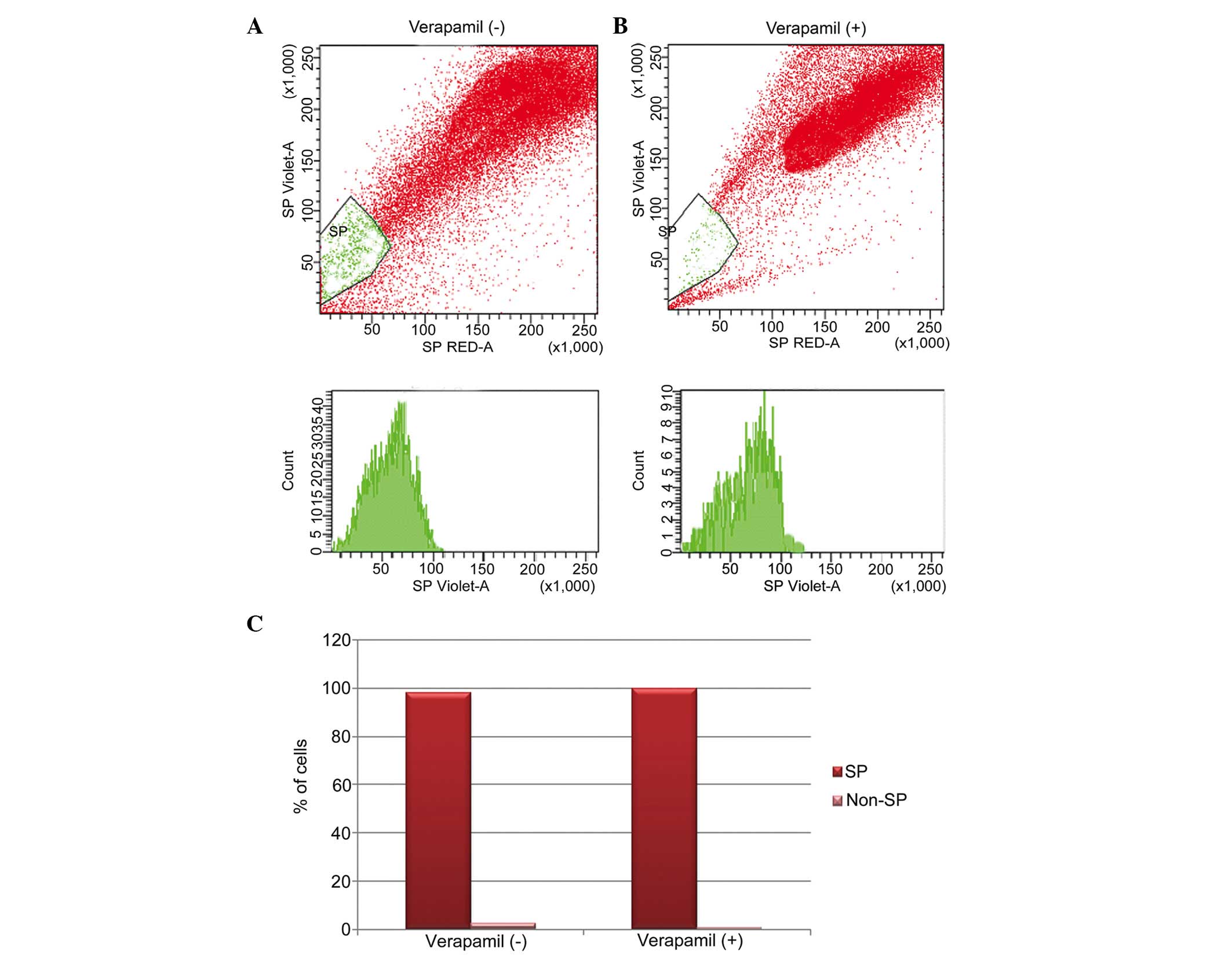
Identification of osteosarcoma SP cells
using Hoechst 33342 dye
The present study investigated the presence of
cancer stem-like SP cells in osteosarcoma samples. Using FACS, a
small population of SP cells of ~2.1% were found to exclude the
Hoechst 33342 dye (Fig. 1A; gated
region). The action of pumping out Hoechst 33342 dye is actively
performed by over-expression of the ABC transporter protein, ABCG2,
which was confirmed by treatment of cells with verapamil. Upon
verapamil treatment, the population of SP cells were significantly
reduced to 0.7% (Fig. 1B; gated
region). These data indicated that the SP cells were resistant to
drug uptake due to the overexpression of ABC transporter proteins
(Fig. 1C).
