Introduction
Endometriosis is an extremely prevalent
gynaecological disorder defined by the presence and growth of
endometrial tissue outside the uterine cavity. It occurs in 10–20%
of females of reproductive age worldwide (1) and often results in severe pelvic pain
(2) and reduced fecundity
(3). It is a benign disease;
however, it expresses malignant features, including angiogenesis,
abnormal apoptotic patterns, unrestrained cellular proliferation
and in rare cases, invasion of distant organs (4,5).
Sampson's postulation (6) of
retrograde menstruation of viable endometrial cells, which implant
and grow at ectopic sites is still the most widely accepted theory
for the histogenesis of endometriosis (7). The attachment of single cells to a
substrate, their invasion, proliferation and vascularisation are
properties that occur in carcinogenesis, and are similar or
identical to the implantation of the embryo in the endometrium and
to endometriosis.
An association between endometriosis and ovarian
cancer has been previously demonstrated (8,9). Due
to a lack of effective early detection methods and minimal physical
symptoms, epithelial ovarian cancer is commonly diagnosed at
advanced stages, resulting in one of the highest mortality rates
amongst all gynaecological malignancies (10). The use of oral contraceptives was
demonstrated to be beneficial on the incidence of ovarian cancer
(11,12). A possible explanation for an
increased rate of malignant transformation in the ovary is provided
by the 'incessant ovulation' theory which, although suggested a
number of years ago, is currently regaining importance. This theory
suggests a negative impact of each ovulation (13) via the underlying inflammatory
response associated with each occurrence (14). Pregnancy and the use of
contraceptives suppressing ovulation would therefore reduce the
risk of ovarian cancer (15,16).
In the peritoneal fluid (PF) of patients with
endometriosis, a number of cytokines, chemokines and growth factors
have been demonstrated to be expressed at higher levels compared
with females without the disease (17–21).
The same has been suggested for the expression levels of CA125, a
coelomic epithelial membrane glycoprotein antigen of unknown
biological function, which is widely used as a serum marker for
ovarian cancer and other types of tumour. Certain studies have
suggested the importance of CA125 as a marker for endometriosis
(22,23) and other previous studies have
confirmed this (24,25). Additionally, CA125 has been
demonstrated to be produced in vitro by endometrial tissue
in explant cultures (26). CA125
is an established serum marker for epithelial ovarian cancer
(27,28). Serum CA125 has also been
demonstrated to be increased in non-malignant disorders (29). The correlation between serum and PF
CA125 has been previously demonstrated (25). Human epididymis protein 4 (HE4), a
small glycoprotein and protease inhibitor first identified in
epididymal epithelium, has been identified as an ovarian cancer
marker, particularly for the serous and endometrioid type (30), and is superior to CA125 (31) either on its own or in combination
with CA125 as defined by a higher detection rate (32–34).
The increased specificity of HE4 over CA125 was previously
confirmed in a large patient cohort with benign diseases (35).
The current available information regarding HE4 in
the context of endometriosis in the literature is scarce and
pertains exclusively to its concentration in the circulation.
Additionally, to the best of our knowledge, no information exists
with regards to HE4 in the PF of patients with endometriosis. The
present study aimed to investigate the two ovarian cancer markers,
CA125 and HE4, in the PF of patients with endometriosis, with or
without treatment with oral contraceptives (combined or
gestagen-only) or with GnRH agonists, and control individuals.
Materials and methods
Patient information
A total of 358 women were involved in the present
study. They underwent laparoscopic surgery in the Department of
Obstetrics and Gynaecology, University of Berne (Berne,
Switzerland) between July 2007 and July 2014 for reasons of chronic
abdominal or menstrual pain, or as a result of unexplained
infertility. Information regarding the presence and staging, or
absence of endometriosis, hormonal treatment administration, and/or
menstrual cycle stage was obtained together with the biological
samples, or retrieved post hoc from the medical records. All
enrolled women provided informed, written consent. The study was
approved by the ethics committee of the Canton of Berne (approval
no. KEK14903).
