Introduction
Ischemic diseases remain a challenging problem of
clinical relevance, despite advances in relevant medical
intervention. A number of patients with cardiovascular or
peripheral ischemia are not suitable candidates for conventional
revascularization procedures. For those with no alternative,
therapeutic angiogenesis has been indicated to be a promising
treatment strategy. Several successful experimental and clinical
trials of pro-angiogenic therapy have been conducted, with genes
such as hypoxia-inducible factor (1), nerve growth factor (2) and placental growth factor (3). Since the natural process of
angiogenesis is complex and multi-factorial, single factor-based
therapy often fails to promote the formation of mature and stable
vasculature, and may even have detrimental effects. Sustained
expression of vascular endothelial growth factor (VEGF) has been
demonstrated to lead to extensive edema and destroy the normal
organ architecture (4). Therefore,
the combination of different pro-angiogenic factors, including VEGF
and fibroblast growth factor (FGF) (5), granulocyte-colony stimulating factor
and hepatocyte growth factor (6),
and VEGF and mono-cyte chemoattractant protein 1 (7) has also been investigated.
Basic FGF (FGF2) is a powerful mitogen in a variety
of cell types, including endothelial and smooth muscle cells
(8), and has been used as a
stimulator of angiogenesis and arteriogenesis (9). Platelet-derived growth factor (PDGF)
has the ability to recruit smooth muscle cells and participate in
arteriogenesis (10). Blood vessel
formation not only requires endothelial cells, but also pericytes
and smooth muscle cells (11). The
synergistic pro-angiogenic effect of FGF2 and PDGF-BB in the
revascularization process have previously been reported (12,13),
and when simultaneously administered into ischemic tissues, the two
factors can promote mature and stable vessel formation.
Cell therapy is another promising therapeutic
approach to ischemic tissue regeneration and repair (14,15).
The use of mesenchymal stem cells (MSCs) in angiogenic stem cell
transplantation has been widely exploited, due to characteristics
such as easy isolation and expansion. MSCs can undergo multipotent
differentiation in vivo and in vitro, can home to and
incorporate into sites of neovascularization, secrete angiogenic
factors and promote neovacularization through paracrine mechanisms
(16,17). Due to its unique feature of little
or low immunogenicity, MSCs can be administered without the
requirement of human leukocyte antigen (HLA) matching (18). MSCs, isolated from bone marrow and
adipose tissue and expanded in vitro, have already been used
for therapeutic angiogenesis (16,19).
However, the paucity of these cells is hampering their application.
Along with easy accessibility and abundant MSCs, the placenta has
proven to be an attractive cell source for cell therapy (20–22).
Additionally, MSCs isolated from the placenta have similar
biological characteristics to those from the bone marrow (23). Previous studies have indicated that
hPDMSCs can be used for the treatment of ischemic diseases
(24–27).
In the present study, hPDMSCs were isolated and
expanded in vitro, and transfected with adenoviral
bicistronic vectors carrying the FGF2 and PDGF-BB genes. It was
hypothesized that this strategy of a combination of gene therapy
and stem cell therapy may more effectively enhance
neovascularization compared with previously tested angiogenic
factors.
Materials and methods
Harvest, culture and isolation of
hPDMSCs
A term gestation placenta from a healthy donor
mother was obtained with informed consent from the West China
Second Hospital (Chengdu, China). Placental tissues were harvested
and washed several times with low-glucose Dulbecco's modified
Eagle's medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA)
under sterile conditions. It was then minced into a coarse slurry
with scissors in a Petri dish, followed by enzymatic digestion with
1 mg/ml collagenase (Sigma-Aldrich, St. Louis, MO, USA) for ~2 h at
37°C. The homogenate was centrifuged at 188 × g for 3 min. The
predispositions were suspended and cultured in the T75 flasks with
low-glucose DMEM supplemented with 20% fetal bovine serum (Gibco
Life Technologies). Cell cultures were maintained in a humidified
5% CO2 atmosphere at 37°C. Cells were identified by
their typical fibroblast-like morphology under an AxioVert 200
inverted phase microscope (Zeiss, Thornwood, NY, USA). Under daily
observation, the initial medium was changed ~6 days after plating,
and nonadherent cells were removed. Thereafter, media were changed
every 3 days. Cells were passaged at 80–90% confluence with 0.25%
trypsin (Gibco Life Technologies).