Phenotypic characterization of
osteosarcoma SP cells
In order to characterize the FACS-sorted SP cells,
the SP and non-SP cells were subsequently subjected to in
vitro cell proliferation and multi-drug resistance assays.
Notably, the osteosarcoma SP cells (Fig. 2A) underwent rapid cell
proliferation, beginning on the third day (D3), and became more
confluent on the eight day (data not shown). However, the growth
rates of the non-SP cells were significantly lower, compared with
the SP cells (Fig. 2A). Similarly,
the SP cells were resistant to uptake drugs, including etoposide,
gemcitabine, 5-flurouracil (5-FU), cisplatin, paclitaxel and
oxaliplatin. Upon treatment with these drugs, the survival rate of
the SP cells was significantly higher, compared with the non-SP
cells (Fig. 2B). The increased
drug resistance of the SP cells was most likely due to
overexpression of ABCG2 in the SP cells. As shown in the Fig. 2C, the SP cells were more positive
to ABCG2 than the non-SP cells. Therefore, these findings
demonstrated that the osteosarcoma SP cells underwent marked
proliferation and exhibited enhanced survival rates following
chemotherapy.
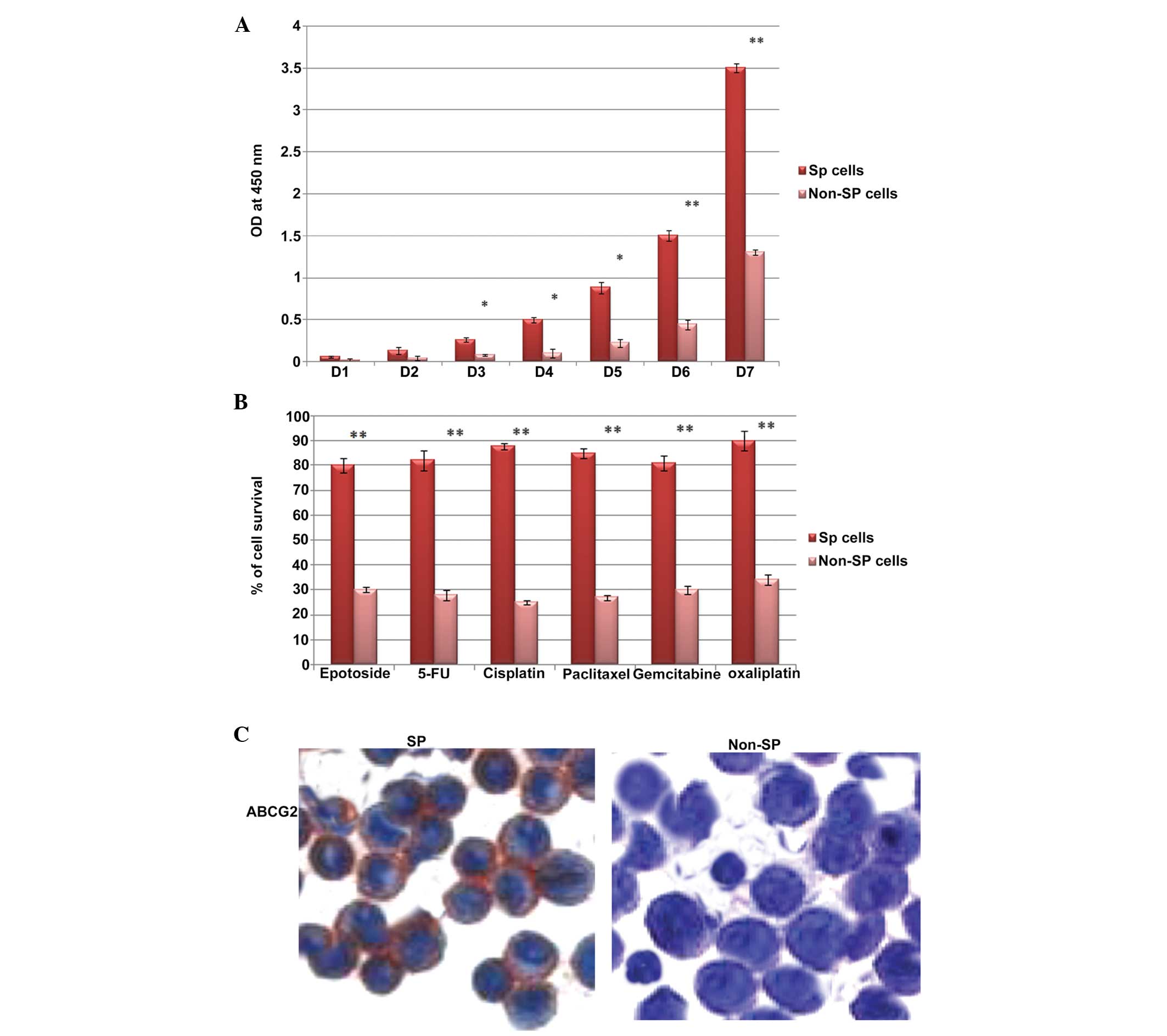 | Figure 2Phenotypic characterization of