Patient exclusion criteria
PF was quantitatively collected from the Pouch of
Douglas and clarified by centrifugation at 800 x g for 10 min. The
supernatant was stored at −80°C in aliquots after recording the
total volume. The total protein content was determined using a
micro-bicinchoninic assay (Quanti-Pro® BCA,
Sigma-Aldrich, St. Louis, MO, USA) to ascertain absence of dilution
with abdominal flushing medium under the procedure. The PF samples
with a total protein content of <10 mg/ml were excluded from the
present study. Other exclusion criteria were as follows: The
presence of haemolysis in the PF, the diagnosis of malignancies or
the use of hormonal therapy in the three months prior to the
procedure.
A total of 258 patients with endometriosis and 100
control individuals without endometriosis were included. The group
of cases included patients who did not receive any hormonal
treatment in the 3 months prior to laparoscopy (n=107). Of these
patients, 67 were in the proliferative and 28 were in the secretory
phase of the menstrual cycle (cycle stage was peri-ovulatory or
unknown in 12 cases). The remaining cases were divided into three
groups: Treatment with combined oral contraceptives (n=45),
continuous progesterone (n=56) or GnRH agonists (n=50), all for at
least 3 months leading up to the day of surgery. The control group
(without endometriosis, n=100) consisted of 67 women in the
proliferative menstrual cycle stage and 33 in the secretory
menstrual cycle stage. The demographic information and PF
characteristics are shown in Table
I.
 | Table IDemographic data and PF
characteristics. |
Table I
Demographic data and PF
characteristics.
| Characteristic | N | Age (years) | BMI
(kg/m2) | PF volume (ml) | PF protein
(mg/ml) |
|---|
| No endometriosis | 100 | 34.3±8.0 | 24.4±4.0 | 9.7±8.0 | 34.47±10.63 |
| Proliferative
phase | 67 | 33.6±8.0 | 24.3±4.5 | 8.5±8.1 | 32.96±9.87 |
| Secretory phase | 33 | 35.7±7.9 | 24.7±3.0 | 12.2±7.3 | 37.57±11.59 |
| Endometriosis | 258 | | | | |
| Not treated | 107 | 34.5±5.8 | 23.7±4.0 | 10.1±8.1 | 34.18±10.29 |
| Proliferative
phase | 67 | 33.6±5.7 | 23.8±3.9 | 9.5±8.0 | 31.60±10.39 |
| Secretory
phase | 28 | 35.2±5.2 | 22.9±3.3 | 12.6±8.7 | 38.62±7.03 |
| Peri-ovulatory
or unknown | 12 | | | | |
| OC treated | 45 | 28.7±5.1 | 22.3±4.4 | 7.2±5.9 | 33.19±8.00 |
| Gestagen only
treated | 56 | 31.5±5.9 | 22.6±3.3 | 6.9±6.5 | 38.46±8.25 |
| GnRHa treated | 50 | 31.6±5.7 | 22.2±3.6 | 8.1±7.6 | 36.78±7.67 |
ELISA
CA125 and HE4 were quantified manually in the PF
samples using commercially available microplate ELISA kits. For
CA125, the TM-CA125 ELISA kit (cat. no. EIA-5072; DRG Instruments,
Marburg, Germany) was used, according to the manufacturer's
instructions. Incubation temperature was maintained at 28°C without
agitation. The functional sensitivity was 0.25 U/ml and the intra-
and inter-assay coefficients of variance at 30 U/ml were 5.8 and
10.6%, respectively. The PF samples were diluted 1:21 with
phosphate-buffered saline, containing 0.1% (w/v) bovine serum
albumin (Sigma-Aldrich, Buchs, Switzerland). For HE4, the EIA kit
404-10 (Fujirebio AB, Göteborg, Sweden) was used, according to the
manufacturer's instructions. Incubations were performed at 28°C
with an agitation speed of 300 rpm. The detection limit was 15 pM
(functional sensitivity, <2.5 pM), and the coefficient of
variance (intra-assay) was 2.4%. The PF samples were diluted 1:26
with the zero calibrator of the assay kit.