Immunophenotyping of hPDMSCs
To detect the immunophe-notype of hPDMSCs, flow
cytometric analyses were performed. Aliquots of cells were
incubated with the following antibodies: Monoclonal anti-human CD29
(cat. no. MA1-82635; Thermo Fisher Scientific, Inc., Waltham, MA,
USA); monoclonal anti-human CD90 (cat. no. MA1-24985; Thermo Fisher
Scientific, Inc.); monoclonal anti-human CD105 (cat. no. MS-1290-P;
Thermo Fisher Scientific, Inc.); monoclonal anti-human CD31 (cat.
no. ab9498-500; Abcam, Cambridge, MA, USA), followed by fluorescein
isothiocyanate- and phycoerythrin-conjugated secondary anti-mouse
IgG antibodies (cat. nos. 11-4011 and 12-4010; eBioscience, Inc.,
San Diego, CA, USA). Isotype-identical antibodies were used as
controls. Labeled cells were acquired by flow cytometry using a BD
FACSCalibur cell analyzer and Cellquest software 6.0 (BD
Biosciences, San Jose, CA, USA).
Adenoviral bicistronic vector
construction and transfection of hPDMSCs
hPDMSCs at passages 5–8 were used in the current
experiments. Adenoviral bicistronic vectors were constructed
containing human FGF2 and PDGF-BB genes, in which those two genes
were separated by a cis-acting region designated internal ribosome
entry site element. Therefore, the vectors could secrete both FGF2
and PDGF-BB. Ad-null vectors were constructed without the FGF-2 and
PDGF-BB genes. The cells were cultured at a density of
2×106 cells/75 cm2 tissue culture flask and
incubated with Ad-F-P or Ad-null at a multiplicity of infection of
1,500 with the addition of Lipofectamine® 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA) when cells were
at 70–80% confluence. Following transduction for 4 h, cells were
incubated for another 48 h prior to the in vitro experiment
and cell transplantation.
Hindlimb ischemia model and cell
delivery
All animal interventions were performed in
accordance with guidelines of the Sichuan University Institutional
Animal Care and Use Committee (Chengdu, China). Male New Zealand
White rabbits (4–5 months old and 2.5–3.0 kg in weight; Animal
Experimental Center of the West China Hospital, Sichuan, China)
were used to produce a hindlimb ischemia model. Briefly, the
rabbits were anesthetized with 3% pentobarbital sodium (30 mg/kg;
Animal Experimental Center of West China Hospital), the femoral
artery of the right hindlimb was exposed and freed from the
inguinal ligament to the point where it bifurcates into the
popliteal and saphenous arteries. All branches were ligated prior
to excision of the whole femoral artery. A total of 20,000 U/kg
penicillin (Shiyao Pharmaceutical Group Co., Ltd., Shijiazhuang,
China) were administered intramuscularly for 3 days subsequent to
surgery. Seven days after unilateral femoral artery excision, one
of the following was injected into the right ischemic adductor
muscles (n=5 in each group): 5×106 Ad-F-P-hPDMSCs,
5×106 Ad-null-hPDMSCs, 5×106 hPDMSCs or
low-glucose DMEM medium. After the initiation of cell therapy,
observations of edema, inflammation, and limb and toe necrosis in
the ischemic hindlimbs were performed daily.
Angiographic assessment
Four weeks subsequent to treatment, the animals were
subjected to angiography. Briefly, under anesthetization, a
catheter was inserted into the left femoral artery and advanced
into the aorta. Serial filming of the right hindlimb was performed
with a Philip-FD-201000 mA angiography machine (Philips, Amsterdam,
Netherlands). Quantitative angiographic analysis was performed with
previously described methods (28). Briefly, a composite of grids was
placed over the ischemic area. The number of grid intersections
that were crossed by opacified vessels was counted. The
angiographic score in each film was calculated as the ratio of grid
intersections crossed by opacified vessels divided by the total
number of grid intersections in this area.
