Introduction
Globally, liver disease, which is frequently
associated with persistent hepatitis B virus (HBV) infection,
remains a signifi-cant health problem. More than 350 million people
are chronic carriers, despite the existence of effective
vaccinations (1). As a member of
the hepadnavirus family, HBV has a genome that is a
partially-double stranded circular DNA, of ~3.2 kb in length. The
viral genome contains four partially overlapping open reading
frames (ORFs), including ORF P, which codes for the polymerase
gene; ORF C, for the core gene; ORF S, for the surface or envelope
gene; and ORF X, for the regulatory X gene. The HBV C gene is
divided into two parts by two initiation cordons (ATG); the core
region and the pre-core (PC) region (2). The PC region is located upstream of
the HBV C gene. The mRNA transcription from the PC region is
translated into the HBeAg protein and released into the
bloodstream. A proportion of mRNAs transcribed from the core region
result in the production of pregenomic RNA, which is wrapped into
the core granules and reverse transcribed into viral DNA. The
remainder is translated into HBcAg following splicing. The
nucleotide near the C gene segment is an important component in HBV
replication, containing a variety of regulation sequences. The C
gene promoter (Cp) partially overlaps with the 3′-terminus of the X
gene (~nt1837) and the 5′-terminus of the PC region in the C gene
(nt1838~). Cp is the most important regulatory factor for HBV
transcription, and is composed of two parts: The core upstream
regulatory sequence (CURS, nt1643-1742) and the basal core promoter
(BCP, nt1742-1849). The double nucleotide A1762T and G1764A
exchange is the most common BCP mutation, and is also termed the TA
mutation (3). Clinically, patients
with chronic hepatitis B infection, who are serum HBeAg-negative
but HBV DNA-positive, often exhibit the TA mutation. A number of
reports have shown that the reduction in the PC region mRNA
transcription appears to result in a reduction in HBeAg expression,
while changes in the ability of the transcriptional regulatory
factor to bind with the promoter, and the composition of precore
and pregenomic RNA, may affect HBV DNA replication (4–6).
A large number of clinical studies have shown that
continuous HBV replication within the body is an important cause of
the development and progression of liver disease (7–9).
Therefore, antiviral therapy has become the mainstay of treatment
for HBV. Interferon α and nucleoside analogues remain the primary
treatment options for chronic hepatitis B. A number of clinical
studies have shown that, in patients treated with current
antivirals for one year, <30% had achieved HBeAg seroconversion,
and ~70–80% required long-term antiviral therapy (10). However, treatment with long-term
nucleoside analogues may result in the development of drug
resistance. The basal core promoter mutation (A1762T and G1764A;
TA) is one of the commonest HBV variants, and treatment of this
particular strain remains challenging (11,12).
Currently, among the five nucleoside analogues approved for chronic
hepatitis B treatment, entecavir (ETV) and tenofovir disoproxil
fumarate (TDF) exhibit potent efficacy in the inhibition of viral
replication and low incidence of resistance, and are thus
recommended as first-line agents for the initial treatment of
hepatitis B in Europe and the United States. However, the
sensitivity of the TA mutation for ETV and TDF remains unclear. To
the best of our knowledge there have been no in vivo studies
demonstrating their effectiveness in this strain.
In the life cycle of the hepatitis B virus, the
3.5-, 2.4-, 2.1- and 0.7-kb mRNAs are the central transcription
products of the viral genome. The pregenomic 3.5-kb mRNA is reverse
transcribed to encapsidated viral genomic DNA by the viral
polymerase (13). Viral
transcription is regulated by numerous host factors. Hepatocyte
nuclear factor 4 (HNF4), which contains a zinc finger region and is
expressed primarily in the liver, is one of the principal host
transcription factors that binds DNA as a dimer and regulates a
number of liver-specific genes. HNF4α is a member of the HNF4
subfamily (14). A gene chip-based
study demonstrated that HBV infection increased the transcription
of numerous genes, including HNF4 (15). In a recent study by this group, the
detection of liver tissue samples from HBV-infected patients
indicated that high-expression of HNF4α may be associated with the
occurrence of severe hepatitis B (SHB) (16). Furthermore, clinical data have
shown that the TA mutation is related to an increased risk of SHB
in patients with chronic HBV infection (17). Therefore, it was hypothesized that
there may be an association between the TA mutation and HNF4α
expression. In studies using HepG2 and Huh7 hepatoma cell lines,
HNF4α has been shown to be involved in HBV 3.5 kb pregenomic RNA
synthesis and viral replication (18). There is evidence that the TA
mutation is located in the proximal nuclear hormone receptor
binding site of the core promoter region (3). This suggests that the mutation may
exert a degree of influence on binding between HNF4 and the core
promoter region. However, the majority of studies on the TA
mutation and HNF4α were conducted in vitro, and the
association in vivo remains unclear. In the present study,
the influence of HNF4α on TA mutant and wild-type HBV transcription
and replication was investigated via liver-specific silencing of
HNF4α expression in vivo.