FACS-sorted osteosarcoma SP cells. (A) In vitro cell
proliferation assay. The cell proliferation rates of the SP cells
were significantly higher than those of the non-SP cells. The
x-axis represents days (D) 1–7, the y-axis indicates the
corresponding OD value at 450 nm. (B) Comparison of cell survival
rates of the SP cells and non-SP cells following treatment with
etoposide, gemcitabine, 5-FU, cisplatin, paclitaxel and
oxaliplatin. The SP cells exhibited increased resistance and
increased survival rates (>80%), compared with the non-SP cells
(<35%). (C) Immunocytochemistry analysis of the sorted SP cells
(magnification, x100). SP cells exhibited enhanced expression of
ABCG2 (stained red), compared with the non-SP cells. Cells were
counterstained with hematoxylin. The data are expressed as the mean
± standard deviation. *P<0.05; **P<0.01
vs. non-SP cells. SP, side population; 5-FU, 5-fluorouracil; OD,
optical density. |
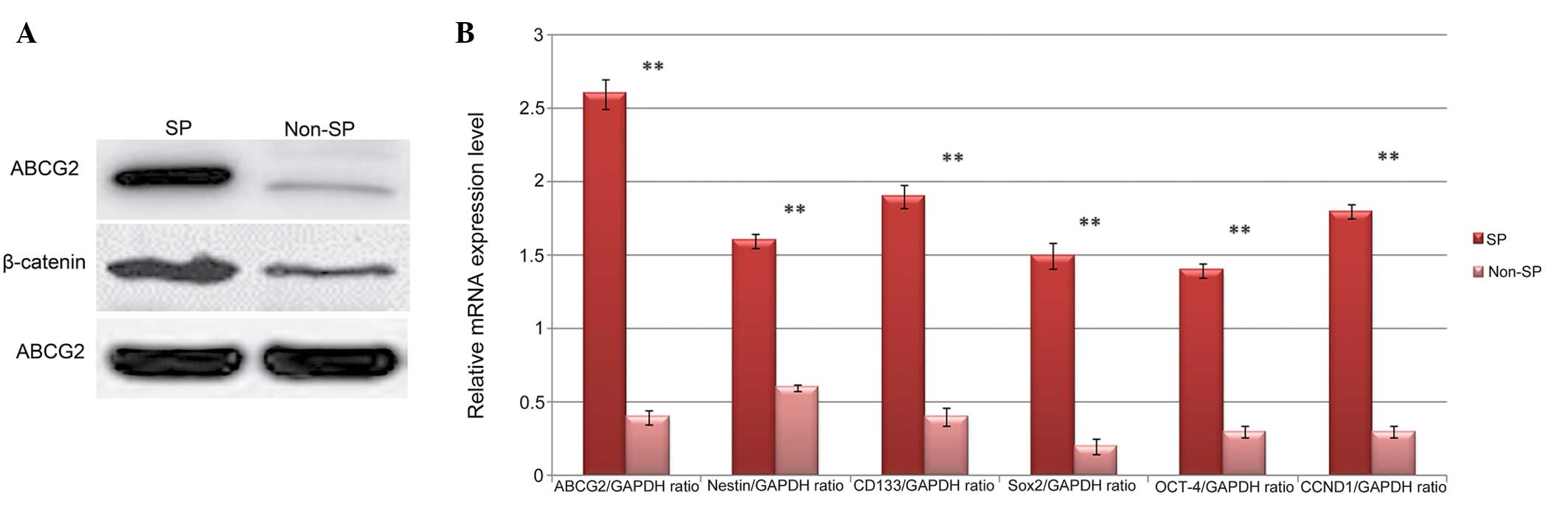
Elevated Wnt/β-catenin signaling and
upregulation of Oct-4 in SP cells
Previous studies investigating different types of
cancer have reported that hyperactivation of the Wnt/β-catenin
pathway results in elevated expression levels of stem cell surface
proteins and its downstream signaling pathways (22,23).