Statistical analysis
The concentrations of CA125 and HE4 were compared
non-parametrically between the different groups using Mann-Whitney
U test. Correlation analyses were performed using Spearman's rank
correlation, following log transformation of the X (HE4) and Y
(CA125) axes, using Graph-Pad Prism® v.5.04 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Levels of CA125 and HE4 vary in different
patient samples
The levels of CA125 and HE4 were below the above
mentioned detection limits in 2/358 and 1/358 PF samples,
respectively. In the non-parametrical statistical analysis these
values were included as 10 U/ml for CA125 and 100 pmol/l for HE4.
All results for CA125 and HE4 in the different groups and
sub-groups are shown in Table II.
Marked patient-to-patient variations for the two markers were
detected over two (CA125 in controls only, <2) to three orders
of magnitude. This is shown for CA125 and HE4 in Fig. 1A and B, respectively.
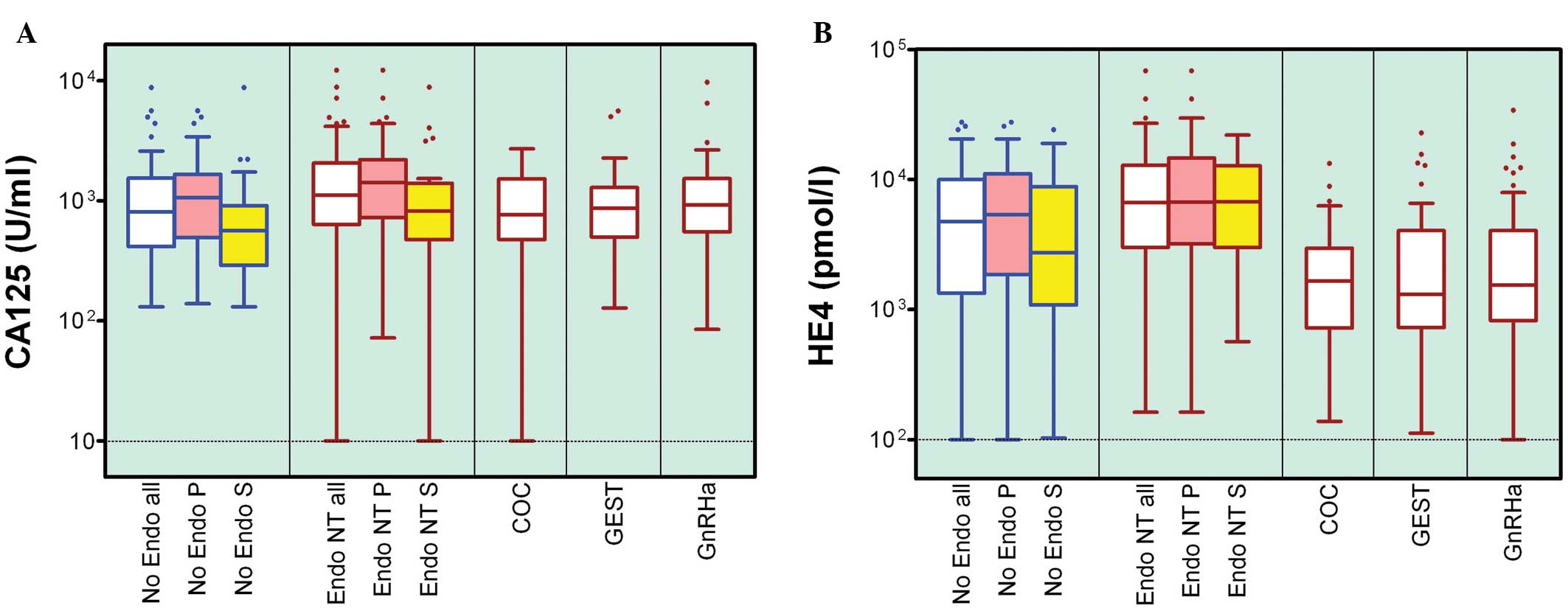 | Figure 1Tukey box and whisker plot of (A)
CA125 and (B) HE4 concentrations in the PF of No Endo women,
patients with endometriosis without hormonal treatment, under
treatment with COC, GEST or GnRHa for at least 3 months prior to PF
collection. Number of women per group and statistical significance
are stated in Table II. P,
proliferative phase of the menstrual cycle; S, secretory phase; PF,
peritoneal fluid; No Endo, endometriosis-free; NT, non-treated;
COC, combined oral contraceptives; GEST, gestagen-only; GnRHa, GnRH
agonist. |
 | Table IIOvarian cancer marker concentrations
in peritoneal fluid. |
Table II
Ovarian cancer marker concentrations
in peritoneal fluid.