In the current study, a Balb/c mouse model for the
replication of the HBV TA mutant was established via a
hydrodynamic-based procedure. Using the model and corresponding
experimental techniques, the differences in transcription and
replication levels between the wild-type and the TA mutant strains
were examined. Furthermore, the differences in the effects of ETV,
TDF and HNF4 on HBV replication were investigated.
Materials and methods
Ethics statement
Ethical approval was obtained from the Laboratory
Animal ethics committee of Sichuan University (Chengdu, China).
Plasmid
The HBV DNA 4.1-kb plasmid (pHBV4.1 wt) construct is
an HBV transcription, replication and expression competent plasmid,
which contains 1.3 copies of the HBV genome (subtype ayw) (19,20).
The TA mutation, with two nucleotide substitutions (A1762T and
G1764A) in the proximal nuclear hormone receptor binding site of
the core promoter region, was introduced into the wild-type pHBV4.1
wt via site-directed mutagenesis. The resulting mutant plasmid was
named pHBV4.1TAmut. In a previous study by this group, a
liver-specific RNA interference (RNAi) plasmid targeting HNF4α
(named pHNF4sh-EP) was successfully constructed and was inserted by
directional cloning liver-specific regulatory elements AFPe-ALBp
(referred as EP) into the vector plasmid HNF4sh-CMV. pHNF4sh-EP is
able to efficiently and liver-specifically silence HNF4α expression
(21).
Mouse model for HBV replication
Male BALB/C mice, which were purchased from the
Huaxi Laboratory Animal Center of Sichuan University, were 18–20 g
and 6–8 weeks old, and were maintained under specific-pathogen-free
(SPF) conditions. All mice received canonical care under the
Institutional Review Board, according to Animal Protection Art of
Sichuan University, including keeping mice in a 12 h light-dark
cycle, and at a constant temperature and humidity.
In order to establish the HBV replication mouse
model, 10 µg (pHBV4.1 wt or pHBV4.1TAmut) plasmids,
dissolved in phosphate-buffered saline (PBS), were injected into
the mouse tail vein over 5–8 sec (hydrodynamic in vivo
transfection) (21). The volume of
PBS injected was 10% of the mouse body weight. Mice were sacrificed
by cervical dislocation at 72 h post-injection, and liver tissues
were stored at −70°C, prior to DNA and RNA extraction and analysis.
Blood samples were allowed to stand overnight at 4°C, and the serum
was then separated and stored at −20°C
Inhibition of HNF4α expression via
RNAi
The expression of HNF4 in the liver tissue was
specifically inhibited using an HNF4sh-EP plasmid. The pHNF4sh-EP
(50 µg) dissolved in 2 ml PBS was rapidly injected into
mouse tail veins. After 4 days, pHBV4.1wt and pHBV4.1TAmut were
injected in the same manner. Mice were sacrificed 3 days later by
cervical dislocation. Samples of liver tissues were frozen at −70°C
and serum was saved at −20°C.
Detection of antiviral effects
24 h after the injection of wild-type (pHBV4.1 wt)
and TA mutant-type (pHBV4.1TAmut) plasmid, ETV (Bristol-Myers
Squibb, New York City, NY, USA; 0.075 mg/kg/day), TDF (Gilead
Sciences, Foster City, CA, USA; 45 mg/kg/day) or normal saline
(control group) were administrated via oral gavage three times at
24 h intervals. The drug dose was determined, based on the
recommended human dosage. Mice were sacrificed 4–6 h after the
final oral gavage. Liver tissues were conserved as described
previously.
Detection of HBV RNA by northern
blotting
Frozen mouse liver tissues were mechanically
pulverized in liquid nitrogen. Liver tissue powder (0.03–0.05 g)
was dissolved in 1 ml TRIzol™ reagent (Beijing Solarbio Science and
Technology Co., Ltd., Beijing, China). HBV RNA was isolated as
described previously (22). After
blending, Turbid liquid was extracted twice with chloroform
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). The
supernatant was precipitated with 0.5 ml of isopropanol (Sinopharm
Chemical Reagent Co., Ltd.), and resuspended in 100 µl of
diethylpyrocarbonate water. Using probes for GAPDH and HBV, 30
µg HBV RNA was analyzed by northern blotting hybridization,
with the GAPDH serving as an internal control. The levels of HBV
RNA were calculated using Quantity One software, according to the
manufacturer's instructions (Quantity One 1-D version 4.6.2;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Detection of HBV DNA replication
intermediates by southern blotting
Frozen liver tissues were ground to powder in liquid
nitrogen, and HBV DNA replication intermediates were isolated from
0.12 g liver tissue powder, as described previously (21). Viral DNA replication intermediates,
resuspended in 30 ul of 10 mmol/l Tris hydrochloride (pH 8.0) and 1
mmol/l EDTA, were analyzed by southern blotting. Membranes were
hybridized with digoxigenin-labeled (Roche Applied Science) HBV ayw
genomic DNA (Sigma-Aldrich, St. Louis, MO, USA) in order to detect
HBV sequences. HBV sequences were detected by analysis of
hybridization of the membranes with digoxigenin-labeled (Roche
Applied Science) HBV ayw genomic DNA. The levels of HBV DNA
replication intermediates were calculated using Quantity One
software, according to the manufacturer's instructions.