Therefore, the presents study evaluated the activation of
Wnt/β-catenin signaling and the expression of stemness genes in the
in the FACS-sorted SP cells. Western blot analysis revealed that
the protein level of β-catenin was higher in the SP cells, compared
with the non-SP cells (Fig. 3A).
Similarly, the expression of the ABCG2 ABC transporter protein was
significantly higher in the SP cells. In addition, the results of
the RT-qPCR analysis revealed that the relative mRNA expression
levels of the wnt target gene cyclin D1, ABCG2 and stem cell genes,
including CD133, nestin Oct-4, Sox-2 and Nanog were significantly
elevated in the SP cells, compared with the non-SP cells (Fig. 3B). Therefore, these data suggested
that elevated levels of Wnt/β-catenin signaling may be a trigger
for the increased expression levels of ABCG2 and stem cell surface
proteins, involved in multi-drug resistance and tumori-genic
properties of the SP cells.
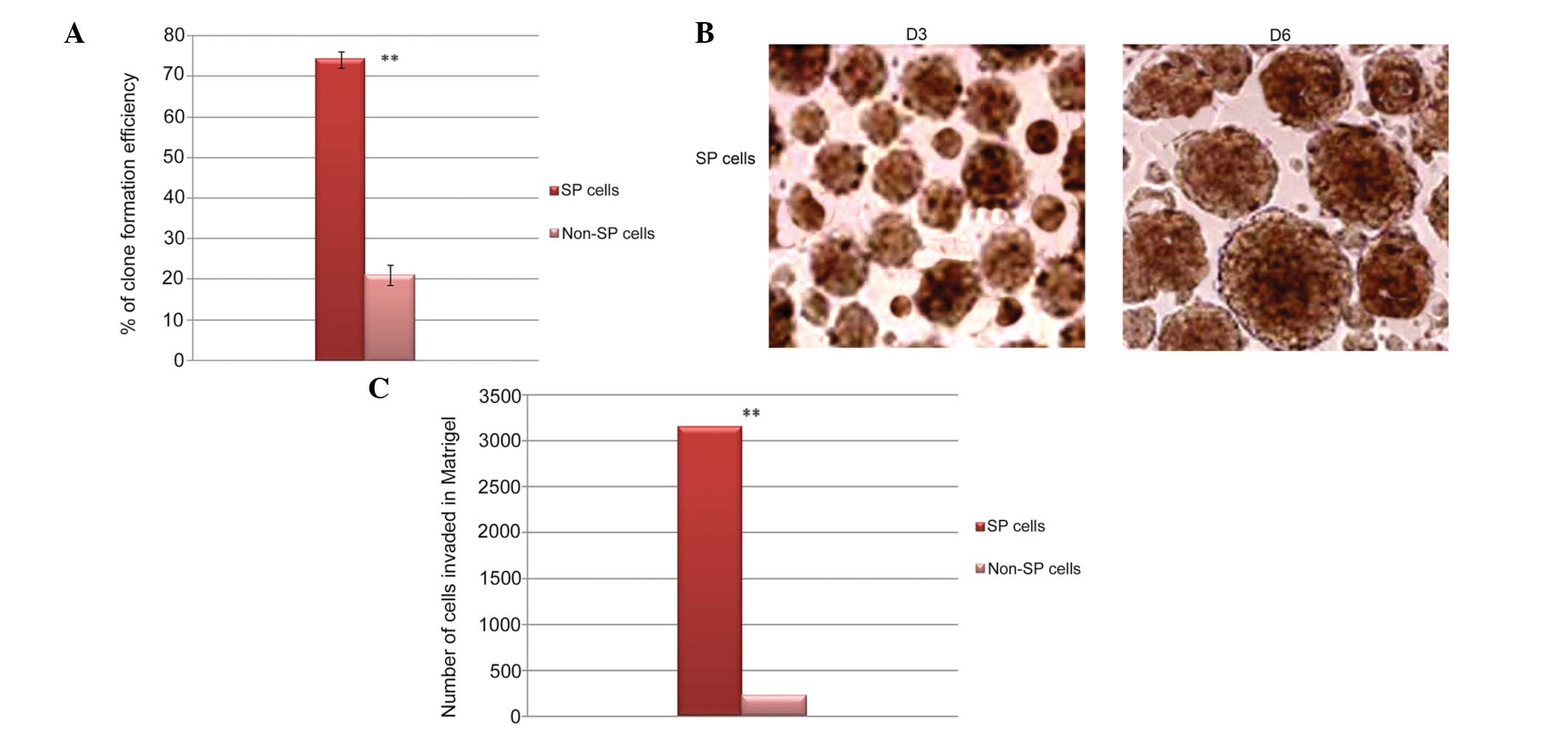
SP cells exhibit high levels of
self-renewal and invasion
In order to compare the clone formation efficiency
of the FACS-sorted SP and non-SP cells, sphere formation and
invasion assays were performed. The total number of sarcospheres
generated by the osteosarcoma SP cells was significantly higher,