| Characteristic | N | CA-125 (U/ml)
median (range) | P-value | HE-4 (pmol/l)
median (range) | P-value | CA-125xHE-4
P-value |
|---|
| No
endometriosis | 100 | 806 (131–8798) | 0.0185a | 4731
(<100–27543) | 0.0105a | 0.0061a |
| Proliferative
phase | 67 | 1064
(139–5625) | | 5371
(<100–27543) | | |
| Secretory
phase | 33 | 566 (131–8798) | 0.0020b | 2731
(103–24138) | 0.1826b | 0.0394b |
| Endometriosis | 258 | | | | | |
| Not treated | 107 | 1114
(<10–12229) | | 6667
(162–68506) | | |
| Proliferative
phase | 68 | 1416
(72–12229) | | 6707
(162–68506) | | |
| Secretory
phase | 30 | 818
(<10–8853) | 0.0175b | 6641
(567–21918) | 0.6555b | 0.3969b |
| OC treated | 45 | 762
(<10–2709) | 0.0178c | 1652
(138–13274) | <0.0001c | <0.0001c |
| Gestagen only
treated | 56 | 865 (128–5602) | 0.0233c | 1306
(112–22782) | <0.0001c | <0.0001c |
| GnRHa treated | 52 | 926 (85–9734) | 0.2419c | 1546
(<100–34123) | <0.0001c | <0.0001c |
Levels of CA125 and HE4 are affected by
hormone treatment
The concentrations of the markers were demonstrated
to be significantly increased in the group of non-treated
endometriosis cases compared with the controls without any sign of
the disease. The P-values were calculated for CA125 and HE4, and
were revealed to be 0.0185 and 0.0105, respectively (Mann-Whitney U
test). Within the endometriosis cases, exposure to combined oral
contraceptives and continuous progesterone resulted in the PF
levels of CA125 returning to that of the control group (P=0.02). No
effect on CA125 was observed following treatment with GnRH
agonists. The levels of HE4 were markedly decreased in all three
hormone-treated groups compared with the non-treated controls
(P<0.0001), and were even lower compared with the levels
observed in the control group (Table
II). CA125 significantly differed in concentration between the
prolif-erative and secretory cycle phases in the controls and
non-hormone-treated cases of endometriosis; however, this was not
the case for HE4 (Fig. 1).
Effect of assessing both markers
The product of both markers (HE4 x CA125) was also
compared between the different groups. When the non-treated
endometriosis cases were compared with the control group, the
increase in this product was more pronounced (P=0.0061) compared
with the two markers individually (Table II). When comparing proliferative
and secretory phases the results for the product came to lie
between those for the individual markers and the difference was
significant in the control but not in the untreated endometriosis
group. Conversely, the hormone treated groups demonstrated markedly
decreased values for the product (P<0.0001 for all three
hormones).
The concentrations of the two markers were revealed
to be correlated with each other in all five groups and subgroups.
Spearman P-values were between 0.0101 (gestagen treated group;
r=0.3411) and <0.0001 (controls; r=0.5816). The Spearman
r-values were not identified to be significantly different between
the five populations. These findings are shown in Fig. 2, following logarithmic
transformation.
Discussion
The present study findings not only confirm
increased PF concentrations of the ovarian cancer marker CA125 in
patients with endometriosis (24,25),
but also demonstrate for the first time, to the best of our
knowledge, similarly increased levels of the novel marker HE4 in
this compartment. The two markers are shown to be closely
correlated (P<0.01; Fig. 2),
which is consistent with the results of a previous study that
measured the concentration of the two markers in the serum of
patients with ovarian cancer (36). Therefore, it may not be necessary
to determine each of these markers, however, this will depend on
the question under investigation. For the monitoring of an existing
or suspected ovarian malignant pathology, it was demonstrated that
the combination of the two markers provided an improved result
compared with a single marker (32,34).