Immunohistochemical studies
Following angiography, the animals were sacrificed
by air embolism, and the right adductor muscle tissues were
harvested immediately and fixed in 4% paraformaldehyde or preserved
at −80°C. The dissected adductor muscles were embedded in paraffin
and sectioned at 5 µm. Hematoxylin and eosin (HE) staining
was performed. To evaluate the density of capillaries and
arterioles following cell therapy, sections were stained with mouse
monoclonal anti-rabbit CD31 (1:100; cat. no. ab9498-500; Abcam) and
mouse monoclonal anti-rabbit α smooth muscle actin (αSMA) (1:100;
cat. no. ab18147-250; Abcam). Identification of the survival of
xenografted hPDMSCs was performed with mouse monoclonal primary
antibody anti-human surface of intact mitochondria protein (1:800;
cat. no. MAB1273; EMD Millipore, Billerica, MA, USA). Biotinylated
polyclonal goat anti-mouse IgG was used as the secondary antibody
(cat. no. PV-6002; ZSGB-BIO ORIGENE, Beijing, China), followed by
streptavidin-biotin horseradish peroxidase complex, and
colorimetric detection was performed with diaminobenzidine (Fuzhou
Maixin Biotechnology Development Co., Ltd., Fuzhou, China)
supplemented with 0.03% hydrogen peroxide. Slides were
counter-stained with hematoxylin solution, dehydrated and mounted.
The capillary and arteriole densities were evaluated under a LEICA
DM 2500 microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was conducted with SPSS 13.0
software (SPSS, Inc, Chicago, IL, USA). Comparisons between groups
were conducted by analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
hPDMSCs have the typical characteristics
of MSCs
Isolated cells were cultured on plastic in
low-glucose DMEM selected for MSCs outgrowth. Roughly 6 days
subsequent to seeding, adherent cells in small colonies with a
spindle-like phenotype were visualized. The cells presented a large
expansive potential following subculture. The spindle-like
morphology, as displayed in Fig.
1A, was maintained throughout the culture period.
Immunophenotype examination was performed to further identify the
hPDMSCs. hPDMSCs expressed CD29, CD90 and CD105, and were negative
for CD31 (Fig. 1B).
Ad-F-P-hPDMSCs promote collateral vessel
formation
To test whether genetically modified hPDMSCs were
able to enhance collateral vessel formation in a model of ischemia,
angiography was performed at 28 days subsequent to cell therapy.
Greater collateral vessel formation was observed in the hindlimbs
of Ad-F-P-hPDMSC-treated rabbits compared with those of other
groups (Fig. 2A). Quantitative
analysis of collateral vessels demonstrated that the angiographic
score in the Ad-F-P-hPDMSC group was significantly higher than that
of the control group (Fig. 2B).
hPDMSCs alone did not significantly induce collateral vessel
formation, although the average angiographic score was higher in
the hPDMSC group compared with that of the control group. These
results suggest that the delivery of genetically-modified hPDMSCs
with FGF2 and PDGF-BB has a greater ability to induce robust
arteriogenesis compared with hPDMSCs alone.