Analysis of hepatitis B e antigen (HBeAg)
by enzyme-linked immunosorbent assay (ELISA)
Three days after the HBV replication mouse model was
established, >50 µl serum was collected from each mouse.
The levels of HBeAg in mouse sera were measured using HBeAg
detection kits, according to the manufacturer's instructions
(Shanghai Shiye Kehua Company, Shanghai, China). Absorbance was
measured with dual-wavelength measurement (450/645 nm) on a
microtiter plate reader. Results were considered positive if the
OD450 was above the cutoff value (2.1 × negative control
value).
Analysis of HBsAg and HBcAg by
immunohistochemistry
The liver tissue in the embedding cassette was fixed
in 10% formaldehyde for 24 h in order to confirm that the model had
been established successfully. The samples were
immunohistochemically stained (23). Paraffin sections were
deparaffinized, rehydrated and blocked. Sections were incubated
with specific antibodies against HBsAg (mouse anti-HBs, monoclonal,
1:100 dilution; cat. no. MA5-13059; Thermo Fisher Scientific,
Rockford, IL, USA) and HBcAg (rabbit anti-HBc, monoclonal, 1:150
dilution; cat. no. RB-1413-A; Neomarkers, Inc., Portsmouth, NH,
USA). The secondary antibody consisting of polymer-horseradish
peroxidase anti-mouse (cat. no. sc65890) or anti-rabbit (cat. no.
sc896; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China) was applied at 1:350 dilution for 50 min at 37°C,
according to the manufacturer's instructions. Following
counter-staining and finalizing, the liver cells were observed
under a microscope (Olympus BX51; Olympus, Tokyo, Japan).
Statistical analysis
Quantity One 1-D analysis software version 4.6.2 was
used for calculation and analysis of HBV DNA replication
intermediate levels and HBV RNA levels. The data of HBV DNA
replication intermediate levels and HBV RNA levels are expressed as
the mean ± standard deviation from three independent experiments
using the SPSS software package version 13.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Biological characteristics of the HBV TA
mutant strain in vivo
In order to investigate the transcription and
replication characteristics of the HBV TA mutant strain in
vivo, HBV RNA and DNA replication intermediate levels were
evaluated by northern blotting and southern blotting, respectively
(Fig. 1). As shown in Fig. 1A and C, the expression level of the
3.5 kb HBV mRNA (transcription level) in mouse liver tissue