The findings of the present study confirm that the multiplication
product (CA125 x HE4, in arbitrary units) provides an improved
ability to discriminate between cases and controls (P=0.0061;
Table II). No information exists
in the literature regarding the levels of HE4 in the PF of patients
with endometriosis; however, a small number of previous studies
have identified HE4 in the serum. A previous case report associated
increasing levels of CA125, and low and stable serum levels of HE4
with the disease (37) indirectly,
suggesting unchanged HE4 levels in endometriosis. A previous study
observed no differences in the serum levels of HE4 between patients
with endometriosis and controls, or between the proliferative and
secretory menstrual cycle phases (38). The same result was obtained
following different hormone treatments, however, in these groups,
particularly for GnRHa, the number of patients assessed was
significantly smaller compared with that of the present study. All
these absences of variations were advocated as an advantage of the
HE4 test compared with the CA125 in patients with suspected ovarian
cancer. A previous study investigated a large patient cohort
(>1,000 patients) with benign gynaecological diseases, including
endometriosis, and revealed an increased specificity of HE4 over
CA125 as a result of less frequently increased levels in these
females, particularly in the pre-menopausal group (35). The present findings in the PF are
parallel with a previous study in the serum (38) regarding the cycle phases and the
two markers, however, not for HE4 regarding the presence of
endometriosis. Another previous study compared the serum levels of
these markers between ovarian cancer and different endometriotic
pathologies, and demonstrated that measuring both markers in the
same sample made it possible to distinguish between the two
pathologies, since HE4 but not CA125, was shown to be increased in
endometriotic pathology (36). The
mentioned study has the advantage of including cancerous and
endometriosis groups, however, hormone exposure or cycle variations
were not investigated. Further studies observing serum and PF from
the same females are required to determine whether the differences
between the present results and the few reports in the literature
regarding HE4 increases in endometriosis are a consequence of the
different compartments assessed. If so, the serum test for HE4 will
be robust towards (independent from) menstrual cycle parameters or
endometriotic pathologies; however, care may be required to ensure
that no hormonal medication was used, since HE4 reacts even more
significantly to these treatments compared with CA125.
Notably, a significant difference in the CA125
levels was demonstrated between the proliferative and secretory
cycle phases in the controls and cases (which have not been exposed
to hormonal treatment in the 3 months prior to PF collection). This
is not in agreement with a previous report (24) in which the sample numbers were
lower (11–22) and the assay was based on a
different, older antibody, which may be less specific. Care is
required and the cycle phase must be taken into account. The
present study hypothesised that HE4 (which in this study, in
contrast to CA125, failed to distinguish between the cycle phases),
may be a more 'robust' marker individually when the cycle phase is
unknown or the population is distributed over the entire menstrual
cycle. However, CA125 and HE4 may exhibit better predictive ability
alone when an exclusively proliferative or an exclusively secretory
phase population is investigated, respectively (Table II). However, this requires further
confirmation using larger groups of samples and patients.
Treatment of endometriosis may reduce the risk of
developing ovarian cancer. Current treatments include the
administration of contraceptives, combined-sequential or
continuous, which inhibit ovulation and therefore, an additional
inflammatory episode on the surface of the ovary and possibly in
surrounding tissues. The use of contraceptives has also been
demonstrated to reduce ovarian cancer risk independently of the
presence of endometriosis (11).