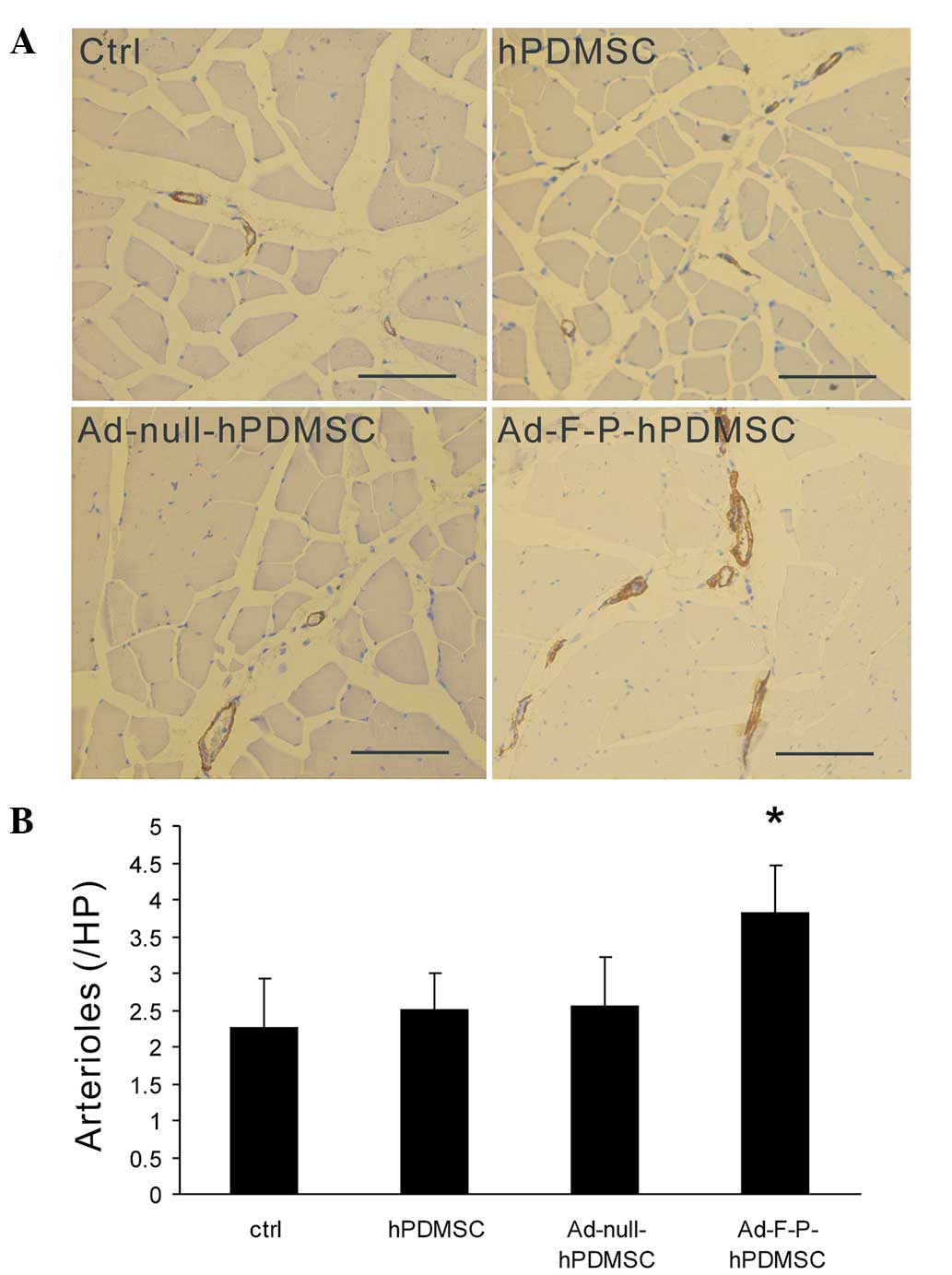
Ad-F-P-hPDMSCs increase mature artery
formation
FGF2 and PDGF have been demonstrated to establish
stable and mature vessels associated with recruitment of mural
cells (12). To further determine
whether Ad-F-P-hPDMSCs are able to promote mature arteriole
formation, muscle sections were stained with antibodies against
αSMA. The arteriole density was higher in the Ad-F-P-hPDMSC group
compared with that of other groups (Fig. 3A). An increase in αSMA-covered
vessels was observed in the Ad-F-P-hPDMSC group. However, there was
no significant difference between the Ad-null-hPDMSC, hPDMSC and
control groups in the arteriole density (Fig. 3B). These results suggest that
Ad-F-P-hPDMSCs promote arteriole maturation in ischemic areas.
