Introduction
Rheumatoid arthritis (RA) is an autoimmune,
inflammatory joint disease, characterized by hyperplasia of the
synovial tissue and the formation of pannus tissue, which grows
invasively into the cartilage, causing cartilage and bone
destruction. Synovium is composed of two types of synoviocytes:
fibroblast-like synoviocytes (FLS) and macrophage-like
synoviocytes. Analyses of hyperplastic synovial tissue from
patients with RA demonstrated the presence of cells with numerous
features similar to transformed long-living cells, including the
presence of somatic mutations, oncogene expression and resistance
to apoptosis (1–5).
Our previous study demonstrated that decoy receptor
3 (DcR3), termed TR6/M68/tumor necrosis factor receptor (TNFR)
superfamily member 6, is expressed in RA-FLS, and that tumor
necrosis factor (TNF)α-induced expression of DcR3 in RA-FLS
protects the cells from Fas-induced apoptosis (6). DcR3 lacks the transmembrane domain of
conventional TNFRs and can, therefore, be secreted (7). DcR3 is typically overexpressed in
tumor cells and is also expressed in certain types of normal
tissue, including the colon, stomach, spleen, lymph nodes, spinal
cord, pancreas and lungs (7,8).
DcR3 exerts numerous biological roles, including osteoclast
formation (9), monocyte adhesion
(10) and inhibition of apoptosis
(11).
DcR3 has three ligands: Fas ligand (FasL), LIGHT,
and TNF-like ligand 1A (TL1A), all of which are members of the TNF
superfamily (12). The
overexpression of DcR3 may benefit tumors by helping them to avoid
the cytotoxic and regulatory effects of FasL (7,13),
LIGHT (14), and TL1A (15). Our previous study suggested that
DcR3 may be one of the key molecules, which regulates RA-FLS
proliferation (6). In addition,
our previous study reported that DcR3 can bind to membrane-bound
TL1A, which is expressed on RA-FLS, resulting in the negative
regulation of inflammatory cytokine-induced cell proliferation
(16). These results suggested
that DcR3 may have a role in the pathogenesis of RA, not only as a
decoy receptor, but also as a ligand for TL1A on RA-FLS.
By comprehensive genetic analysis using microarrays,
our previous study demonstrated that DcR3 may regulate gene
expression in RA-FLS (17). From
these gene expression profiles, tryptophan hydroxylase 1 (TPH1) was
identified as one of the genes whose expression was suppressed in
RA-FLS by DcR3 (17). TPH
catalyzes the hydroxylation of L-tryptophan, and is the
rate-limiting enzyme for the synthesis of serotonin. TPH has two
isoforms: TPH1, which is expressed in peripheral tissues expressing
serotonin, including the skin, and in the intestine, the pineal
gland and the central nervous system (CNS); and TPH2, which is
exclusively expressed in the CNS where it predominates over TPH1.
Numerous previous studies have suggested that serotonergic systems
have an important role in modulating inflammatory pain and bone
remodeling (18–20).
The aim of the present study was to determine the
specificity of the effects of DcR3 on TPH1 in RA-FLS and therefore,
determine whether DcR3 had the potential to modulate the
pathogenesis of RA. The present study also aimed to compare the
effects of DcR3 and inflammatory cytokines on the expression of
TPH1 in RA-FLS and osteoarthritis (OA)-FLS.