The present study demonstrates reduced levels of CA125 and
significantly reduced levels of HE4. This suggests the usefulness
of contraceptives, including gestagens, when administered alone in
the treatment of endometriosis and potentially in the context of
ovarian cancer, since these two proteins are associated with this
pathology. In the present study the patients with endometriosis
were not sub-grouped as a function of lesion location, however, our
previous study observed that women were less likely to develop
ovarian lesions compared with lesions in other locations
(peritoneal or recto-vaginal) following the use of oral
contraceptives (39). This is
particularly notable in the context of the ovarian cancer risk
discussed in the present study. Gonadotropin releasing hormone
agonists (GnRHa) induce a hypo-estrogenic state and therefore can
be used in the treatment of endometriosis as long as the patient
does not wish to become pregnant; this is also the case with other
hormonal medications. The increased incidence of ovarian cancer in
patients with endometriosis provides a reason to identify
endometriosis as soon as it occurs. It is difficult to suggest
whether the sub-population with an increased risk may indeed be
specifically characterised by increased levels of the ovarian
cancer markers, CA125 and possibly HE4, in the phase of
pre-malignancy. The present study, nevertheless, suggests that the
treatment of endometriosis is required as a result of its potential
to reduce the risk of ovarian cancer, as well as to reduce symptoms
such as pain.
The present results confirm those of a previous
study (40) demonstrating that
GnRHa, administered for the reduction of pain (41), resulted in a significant reduction
in peritoneal pro-inflammatory cytokine and growth factor levels,
therefore mediating a regression in the inflammatory activity in
the peritoneal environment in patients with endometriosis (42). The present study shows that several
pro-inflammatory cytokines and growth markers exhibit reduced PF
concentrations following GnRHa treatment in patients with
endometriosis (42). It also
reveals that CA125 levels are not reduced following such treatment
while those of HE4 are (P<0.0001). HE4, therefore, may be an
ideal marker, superior to CA125, for the success of medical
treatment of endometriosis, since it responds similarly to the
different categories of hormones and, as mentioned previously, does
not depend on the menstrual cycle phase in the group of non-treated
patients.
A drawback of the present study is the absence of
data regarding the occurrence of ovarian cancer in the study
population. It is extremely difficult to gather samples from
patients with ovarian cancer, together with the complete
information regarding the presence or absence of past
endometriosis. Additionally, such a project would likely further
benefit from the analysis of serum samples obtained during the
endometriosis and the cancer phases. This was not performed in the
present study where an increased specificity by the restriction to
the peritoneal compartment was targeted.
In conclusion, the present study shows that the
treatment of patients with endometriosis with sex steroids or GnRHa
reduced the exposure of ovarian epithelial cells to an inflammatory
environment. Whether this may later have a beneficial, negative
effect on the risk of developing ovarian cancer remains to be
elucidated. The response of the peritoneal environment to such
treatment may be monitored by ovarian cancer markers, including
CA125 and HE4, with the latter being slightly superior to the
former since it does not react to menstrual cycle variations and
the reductions in PF concentrations are more pronounced. Variance
of the results within one group, however, is not better (smaller)
for HE4 than for CA125. Long-term studies, including observation of
serum levels, are required to gain further insight.
Acknowledgments
The authors would like to thank Dr Magnus Lövenklev
(Fujirebio Diagnostics, Gothenburg, Sweden) for providing the assay
kits for HE4 free of charge, Ms. Barbara Haldemann and her team for
the collection of the PF samples and to Ms. Anne Vaucher for
technical assistance in the laboratory. This study was funded, in
part, by the Swiss National Science Foundation (grant no.
320030_140774).
References
|
1
|
Eskenazi B and Warner ML: Epidemiology of
endometriosis. Obstet Gynecol Clin North Am. 24:235–258. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Evans S, Moalem-Taylor G and Tracey DJ:
Pain and endometriosis. Pain. 132(Suppl 1): S22–S25. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
D'Hooghe TM, Debrock S, Hill JA and
Meuleman C: Endometriosis and subfertility: Is the relationship
resolved. Semin Reprod Med. 21:243–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung SY, Kim SJ, Kim TH, et al: Computed
tomography findings of pathologically confirmed pulmonary
parenchymal endometriosis. J Comput Assist Tomogr. 29:815–818.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fraser IS: Recognising, understanding and
managing endometriosis. J Reprod Sci. 1:56–64. 2008. View Article : Google Scholar
|
|
6
|
Sampson J: Peritoneal endometriosis due to
the menstrual dissemination of endometrial tissue into the
peritoneal cavity. Am J Obstet Gynecol. 14:422–469. 1927.
|
|
7
|
Halme J, Hammond M, Hulka J, Raj S and
Talbert L: Retrograde menstruation in healthy women and in patients
with endometriosis. Obstet Gynecol. 64:151–154. 1984.PubMed/NCBI
|
|
8
|
Brinton LA, Gridley G, Persson I, Baron J
and Bergqvist A: Cancer risk after a hospital discharge diagnosis
of endometriosis. Am J Obstet Gynecol. 176:572–579. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Swiersz L: Role of endometriosis in cancer
and tumour development. Ann NY Acad Sci. 955:281–292. 2002.