injected with the pHBV4.1TAmut was higher than that of mice
injected with pHBV4.1 wt. The expression level of HBV DNA
replication intermediates (replication level) of the TA mutant
virus was 1.35-fold higher than that of the wild-type virus
(Fig. 1B and D).
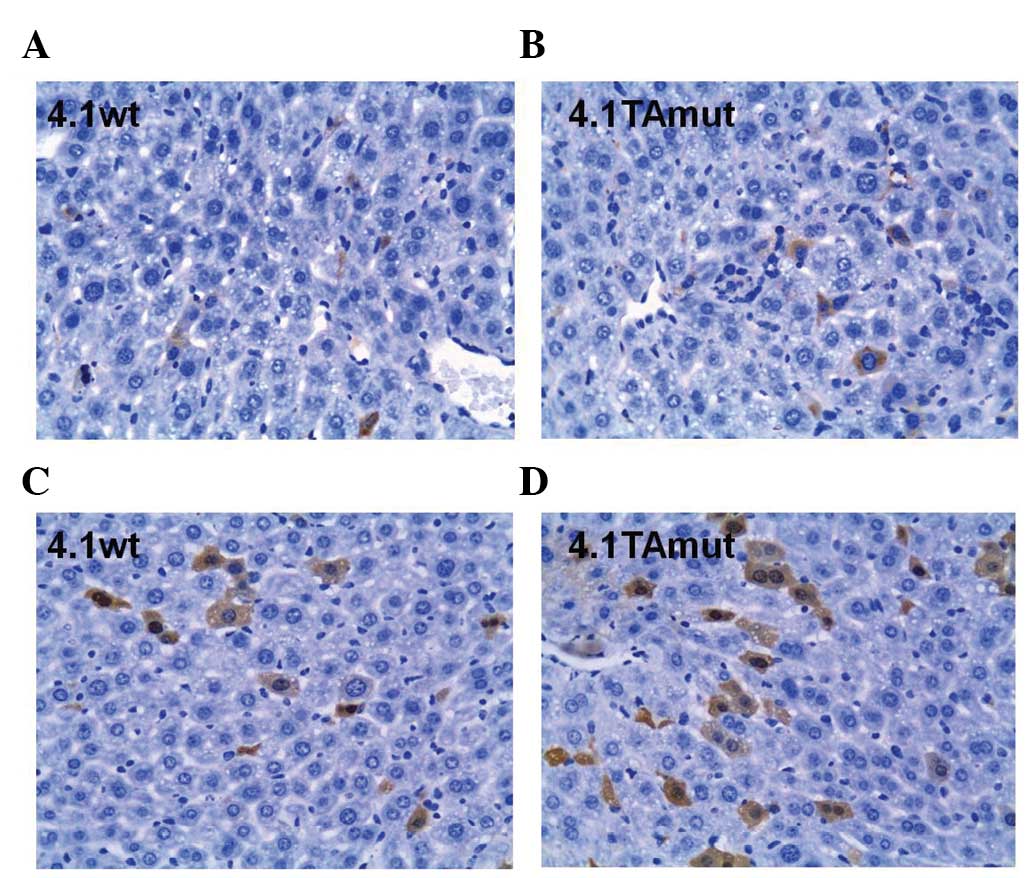
As shown in Fig. 2,
HBsAg and HBcAg in hepatocytes were stained brown. HBsAg was only
detected in the cytoplasm of hepatic cells, whereas HBcAg was
detected in the nucleus and the cytoplasm. The number of
HBsAg-positive cells in mouse liver tissue injected with
pHBV4.1TAmut was greater than that injected with pHBV4.1 wt
(Fig. 2A and B). Similarly the
number of cells that stained positive for HBcAg in the cytoplasm
and nuclei was greater in cells treated with pHBV4.1TAmut than
cells treated with pHBV4.1 wt (Fig. 2C
and D). These results suggest that the expression of HBsAg and
HBcAg from the TA mutant virus was increased compared with that of
the wild-type HBV.
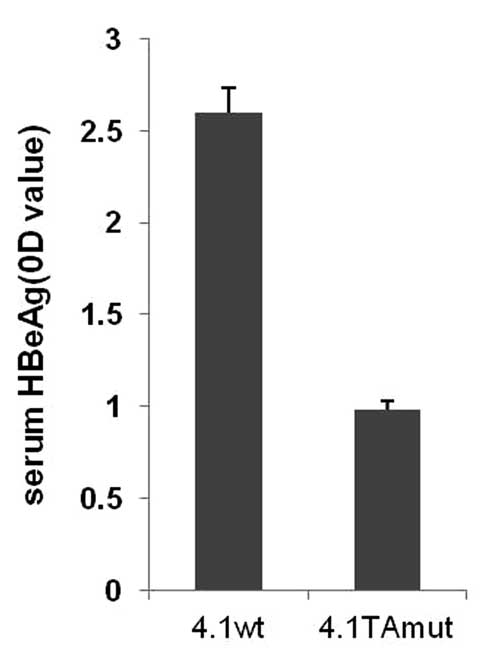
Following injection of the different plasmids, the
level of HBeAg in mouse serum was detected (Fig. 3). An ELISA assay demonstrated that
the level of serum HBeAg in wild-type mice was higher (OD=2.6)
compared with that in mice injected with the TA-mutant plasmid
(OD=0.98). The results demonstrated that the TA mutation may have
an impact on HBeAg expression, which is consistent with our
clinical observation.
Inhibitory effects of antiviral drugs on
variant HBV
In order to analyze the effect of antiviral drugs,
two nucleoside analogues, ETV and TDF, were used to treat mice
infected with wild type or variant virus. The level of
transcription and replication was measured (Figs. 4 and 5). In the ETV-treated group,
transcription and replication levels of the wild-type strain were
reduced by 24 and 61%, respectively, compared with the control
group, and in the TA mutant strain, the transcription and
replication levels decreased by 26 and 62%, respectively (Fig. 4). In the TDF-treated group, the
transcription and replication levels of the wild-type strain were
reduced by 40% and 86%, respectively, compared with the control
group, and the transcription and replication levels in the TA
mutant strain decreased by 42% and 85%, respectively (Fig. 5). The results indicate that the HBV
TA mutant strain remains sensitive to ETV and TDF. Furthermore,
both the strains were more sensitive to TDF than they were to
ETV.