Ad-F-P-hPDMSCs increase capillary
formation
To further evaluate the impact of genetically
modified hPDMSCs on the formation of capillaries in the ischemic
area, histological examination of ischemic adductor muscle was
performed. Capillary density in the Ad-F-P-hPDMSC group was higher
than in other groups (Fig. 4).
However, no significant difference was detected between the
Ad-null-hPDMSC, hPDMSC and control groups.
Xenografted hPDMSCs survive in ischemic
areas
H&E staining was performed to detect the
pathological changes in the ischemic muscles. No muscular atrophy
or hemangioma was detected in any of the groups. In the
Ad-F-P-hPDMSC group, numerous large cells aggregated in the
intermuscular area. To evaluate whether the cells were transplanted
ex vivo, sections were immunostained with a specific
antibody against human mitochondria. The results indicated that
several cells were positive for a human-specific marker (Fig. 5). These results suggest that the
xenotransplanted hPDMSCs existed for at least 4 weeks in the
ischemic muscle.
Discussion
In the present study, MSCs from human term placenta
(hPDMSCs) that had a spindle-like, plastic-adherent phenotype, were
isolated. Flow cytometry suggested that hPDMSCs expressed CD29,
CD90 and CD105, but not CD31. The hPDMSCs were genetically modified
with adenoviral bicistronic vectors that carried the FGF2 and
PDGF-BB genes simultaneously. Local delivery of the genetically
modified hPDMSCs into the adductor muscles of the ischemic hindlimb
significantly enhanced collateral formation and increased the
capillary and arteriole density. Therefore, a combination of FGF2
and PDGF-BB gene therapy with hPDMSC therapy is able to enhance
neovascularization, which requires angiogenesis and
arteriogenesis.
hPDMSCs were genetically modified to overexpress
FGF2 and PDGF-BB in the ischemic muscle tissue. A greater level of
capillary and arteriole formation was observed in the Ad-F-P-hPDMSC
group. FGF2 is a powerful stimulator of angiogenesis and
arteriogenesis. As the most pluripotent form of PDGF (28), PDGF-BB is an effective stimulator
of qsmooth muscle cell proliferation and migration, and
participates in the process of arteriogenesis. The synergistic
effects of FGF2 and PDGF-BB in the revascularization process have
previously been reported in different animal models of angiogenesis
(12,13). Reciprocal interplay between
receptor signaling systems contributes to their pro-angiogenic
synergism. FGF2 upregulates PDGFR expression in endothelial cells;
PDGF-BB induces the release of FGF2 and activation of FGFR-1, and
upregulates FGFR-1 expression in vascular smooth muscle cells
(12,29,30).
In addition, compared with other factors, the
combination of FGF2 and PDGF-BB achieves the strongest
pro-angiogenic effect (11). The
protein infusion and gene delivery of FGF2 and PDGF-BB have already
been used to enhance therapeutic neovascularization, and can
promote angiogenesis and arteriogenesis. However, compared with
previously used combinatorial factors, a bicistronic vector
carrying FGF2 and PDGF-BB genes together was constructed in the
present study, which simplified therapeutic procedures.
Stem cell therapy provides a promising alternative
and adjuvant therapy to current pro-angiogenic therapy (31). Currently, the mechanisms of
therapeutic angiogenesis with MSCs have been demonstrated to
involve two aspects as follows: MSCs secrete a wide spectrum of
angiogenic and arteriogenic cytokines, and enhance
neovascularization by a paracrine mechanism (16). Additionally, MSCs differentiate
into endo-thelial and smooth muscle cells in the ischemic tissues,
and enhance neovascularization directly (32). hPDMSCs have been reported to
secrete a large amount of bioactive VEGF (33). However, in the present study, no
significant improvement was observed in collateral vessel
formation, which may be attributable to the low power of hPDMSCs.
In the present study, genetically modified PDMSCs had greater
efficacy than pure hPDMSCs with regards to therapeutic
angiogenesis. To achieve a similar magnitude of therapeutic
neovascularization, cell therapy alone requires 30 times more cells
than ex vivo-transduced cells (34). It is possible that the
administration of a higher density of hPDMSCs is required to
achieve a similar effect as was created with Ad-F-P-hPDMSCs. The
combination of exogenous genes of angiogenic factors with the MSCs
may be able to achieve superior revascularization (35).