View Article : Google Scholar
|
|
10
|
Martin DC: Cancer and endometriosis: Do we
need to be concerned? Semin Reprod Endocrinol. 15:319–324. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gwinn ML, Lee NC, Rhodes PH, Layde PM and
Rubin GL: Pregnancy, breast feeding, and oral contraceptives and
the risk of epithelial ovarian cancer. J Clin Epidemiol.
43:559–568. 1991. View Article : Google Scholar
|
|
12
|
Fraser IS and Kovacs GT: The efficacy of
non-contraceptive uses for hormonal contraceptives. Med J Aust.
178:621–623. 2003.PubMed/NCBI
|
|
13
|
Fathalla MF: Incessant ovulation - a
factor in ovarian neoplasia? Lancet. 2:1631971. View Article : Google Scholar
|
|
14
|
Ness RB, Cramer DW, Goodman MT, et al:
Infertility, fertility drugs, and ovarian cancer: A pooled analysis
of case-control studies. Am J Epidemiol. 155:217–224. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Booth M, Beral V and Smith P: Risk factors
for ovarian cancer: A case-control study. Br J Cancer. 60:592–598.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Risch HA, Marrett LD and Howe GR: Parity,
contraception, infertility, and the risk of epithelial ovarian
cancer. Am J Epidemiol. 140:585–597. 1994.PubMed/NCBI
|
|
17
|
Ryan IP, Tseng JF, Schriock ED, Khorram O,
Landers DV and Taylor RN: Interleukin-8 concentrations are elevated
in peritoneal fluid of women with endometriosis. Fertil Steril.
63:929–932. 1995.PubMed/NCBI
|
|
18
|
Lebovic DI, Mueller MD and Taylor RN:
Immunobiology of endometriosis. Fertil Steril. 75:1–10. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bersinger NA, von Roten S, Wunder DM, Raio
L, Dreher E and Mueller MD: PAPP-A and osteoprotegerin, together
with interleukin-8 and RANTES, are elevated in the peritoneal fluid
of women with endometriosis. Am J Obstet Gynecol. 195:103–108.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siristatidis C, Nissotakis C, Chrelias C,
Iacovidou H and Salamalekis E: Immunological factors and their role
in the genesis and the development of endometriosis. J Obstet
Gynaecol Res. 32:162–170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bersinger NA, Dechaud H, Mckinnon B and
Mueller MD: Analysis of cytokines in the peritoneal fluid of
endometriosis patients as a function of the menstrual cycle stage
using the Bio-Plex platform. Arch Physiol Biochem. 118:210–218.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moen MH, Hagen B and Onsrud M: CA-125 in
peritoneal fluid from patients with endometriosis. Hum Reprod.
6:1400–1403. 1991.PubMed/NCBI
|
|
23
|
Koninckx PR, Riitinen L, Seppala M and
Cornillie FJ: CA-125 and placental protein 14 concentrations in
plasma and peritoneal fluid of women with deeply infiltrating
pelvic endometriosis. Fertil Steril. 57:523–530. 1992.PubMed/NCBI
|
|
24
|
Matalliotakis IM, Goumenou AG, Mulayim N,
Karkavitsas N and Koumantakis EE: High concentrations of the
CA-125, CA 19-9 and CA 15-3 in the peritoneal fluid between
patients with and without endometriosis. Arch Gynecol Obstet.