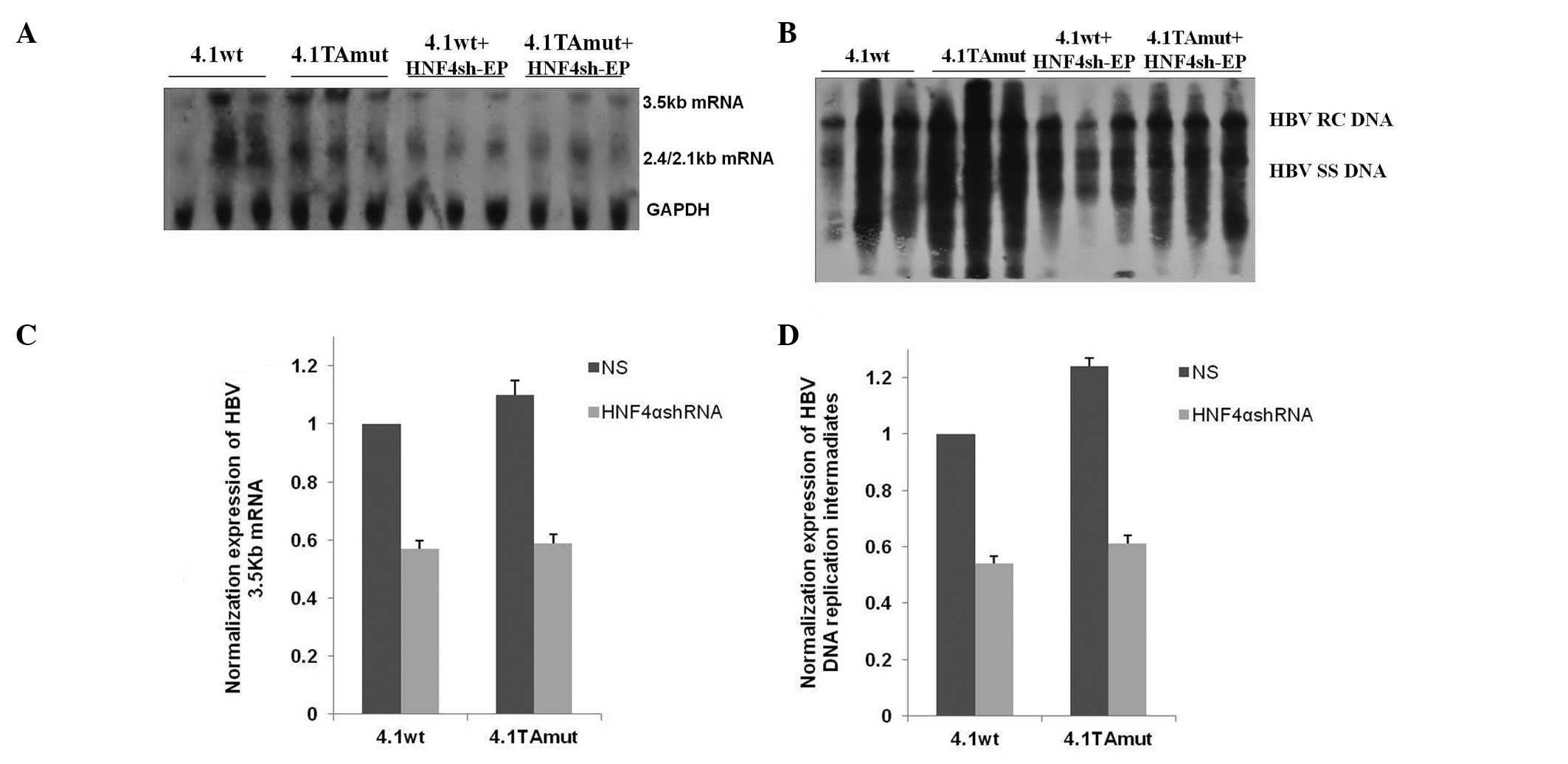
Effects on virus transcription and
replication following inhibition of HNF4α expression in vivo
Following transfection with pHNF4sh-EP, which was
able to specifically downregulate the expression of HNF4 in the
liver, the transcription and replication characteristics of the
wild-type and TA mutant-type HBV variants were investigated. The
relative levels of virus transcription and replication are
presented in Fig. 6. When HNF4α
expression was reduced in mouse liver, transcription levels of
wild-type and TA mutant virus were decreased by 43 and 47%,
respectively (Fig. 6A and C).
Similarly, the expression of HBV DNA replication intermediates
synthesized from wild-type or TA mutant virus was decreased by 46
and 51%, respectively (Fig. 6B and
D). These results demonstrated that HNF4 exerted a more
significant impact on the transcription and replication of the TA
mutant HBV.
Discussion
HBV infection remains a significant global public
health issue. Following infection with HBV, hepatitis, liver
fibrosis, cirrhosis and liver cancer may occur as the disease
progresses (24,25). Each year ~1 million people die from
HBV-related diseases (26).
Therefore, the treatment of hepatitis B, and in particular the
development of antiviral agents, is of worldwide concern. However,
when an anitviral drug is administered for an extended period of
time, there is a greater chance of the development of
drug-resistant strains of the virus, due to viral mutations. The TA
mutation, in which there is an A→T substitution at nt 1762 and a
G→A mutation at nt 1764 in the BCP region of HBV, is a common
mutation that has been found to confer resistance in clinical
trials. The 'immune escape' caused by the reduction in precore
protein RNA and HBeAg synthesis, is an important pathogenic
mechanism for the pre-C mutant strains. The TA mutant strain has
been reported to be associated with the levels of HBV DNA
replication and HBeAg expression (27,28).