Since systemic intravenous delivery of MSCs can be
limited by the entrapment of donor cells in the lung (36,37),
a method involving local implantation of hPDMSCs was used, and a
single therapy was indicated to be sufficient to augment
neovasculariztion. A previous study indicated that premature
cessation of VEGF administration results in regression of acquired
vessels (4). Unlike VEGF, one dose
of FGF2 and PDGF-BB can maintain functional and stable vessels
(12).
MSCs have unique immunological characteristics and
persist in a xenogeneic or allogeneic environment (38). In the present study, the
xenotransplanted hPDMSCs survived in the ischemic muscle tissues
for at least 4 weeks, without the use of immunosuppressive drugs. A
previous study suggested that immunological tolerance of
xenografted amniotic membrane-derived MSCs may be due to HLA-G
expression, and the activation of regulatory T cells (39). A previous study confirmed that
hPDMSCs have low immunogeneity (25), therefore it is possible that this
low immunogeneity and the ability to tolerate the low oxygen
concentration contribute to the survival of hPDMSCs in ischemic
tissues. These properties suggest that hPDMSCs can act as a durable
source of FGF2 and PDGF-BB secretion following implantation into
ischemic tissue, which may produce relatively long-lasting
expression of potent factors. Ex vivo transduction may
preclude the exposure of adenovirus to the host immune system, then
MSC-based cell therapy may ameliorate the adverse effect caused by
adenovirus (40). In the present
experiment, Ad-F-P-hPDMSCs was able to secrete long-lasting
pro-angiogenic factors.
There are several limitations to the current study.
The fate of the MSCs was not determined in vivo, but MSCs
have previously been demonstrated to integrate into neovasculature
directly and differentiate into endothelial cells or smooth muscle
cells (32). Although it was
indicated in the present study that the xenografted cells can
survive in the ischemic areas, the molecular mechanisms were not
elucidated. Further investigation is required to clarify these
remaining questions.
In conclusion, the present study demonstrated the
following: (i) Human placenta represents a promising source of
MSCs, and hPDMSCs are an alternative vector for gene therapy; (ii)
local delivery of genetically modified hPDMSCs with the FGF2 and
PDGF-BB genes into ischemic muscle has the ability to enhance
neovascularization in a hindlimb ischemia model; and (iii)
xenografted hPDMSCs survive in ischemic tissue for a minimum of 4
weeks subsequent to the initiation of cell therapy. The present
study provides a novel therapeutical strategy for the treatment of
ischemic diseases.
Acknowledgments
The current study was supported by the National Key
Basic Research Program of China (2010 CB 529900) and the National
High Technology Research and Development Program of China
(2012AA020807).
Abbreviations:
|
FGF2
|
basic fibroblast growth factor
|
|
PDGF-BB
|
platelet-derived growth factor-BB
|
|
hPDMSCs
|
human placenta-derived mesenchymal
stem cells
|
|
Ad-F-P
|
adenoviral bicistronic vectors
carrying FGF2 and PDGF-BB genes
|
References
|
1
|
Vincent KA, Shyu KG, Luo Y, et al:
Angiogenesis is induced in a rabbit model of hindlimb ischemia by
naked DNA encoding a HIF-1alpha/VP16 hybrid transcription factor.
Circulation. 102:2255–2261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Emanueli C, Salis MB, Pinna A, Graiani G,
Manni L and Madeddu P: Nerve growth factor promotes angiogenesis
and arteriogenesis in ischemic hindlimbs. Circulation.
106:2257–2262. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luttun A, Tjwa M, Moons L, et al:
Revascularization of ischemic tissues by PIGF treatment, and
inhibition of tumor angiogenesis, arthritis and atherosclerosis by
anti-Flt1. Nat Med. 8:831–840. 2002.PubMed/NCBI
|
|
4
|
Dor Y, Djonov V, Abramovitch R, et al:
Conditional switching of VEGF provides new insights into adult
neovascularization and pro-angiogenic therapy. EMBO J.