compared with the non-SP cells (Fig.
4A). Similarly, the sarcospheres generated by the SP cells were
increased in size following continuous culture, and attained
maximal size on day 6 (Fig. 4B).
However, the non-SP cells did not attain a mature size. In
addition, the in vitro Matrigel invasion assay demonstrated
that FACS-sorted SP cells were significantly more invasive,
compared with the non-SP cells (Fig.
4C). Taken together, the SP cells were capable of initiating
tumor growth and causing tumor invasion.
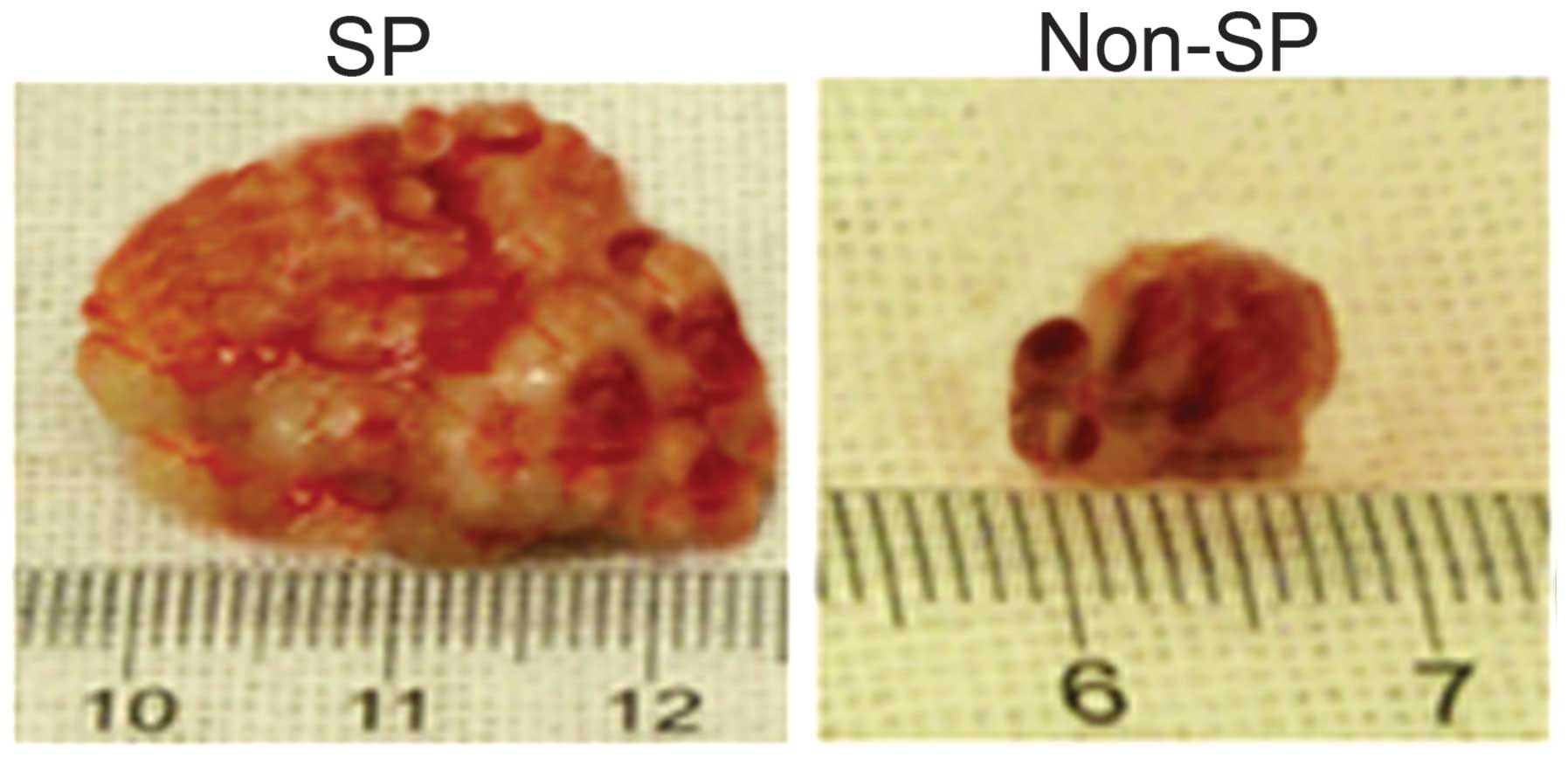
Tumorigenic potential of osteosarcoma SP
cells
The SP cells were able to regenerate tumors even
following transplantation at the lowest cell density (5,000 cells),
however, the non-SP cells were unable to repopulate the tumor cells
at this cell concentration (data not shown). As shown in Fig. 5, injection of the SP cells into the
NOD/SCID mice formed tumor spheres in vivo and these tumors
grew significantly faster than the tumors formed from non-SP cells.
Therefore, the osteosarcoma SP cells exhibited multi-drug
resistance, enhanced survival rate and increased
tumorigenicity.
Discussion
The CSC theory states that the presence of a small
sub-population of cancer cells, termed CSCs, are responsible for
treatment failure and minimal residual disease (MDR). These CSCs
evade current treatment regimens and are capable of initiating
tumor growth following chemoradiotherapy and are considered to be
'tumor initiating cells'. Therefore, it is important to isolate and
characterize CSCs to provide effective treatment in subjecting CSCs
to apoptosis. Based on Hoechst 33342 dye exclusion, CSCs have been
identified in a variety of solid types of tumor, including
glioblastomas, and breast, prostate and lung cancers (21,24–27).
In the present study, the presence of osteosarcoma-initiating cells
was examined using the Hoechst dye exclusion method. The results
revealed osteosarcoma SP cells of ~2.1%, which were analyzed for
stem cell properties and the Wnt/β-catenin signaling pathways. The
SP cells exhibited clonogenic capacities, high levels of
tumorigenicity and self-renewal characteristics. The SP cells
efficiently excluded the Hoechst 33342 dye from the cell due to
overexpression of the ABCG2 ABC transporter protein. Important
characteristic features of cancer stem-like SP cells are multi-drug
and apoptotic resistance, which are particularly tolerant to
cytotoxins. This multi-drug resistance property is predominantly
regulated by ABC transporters, which promote efficient efflux
chemotherapeutic drug and, therefore, are vital in tumorigenesis.