The majority of studies on TA mutation have been conducted in cell
cultures or in clinical studies. However, few studies have
investigated the characteristics of this HBV strain in vivo.
Compared with in vitro experiments, studies using
appropriate animal models may simulate human diseases more closely.
Transgenic mice have been widely used in studies on HBV. The HBV
replication mouse model established in this laboratory, restores
virus transcription and replication process more authentically
(29), and saves time and money,
particularly in the study of HBV mutant strains. Using the HBV
replication mouse model, our group has investigated the biological
characteristics of the TA mutant strain, in addition to the effect
of HNF4 on the mutant HBV.
The present results indicated that the replication
level of HBV DNA with the TA mutation was increased in mouse liver
samples, while the expression level of HBeAg in serum was
decreased. A previous in vitro study demonstrated that a
higher proportion of pregenomic RNAs from the TA mutant HBV genome
were encapsidated, compared with that of the wild-type strain, thus
producing higher levels of replication intermediates of variant
virus (18). This is in accordance
with the results from the present in vitro study. The TA
mutation affects the AT-rich regions in the core promoter, which
are located upstream of the mRNA start points in viral promoters.
These regions are hypothesized to initiate transcription by binding
with RNA polymerase (30). The
synthesized level of mRNA may influence the replication level of
viral DNA. The replication level of the mutant strain may increase
following subtle alteration in the levels of pregenomic mRNA
transcription and encapsidation. In the present study, the
synthesis of HBeAg in vivo was suppressed, although not
fully abolished, in the presence of the TA mutation. HBeAg was
synthesized by pre-core mRNA and secreted into the serum. The TA
mutation inhibited the synthesis of PC mRNA, thereby reducing the
expression of HBeAg (4,31). Therefore, the TA mutation appears
to influence the serum HBeAg level of patients infected with this
strain of HBV (32). As an immune
tolerant antigen, HBeAg is involved in the virus persistence in
infected individuals (33). For
viral variants expressing little or no HBeAg, there is a weaker
selection force from the anti-HBe immune response of hosts
(6). Certain researchers have
postulated that HBeAg in the serum inhibits HBV replication, and
that viral replication level may increase in conjunction with a
decrease in HBeAg levels (30).
The TA mutant strain may be more dominant than the wild-type strain
in vivo.
Nucleoside analogues, which inhibit HBV replication,
are important in antiviral therapy. In recent years, due to
advances in the antiviral treatment of chronic hepatitis B (CHB),
international associations on liver diseases have updated their
guidelines on the management of CHB, to emphasize that the goal of
CHB antiviral treatment is to induce maximal long-term suppression
of viral replication, thereby delaying the progression and
occurrence of cirrhosis, hepatocellular carcinoma and liver
failure. However, following a period of continuous treatment,
virological breakthrough may occur, primarily as a result of the
development of drug resistance. Drug resistance of HBV is
predominantly due to mutations in the P region of the HBV genome.
However, the sensitivity to nucleoside analogues merited further
research in strains with mutation in other regions. In the
multidrug-resistant variant, rtA181 V/T, for example, the drug
sensitivity to lamivudine (LAM), adefovir dipivoxil (ADV), and TDF
was downregulated by a factor of 10, 2–8 and 2–3, respectively,
although the sensitivity of this strain to ETV was undiminished
(34). LAM therapy resulted in the
rapid development of TA mutants in HBeAg-positive patients during
one clinical trial (35). An in
vitro study demonstrated that the TA mutant strain remained
resistant to LAM, while it was sensitive to ADV (2). ETV is a cyclopentanoyloxy guanosine
analogue and TDF is a single adenosine analogue. Clinical trials
have shown that ETV and TDF are safe and effective for long-term
use in patients with CHB (36).
The 2012 guidelines published by the Asian Pacific Association for
the Study of the Liver, recommended ETV and TDF as the nucleoside
analogues of choice. However for viruses with the TA mutation, the
antiviral effect of ETV and TDF is unclear. In the present study,
ETV and TDF were administrated via oral gavage three times. The
results showed that they were similarly effective in wild-type
compared with TA mutant mice models, while TDF showed greater
antiviral efficacy in each strain.