271:40–45. 2005. View Article : Google Scholar
|
|
25
|
Amaral VF, Ferriani RA, Sá MF, Nogueira
AA, Rosa e Silva JC, Rosa e Silva AC and Moura MD: Positive
correlation between serum and peritoneal fluid CA-125 levels in
women with pelvic endometriosis. Sao Paulo Med J. 124:223–227.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bersinger NA, Sinosich MJ, Baber R, Torode
H and Saunders DM: Development of an endometrial explant model for
the investigation of uterine readiness for implantation in the
human. Implantation in Mammals. Serono Symposia; 91. pp. 301–308.
1993
|
|
27
|
Duffy MJ: Role of tumour markers in
patients with solid cancers. A critical review. Eur J Intern Med.
18:175–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anderson MR, Goff BA, Lowe KA, et al:
Combining a symptoms index with CA125 to improve detection of
ovarian cancer. Cancer. 113:484–489. 2008. View Article : Google Scholar
|
|
29
|
Ataseven H, Oztürk ZA, Arhan M, et al:
Cancer antigen 125 levels in inflammatory bowel diseases. J Clin
Lab Anal. 23:244–248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Drapkin R, Von Horsten HH, Lin Y, et al:
Human epididymis protein 4 (HE4) is a secreted glycoprotein that is
overexpressed by serous and endometrioid ovarian carcinomas. Cancer
Res. 65:2162–2169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hellstrom I, Raycraft J, Hayden M, et al:
The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma.
Cancer Res. 63:3695–3700. 2003.PubMed/NCBI
|
|
32
|
Moore RG, Brown AK, Miller MC, et al: The
use of multiple novel tumor biomarkers for the detection of ovarian
carcinoma in patients with a pelvic mass. Gynecol Oncol.
108:402–408. 2008. View Article : Google Scholar
|
|
33
|
Chang X, Ye X, Dong L, et al: Human
epididymis protein 4 (HE4) as a serum tumor biomarker in patients
with ovarian carcinoma. Int J Gynecol Cancer. 21:852–858. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Escudero JM, Auge JM, Filella X, Torne A,
Pahisa J and Molina R: Comparison of serum human epididymis protein
4 with cancer antigen 125 as a tumor marker in patients with
malignant and nonmalignant diseases. Clin Chem. 57:1534–1544. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moore RG, Miller MC and Steinhoff MM:
Serum HE4 levels are less frequently elevated than CA125 in women
with benign gynecologic disorders. Am J Obstet Gynecol.
206:351.e1–e8. 2012. View Article : Google Scholar
|
|
36
|
Huhtinen K, Suvitie P, Hiissa J, et al:
Serum HE4 concentration differentiates malignant ovarian tumours
from ovarian endometriotic cysts. Br J Cancer. 100:1315–1319. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anastasi E, Granato T, Coppa A, et al: HE4
in the differential diagnosis of a pelvic mass: a case report. Int
J Mol Sci. 12:627–632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hallamaa M, Suvitie P, Huhtinen K,
Matomäki J, Poutanen M and Perheentupa A: Serum HE4 concentration
is not dependent on menstrual cycle or hormonal treatment among
endometriosis patients and healthy premenopausal women. Gynecol
Oncol. 125:667–672. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McKinnon BD, Bertschi D, Wanner J,
Bersinger NA and Mueller MD: Hormonal contraceptive use and the
prevalence of endometriotic lesions at different regions within the
peritoneal cavity. Biomed Res Int. 2014:5909502014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kupker W, Schultze-Mosgau A and Diedrich
K: Paracrine changes in the peritoneal environment of women with
endometriosis. Hum Reprod Update. 4:719–723. 1998. View Article : Google Scholar
|
|
41
|
Brown J, Pan A and Hart RJ:
Gonadotrophin-releasing hormone analogues for pain associated with
endometriosis. Cochrane Database Syst Rev. CD0084752010.PubMed/NCBI
|
|
42
|
Nirgianakis K, Bersinger NA, McKinnon B,
Kostov P, Imboden S and Mueller MD: Regression of the inflammatory
microenvironment of the peritoneal cavity in women with
endometriosis by GnRHa treatment. Eur J Obstet Gynecol Reprod Biol.
170:550–554. 2013. View Article : Google Scholar : PubMed/NCBI
|
