It is also possible that these SP cells also exhibit reduced
apoptosis, and these factors together produce CSCs with drug,
apoptosis resistance and enhanced survival rates (28). The immunofluorescence and RT-qPCR
analysis in the present study demonstrated that the osteosarcoma SP
cells had relatively higher expression levels of ABCG2, compared
with the non-SP cells, contributing to multi-drug resistance.
The primary difference between the SP and non-SP
cells was the ability to generate tumor spheres. The SP cells
exhibited marked self-renewal abilities and formed sarcospheres
rapidly, compared with the non-SP cells. Another notable difference
was that the SP cells are particularly tumorigenic and were able to
initiate tumor growth in vivo at the lowest cell
concentration, which failed to form tumor growth in the the non-SP
cells. The SP cell-derived tumors were identical to primary tumors.
Similar to these findings, it was previously demonstrated that SP
cells from osteosarcoma cell lines were more clonogenic and
exhibited higher self-renewal capacities, compared with non-SP
cells (8,29).
Another notable feature of SP cells are the elevated
expression levels of stem cell genes. Previously, it was reported
that increased expression levels of stemness genes, including
CD133, CD44, Oct-4 Nanog, in osteosarcoma SP cells contribute to
self-renewal and enhanced the proliferation rate of the SP cells
(8,29–31).
It has also been demonstrated that the Wnt/β-catenin pathway is one
of the most important signal transduction pathways involved in
tumorgenesis, tumor progression and maintenance of CSCs (32–34).
Consistent with these findings, the present study demonstrated that
increased expression levels of β-catenin and cyclin D in the SP
cells, compared with the non-SP. In addition, the SP cells
exhibited significantly higher expression levels of CD133, CD44,
nestin Oct-4, Sox-2 and Nanog, which are important for the
self-renewal properties of the SP cells. However, the precise
molecular mechanism underlying the Wnt/β-catenin mediated
overexpression of stemness genes remains to be fully
elucidated.
In conclusion, the present study demonstrated that
the osteosarcoma samples contained a small population of SP cells,
in which the wnt/β-catenin signaling was markedly elevated,
concomitant with increased stem cell surface proteins. Therefore,
the wnt/β-catenin signaling is crucial in the maintenance of
self-renewal and tumorigenic SP cells and may be a potential target
of novel anticancer drugs in order to eliminate chemoresistance and
tumorigenicity of SP cells.
References
|
1
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Damron TA, Ward WG and Stewart A:
Osteosarcoma, chondrosarcoma and Ewing's sarcoma: National cancer
data base report. Clin Orthop Relat Res. 459:40–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gollard RP and Turner JF: Multimodality
therapy for metastatic sarcomas confined to the lung. Oncol Lett.
4:583–587. 2012.PubMed/NCBI
|
|
4
|
Ando K, Heymann MF, Stresing V, Mori K,
Redini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar
|
|
5
|
Gibbs CP, Kukekov VG, Reith JD,
Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN and
Steindler DA: Stem-like cells in bone sarcomas: Implications for
tumorigenesis. Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berman SD, Calo E, Landman AS, et al:
Metastatic osteosarcoma induced by inactivation of Rb and p53 in
the osteoblast lineage. Proc Natl Acad Sci USA. 105:11851–11856.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tirino V, Desiderio V, d'Aquino R,
Francesco DF, Pirozzi G, Galderisi U, Cavaliere C, De Rosa A and
Papaccio G: Detection and characterization of CD133+cancer stem
cells in human solid tumours. PLoS ONE. 3:e34692008. View Article : Google Scholar
|
|
8
|
Wu C, Wei Q, Utomo V, Nadesan P, Whetstone
H, Kandel R, Wunder JS and Alman BA: Side population cells isolated
from mesenchymal neoplasms have tumor initiating potential. Cancer
Res. 67:8216–2822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pardal R, Clarke MF and Morrison SJ:
Applying the principles of stem-cell biology to cancer. Nat Rev
Cancer. 3:895–902. 2003. View
Article : Google Scholar
|
|
10
|
Scharenberg CW, Harkey MA and Torok-Storb
B: The ABCG2 transporter is an efficient Hoechst 33342 efflux pump
and is preferentially expressed by immature human hematopoietic
progenitors. Blood. 99:507–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oates JE, Grey BR, Addla SK, et al:
Hoechst 33342 side population identification is a conserved and
unified mechanism in urological cancers. Stem Cells Dev.