Liver enriched transcription factor is a class of
protein molecule with gene transcription regulation function. A
number of forms of liver enriched transcription factors, such as
hepatocyte nuclear factor 4 (HNF4), HNF1, retinoid X receptor α
(RXRα) and peroxisome proliferator-activated receptor α (PPARα)
heterodimers, are able to bind with four promoters of the HBV
genome, and are involved in regulating HBV gene transcription and
viral replication (37). A
previous study showed that HNF4 and RXRα-PPARα heterodimers
activate the transcription of the HBV core promoter (18). It may be that these nuclear hormone
receptors, which are primarily expressed in hepatocytes, may
restrict viral mRNA transcription and DNA synthesis. Synthesis of
pregenomic 3.5-kb mRNA begins from the nucleocapsid promoter of C
region. The nuclear hormone receptor binding site is located in the
nucleocapsid promoter and encompasses the location of the TA
mutation, with the two nucleotide substitutions of A→T at nt 1762
and G→A at nt 1764 (18). Previous
analysis has indicated that the binding properties of transcription
factors are altered in the nuclear hormone receptor binding site of
the variant virus. RXRα-PPARα and HNF4 may bind to the nuclear
hormone receptor binding sites in the wild-type nucleocapsid
promoter and activate transcription. By contrast, the variant
nuclear hormone receptor sites combined with HNF4 but not
RXRα-PPARα (27). In the current
study, pHNF4sh-EP was transfected into the mice via
hydrodynamic-based injection, in order to knock down HNF4. As the
TA mutation blocked the binding of the RXRα-PPARα heterodimers with
the promoter, the variant viral gene expression was regulated only
by HNF4. Therefore, silencing of HNF4 resulted in a significant
reduction in transcription and replication of the mutant strain.
The effect of specific nuclear hormone receptors on pregenomic RNA
synthesis and viral replication, indicated that RXRα-PPARα in liver
cells, supports high levels of transcription and replication of
wild-type strain, while HNF4 supports high levels of transcription
and replication of the TA mutation strain.
In conclusion, the present study provided further
evidence that the double nucleotide substitutions (A1762T and
G1764A) in the BCP region of the hepatitis B virus may increase
transcription and replication of this virus, and reduce the level
of serum HBeAg patients with HBV infection. ETV and TDF therapy
were found to be effective in decreasing levels of wild-type and TA
mutant-type HBV, which may be significant in clinical practice. In
addition, a high level of HNF4 promoted TA mutant HBV replication
in vivo.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81271811) and
National Science and Technology Major Project of China (grant no.
2012ZX10002007-001-003). The authors would like to thank Alan
McLachlan (Department of Microbiology and Immunology, College of
Medicine, University of Illinois at Chicago, IL, USA) for plasmid,
pHBV4.1.
References
|
1
|
Dandri M, Lutgehetmann M and Petersen J:
Experimental models and therapeutic approaches for HBV. Semin
Immunopathol. 35:7–21. 2013. View Article : Google Scholar
|
|
2
|
Tacke F, Gehrke C, Luedde T, Heim A, Manns
MP and Trautwein C: Basal core promoter and precore mutations in
the hepatitis B virus genome enhance replication efficacy of
Lamivudine-resistant mutants. J Virol. 78:8524–8535. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gunther S, Piwon N and Will H: Wild-type
levels of pregenomic RNA and replication but reduced pre-C RNA and
e-antigen synthesis of hepatitis B virus with C (1653)→ T, A
(1762)→ T and G (1764)→ A mutations in the core promoter. J Gen
Virol. 79:375–380. 1998. View Article : Google Scholar
|
|
4
|
Laras A, Koskinas J and Hadziyannis SJ: In
vivo suppression of precore mRNA synthesis is associated with
mutations in the hepatitis B virus core promoter. Virology.
295:86–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Buckwold VE, Hon MW and Ou JH:
Mechanism of suppression of hepatitis B virus precore RNA
transcription by a frequent double mutation. J Virol. 73:1239–1244.
1999.PubMed/NCBI
|
|
6
|
Parekh S, Zoulim F, Ahn SH, et al: Genome
replication, virion secretion and e antigen expression of naturally
occurring hepatitis B virus core promoter mutants. J Virol.
77:6601–6612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nelson PK, Mathers BM, Cowie B, et al:
Global epidemiology of hepatitis B and hepatitis C in people who
inject drugs: results of systematic reviews. Lancet. 378:571–583.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lavanchy D: Hepatitis B virus
epidemiology, disease burden, treatment and current and emerging
prevention and control measures. J Viral Hepat. 11:97–107. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maynard JE: Hepatitis B: Global importance
and need for control. Vaccine. 8(Suppl): S18–S23. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tseng TC, Liu CJ, Chen CL, et al: Serum
hepatitis B virus-DNA levels correlate with long-term adverse
outcomes in spontaneous hepatitis B e antigen seroconverters. J
Infect Dis. 205:54–63. 2012. View Article : Google Scholar
|
|
11
|
Chu CJ, Keeffe EB, Han SH, et al:
Prevalence of HBV precore/core promoter variants in the United
States. Hepatology. 38:619–628. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ouneissa R, Bahri O, Alaya-Bouafif NB, et
al: Frequency and clinical significance of core promoter and
precore region mutations in Tunisian patients infected chronically
with hepatitis B. J Med Virol. 84:1719–1726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Will H, Reiser W, Weimer T, et al:
Replication strategy of human hepatitis B virus. J Virol.