21:1939–1947. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asahara T, Bauters C, Zheng LP, et al:
Synergistic effect of vascular endothelial growth factor and basic
fibroblast growth factor on angiogenesis in vivo. Circulation.
92(Suppl): II365–II371. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ieda Y, Fujita J, Ieda M, et al: G-CSF and
HGF: Combination of vasculogenesis and angiogenesis synergistically
improves recovery in murine hind limb ischemia. J Mol Cell Cardiol.
42:540–548. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jay SM, Shepherd BR, Andrejecsk JW,
Kyriakides TR, Pober JS and Saltzman WM: Dual delivery of VEGF and
MCP-1 to support endothelial cell transplantation for therapeutic
vascularization. Biomaterials. 31:3054–3062. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lindner V, Lappi DA, Baird A, Majack RA
and Reidy MA: Role of basic fibroblast growth factor in vascular
lesion formation. Circ Res. 68:106–113. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Royen N, Piek JJ, Buschmann I, Hoefer
I, Voskuil M and Schaper W: Stimulation of arteriogensis; a new
concept for the treatment of arterial occlusive disease. Cardiovasc
Res. 49:543–553. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raines EW: PDGF and cardiovascular
disease. Cytokine Growth Factor Rev. 15:237–254. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greenberg JI, Shields DJ, Barillas SG, et
al: A role for VEGF as a negative regulator of pericyte function
and vessel maturation. Nature. 456:809–813. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao R, Bråkenhielm E, Pawliuk R, et al:
Angiogenic synergism, vascular stability and improvement of
hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med.
9:604–613. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao X, Månsson-Broberg A, Gustafsson T, et
al: Angiogenic effects of dual gene transfer of bFGF and PDGF-BB
after myocardial infarction. Biochem Biophys Res Commun.
315:1058–1063. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho SW, Moon SH, Lee SH, et al:
Improvement of postnatal neovascularization by human embryonic stem
cell-derived endothelial-like cell transplantation in a mouse model
of hindlimb ischemia. Circulation. 116:2409–2419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Enis DR, Shepherd BR, Wang Y, et al:
Induction, differentiation, and remodeling of blood vessels after
transplantation of Bcl-2-transduced endothelial cells. Proc Natl
Acad Sci USA. 102:425–430. 2005. View Article : Google Scholar :
|
|
16
|
Kinnaird T, Stabile E, Burnett MS, et al:
Local delivery of marrow-derived sromal cells augments collateral
perfusion through paracrine mechanisms. Circulation. 109:1543–1549.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagaya N, Kangawa K, Itoh T, et al:
Transplantation of mesenchymal stem cells improves cardiac function
in a rat model of dilated cardiomyopathy. Circulation.
112:1128–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozawa K, Sato K, Oh I, et al: Cell and
gene therapy using mesenchymal stem cells (MSCs). J Autoimmun.
30:121–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao Y, Sun Z, Liao L, Meng Y, Han Q and
Zhao RC: Human adipose tissue-derived stem cells differentiate into
endothelial cells in vitro and improve postnatal neovascularization
in vivo. Biochem Biophys Res Commun. 332:370–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matikainen T and Laine J: Placenta - an
alternative source of stem cells. Toxicol Appl Pharmacol.
207(Suppl): 544–549. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Parolini O, Alviano F, Bagnara GP, et al:
Concise review: isolation and characterization of cells from human
term placenta: outcome of the first international Workshop on
Placenta Derived Stem Cells. Stem Cells. 26:300–311. 2008.
View Article : Google Scholar
|
|
22
|
Fukuchi Y, Nakajima H, Sugiyama D, Hirose
I, Kitamura T and Tsuji K: Human placenta-derived cells have
mesenchymal stem/progenitor cell potential. Stem Cells. 22:649–658.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miao Z, Jin J, Chen L, et al: Isolation of
mesenchymal stem cells from human placenta: comparison with human
bone marrow mesenchymal stem cells. Cell Biol Int. 30:681–687.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prather WR, Toren A, Meiron M, Ofir R,
Tschope C and Horwitz EM: The role of placental-derived adherent
stromal cell (PLX-PAD) in the treatment of critical limb ischemia.