18:1515–1522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Challen GA and Little MH: A side order of
stem cells: the SP phenotype. Stem Cells. 24:3–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou S, Schuetz JD, Bunting KD, et al: The
ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem
cells and is a molecular determinant of the side-population
phenotype. Nat Med. 6566:1028–1034. 2001. View Article : Google Scholar
|
|
14
|
Kim CF, Jackson EL, Woolfenden AE,
Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT and Jacks T:
Identification of bronchioalveolar stem cells in normal lung and
lung cancer. Cell. 121:823–835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirschmann-Jax C, Foster AE, Wulf GG, et
al: A distinct 'side population' of cells with high drug efflux
capacity in human tumor cells. Proc Natl Acad Sci USA.
101:14228–33. 2004. View Article : Google Scholar
|
|
16
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpop-ulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar
|
|
17
|
Di Fiore R, Fanale D, Drago-Ferrante R, et
al: Genetic and molecular characterization of the human
osteosarcoma 3AB-OS cancer stem cell line: a possible model for
studying osteosarcoma origin and stemness. J Cell Physiol.
228:1189–1201. 2013. View Article : Google Scholar
|
|
18
|
Kuijjer ML, Namløs HM, Hauben EI, et al:
mRNA expression profiles of primary high-grade central osteosarcoma
are preserved in cell lines and xenografts. BMC Med Genomics.
4:662011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H, Liu W, Feng X, Wang L, et al:
Identification of ABCG2+ cells in nasopharyngeal carcinoma cells.
Oncol Rep. 27:1177–1187. 2012.PubMed/NCBI
|
|
20
|
Shi Y, Fu X, Hua Y, Han Y, Lu Y and Wang
J: The side population in human lung cancer cell line NCI-H460 is
enriched in stem-like cancer cells. PLoS One. 7:e333582012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monzani E, Facchetti F, Galmozzi E, et al:
Melanoma contains CD133 and ABCG2 positive cells with en- hanced
tumourigenic potential. Eur J Cancer. 43:935–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teng Y, Wang X, Wang Y and Ma D:
Wnt/beta-catenin signaling regulates cancer stem cells in lung
cancer A549 cells. Biochem Biophys Res Commun. 392:373–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valkenburg KC, Graveel CR, Zylstra-Diegel
CR, Zhong Z and Williams BO: Wnt/β-catenin signaling in normal and
cancer stem cells. Cancers (Basel). 3:2050–2079. 2011. View Article : Google Scholar
|
|
24
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5551. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumor initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2005.
View Article : Google Scholar
|
|
28
|
Alisi A, Cho WC, Locatelli F and Fruci D:
Multidrug resistance and cancer stem cells in neuroblastoma and
hepatoblastoma. Int J Mol Sci. 14:24706–24725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang M, Yan M, Zhang R, Li J and Luo Z:
Side population cells isolated from human osteosarcoma are enriched
with tumor-initiating cells. Cancer Sci. 102:1774–1781. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ailles LE and Weissman IL: Cancer stem
cells in solid tumors. Curr Opin Biotechnol. 18:460–466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al-Hajj M and Clarke MF: Self-renewal and
solid tumor stem cells. Oncogene. 23:7274–7282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawaguchi-Ihara N, Murohashi I, Nara N and
Tohda S: Promotion of the self-renewal capacity of human acute
leukemia cells by Wnt3A. Anticancer Res. 28:2701–2704.
2008.PubMed/NCBI
|
|
33
|
Khan NI, Bradstock KF and Bendall LJ:
Activation of Wnt/beta-catenin pathway mediates growth and survival
in B-cell progenitor acute lymphoblastic leukaemia. Br J Haematol.
138:338–348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ysebaert L, Chicanne G, Demur C, De Toni
F, et al: Expression of beta-catenin by acute myeloid leukemia
cells predicts enhanced clonogenic capacities and poor prognosis.
Leukemia. 20:1211–1216. 2006. View Article : Google Scholar : PubMed/NCBI
|