61:904–911. 1987.PubMed/NCBI
|
|
14
|
Sladek FM, Zhong WM, Lai E and Darnell JE
Jr: Liver-enriched transcription factor HNF-4 is a novel member of
the steroid hormone receptor superfamily. Genes Dev. 4:2353–2365.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Bishop EP and Burke PA: Expression
profile analysis of the inflammatory response regulated by
hepatocyte nuclear factor 4α. BMC genomics. 12:1282011. View Article : Google Scholar
|
|
16
|
Chen EQ, Sun H, Feng P, et al: Study of
the expression levels of Hepatocyte nuclear factor 4 alpha and 3
beta in patients with different outcome of HBV infection. Virol J.
9:232012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu L, Chen EQ, Lei J, et al:
Pre-core/basal-core promoter and reverse transcriptase mutations in
chronic HBV infected-patients. Hepatogastroenterology. 59:212–215.
2012.PubMed/NCBI
|
|
18
|
Buckwold VE, Xu Z, Chen M, Yen TS and Ou
JH: Effects of a naturally occurring mutation in the hepatitis B
virus basal core promoter on precore gene expression and viral
replication. J Virol. 70:5845–5851. 1996.PubMed/NCBI
|
|
19
|
Liu FJ, Liu L, He F, et al: Establishment
and primary application of a mouse model with hepatitis B virus
replication. World J Gastroenterol. 13:5324–5330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang H and McLachlan A: A pregenomic RNA
sequence adjacent to DR1 and complementary to epsilon influences
hepatitis B virus replication efficiency. Virology. 303:199–210.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu F, Song Y and Liu D:
Hydrodynamics-based transfection in animals by systemic
administration of plasmid DNA. Gene Ther. 6:1258–1266. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang H, Delgermaa L, Huang F, et al: The
transcriptional transactivation function of HBx protein is
important for its augmentation role in hepatitis B virus
replication. J Virol. 79:5548–5556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen EQ, Gong DY, Leng XH, et al:
Inhibiting the expression of hepatocyte nuclear factor 4 alpha
attenuates lipopolysaccharide/D-galactosamine-induced fulminant
hepatic failure in mice. Hepatobiliary Pancreat Dis Int.
11:624–629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ganem D and Prince AM: Hepatitis B virus
infection-natural history and clinical consequences. N Engl J Med.
350:1118–1129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sumi H, Yokosuka O, Seki N, et al:
Influence of hepatitis B virus genotypes on the progression of
chronic type B liver disease. Hepatology. 37:19–26. 2003.
View Article : Google Scholar
|
|
26
|
Pungpapong S, Kim WR and Poterucha JJ:
Natural history of hepatitis B virus infection: An update for
clinicians. Mayo Clin Proc. 82:967–975. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang H, Raney AK and McLachlan A:
Replication of the wild type and a natural hepatitis B virus
nucleocapsid promoter variant is differentially regulated by
nuclear hormone receptors in cell culture. J Virol. 75:8937–8948.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu H, Zhu R, Zhu YZ, Chen Q and Zhu HG:
Effects of mutations in the X gene of hepatitis B virus on the
virus replication. Acta virologica. 56:101–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim do Y, Kim HJ, Lee CK, et al: Efficacy
of adefovir dipivoxil in the treatment of lamivudine-resistant
hepatitis B virus genotype C infection. Liver Int. 27:47–53.
2007.PubMed/NCBI
|
|
30
|
Okamoto H, Tsuda F, Akahane Y, et al:
Hepatitis B virus with mutations in the core promoter for an e
antigen-negative phenotype in carriers with antibody to e antigen.
J Virol. 68:8102–8110. 1994.PubMed/NCBI
|
|
31
|
Scaglioni PP, Melegari M and Wands JR:
Biologic properties of hepatitis B viral genomes with mutations in
the precore promoter and precore open reading frame. Virology.
233:374–381. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim DW, Lee SA, Hwang ES, Kook YH and Kim
BJ: Naturally occurring precore/core region mutations of hepatitis
B virus genotype C related to hepatocellular carcinoma. PloS one.
7:e473722012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seeger C and Mason WS: Hepatitis B virus
biology. Microbiol Mol Biol Rev. 64:51–68. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dai J, Chen EQ, Bai L, et al: Biological
characteristics of the rtA181T/sW172* mutant strain of Hepatitis B
virus in animal model. Virol J. 9:2802012. View Article : Google Scholar
|
|
35
|
Selabe SG, Song E, Burnett RJ and
Mphahlele MJ: Frequent detection of hepatitis B virus variants
associated with lamivudine resistance in treated South African
patients infected chronically with different HBV genotypes. J Med
Virol. 81:996–1001. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koklu S, Tuna Y, Gulsen MT, et al:
Long-term efficacy and safety of lamivudine, entecavir and
tenofovir for treatment of hepatitis B virus-related cirrhosis.
Clin Gastroenterol Hepatol. 11:88–94. 2013. View Article : Google Scholar
|
|
37
|
Su W and T H: Regulation of hepatitis B
virus transcription and replication by liver-enriched
transcriptional factors. World Chinese Journal of Digestology.
15:1237–1240. 2007.
|