Cytotherapy. 11:427–434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kranz A, Wagner DC, Kamprad M, et al:
Transplantation of placenta-derived mesenchymal stromal cells upon
experimental stroke in rats. Brain Res. 1315:128–136. 2010.
View Article : Google Scholar
|
|
26
|
Ventura C, Cantoni S, Bianchi F, et al:
Hyaluronan mixed esters of butyric and retinoic acid drive cardiac
and endothelial fate in term placenta human mesenchymal stem cells
and enhance cardiac repair in infarcted rat hearts. J Biol Chem.
282:14243–14252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishishita T, Ouchi K, Zhang X, et al: A
potential pro-angiogenic cell therapy with human placenta-derived
mesenchymal cells. Biochem Biophys Res Commun. 325:24–31. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shyu KG, Chang H and Isner JM: Synergistic
effect of angiopoietin-1 and vascular endothelial growth factor on
neoangiogenesis in hypercholesterolemic rabbit model with acute
hindlimb ischemia. Life Sci. 73:563–579. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao Y, Cao R and Hedlund EM: Regulation of
tumor angiogenesis and metastasis by FGF and PDGF signaling
pathways. J Mol Med (Berl). 86:785–789. 2008. View Article : Google Scholar
|
|
30
|
Millette E, Rauch BH, Defawe O, Kenagy RD,
Daum G and Clowes AW: Platelet-derived growth factor-BB-induced
human smooth muscle cell proliferation depends on basic FGF release
and FGFR-1 activation. Circ Res. 96:172–179. 2005. View Article : Google Scholar
|
|
31
|
Rafii S and Lyden D: Therapeutical stem
and progenitor cell transplantation for organ vascularization and
regeneration. Nat Med. 9:702–712. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang J, Xie Q, Pan G, Wang J and Wang M:
Mesenchymal stem cells participate in angiogenesis and improve
heart function in rat model of myocardial ischemia with
reperfusion. Eur J Cardiothorac Surg. 30:353–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishishita T, Ouchi K, Zhang X, et al: A
potential pro-angiogenic cell therapy with human placenta-derived
mesenchymal cells. Biochem Biophys Res Commun. 325:24–31. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iwaguro H, Yamaguchi J, Kalka C, et al:
Endothelial progenitor cell vascular growth factor gene transfer
for vascular regeneration. Circulation. 105:732–738. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Phillips MI and Tang YL: Genetic
modification of stem cells for transplantation. Adv Drug Deliv Rev.
60:160–172. 2008. View Article : Google Scholar
|
|
36
|
Barbash IM, Chouraqui P, Baron J, et al:
Systemic delivery of bone marrow-derived mesenchymal stem cells to
the infracted myocardium: feasibility, cell migration, and body
distribution. Circulation. 108:863–868. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vulliet PR, Greeley M, Halloran SM,
MacDonald KA and Kittleson MD: Intra-coronary arterial injection of
mesenchymal stromal cells and microinfarction in dogs. Lancet.
363:783–784. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liechty KW, MacKenzie TC, Shaaban AF, et
al: Human mesenchymal stem cells engraft and demonstrate
site-specific differentiation after in utero transplantation in
sheep. Nat Med. 6:1282–1286. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tsuji H, Miyoshi S, Ikegami Y, et al:
Xenografted human amniotic membrane-derived mesenchymal stem cells
are immunologically tolerated and transdifferentiated into
cardiomyocytes. Circ Res. 106:1613–1623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishii M, Numaguchi Y, Okumura K, et al:
Mesenchymal stem cell-based gene therapy with prostacyclin synthase
enhanced neovascularization in hindlimb ischemia. Atherosclerosis.
206:109–118. 2009. View Article : Google Scholar : PubMed/NCBI
|