Materials and methods
Isolation and culture of synovial
fibroblasts
FLS were obtained during total knee replacement
surgery from patients with RA, who fulfilled the 1987 criteria of
the American College of Rheumatology (21), who had never been treated with
biological drugs (34 females and 5 males, mean age 64.1±18.0
years), and from patients with OA (31 females and 2 males, mean age
71.7±7.5 years) between May 2010 and June 2012. Synovial samples
were collected from the patients who provided written informed
consent for their involvement in the present study, in accordance
with the World Medical Association Declaration of Helsinki Ethical
Principles for Medical Research Involving Human Subjects. The
present study, including consent procedures, was approved by the
ethics committee of Kobe University Graduate School of Medicine
(Kobe, Japan). Tissue specimens were minced using Cooper surgical
scissors and digested in Dulbecco's modified Eagle's medium (DMEM;
Gibco Life Technologies, Grand Island, NY, USA), supplemented with
0.2% collagenase (Sigma-Aldrich, St. Louis, MO, USA), for 2 h at
37°C in an atmosphere containing 5% CO2. The dissociated
synovial cells were subsequently cultured in DMEM, supplemented
with 10% fetal bovine serum (FBS; BioWhittaker®, Lonza,
Walkersville, MD, USA) and 100 U/ml penicillin/streptomycin (Meiji
Seika Pharma Co., Ltd., Tokyo, Japan). Following an overnight
culture, the non-adherent cells were removed, and the adherent
cells were further incubated in fresh medium. All experiments were
performed using cells from passages 3–7 (6).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Individual lines of primary cultured RA- and OA-FLS
were seeded into 6-well plates at a density of
0.5–1.0×106 cells/well in DMEM, supplemented with 10%
FBS, and were cultured for 24 h. The medium was subsequently
replaced with serum-free Opti-MEM (Gibco Life Technologies) and the
cells were incubated with 1.0 μg/ml recombinant human
DcR3-Fc protein (R&D Systems, Minneapolis, MN, USA) or 1.0
μg/ml control human immunoglobulin G (IgG)1 (R&D
Systems) for 12 h at 37°C in an atmosphere containing 5%
CO2, or with 1.0 ng/ml recombinant human TNFα (R&D
Systems) or 1.0 ng/ml recombinant human interleukin (IL)-1β
(R&D Systems) for 24 h at 37°C in an atmosphere containing 5%
CO2. Following incubation, the total RNA was extracted
from the cells using a QIAshredder with the RNeasy Mini kit (Qiagen
GmbH, Hilden, Germany), according to the manufacturer's
instructions.
The total RNA (2 μg) was reverse transcribed
to first-strand cDNA using an Oligo (dT) primer and a High-Capacity
cDNA Reverse Transcription kit (Applied Biosystems Life
Technologies, Foster City, CA, USA), according to the
manufacturer's instructions. The cycling conditions were as
follows: 10 min at 25°C, 2 h at 37°C, 5 min at 85°C and 4°C
thereafter using a PCR thermal cycler (Astec, Fukuoka, Japan).
The relative mRNA expression levels were compared
using TaqMan® Real-Time PCR on a StepOne™ Real-Time PCR
system (Applied Biosystems Life Technologies). The cycling
conditions were as follows: 50°C for 2 min, 95°C for 10 min, 50
cycles of 95°C for 15 sec and 60°C for 1 min. Pre-designed primers
and probes for TPH1 (Hs00188220_m1) and GAPDH
(Hs99999905_m1) mRNA were obtained from Applied Biosystems Life
Technologies. GAPDH was used as a control. Comparative
analyses of each of these genes in individual patients were
performed using a specialized computer program, StepOne™ Software
version 2.1 (Applied Biosystems Life Technologies). All
amplifications were performed at least in duplicates. The mRNA
expression levels of each gene were calculated using the
2−ΔΔCt (comparative threshold cycle, or
CT) method, as detailed by the manufacturer
(Applied Biosystems Life Technologies).
Immunohistochemistry
Frozen synovial tissues from patients with RA and OA
were cut into 9 μm sections using a cryostat (Leica CM3050
S™; Leica Biosystems, Nussloch, Germany). Mouse anti-human
serotonin monoclonal antibody (cat. no. 53842; 1:20 dilution;
AnaSpec, Fremont, CA, USA) was applied to the sections and
incubated overnight at 4°C. The HistoFine® Simple Stain™
kit anti-mouse antibody with peroxidase (cat. no. 414131F; Nichirei
Corporation, Tokyo, Japan) was used as a secondary antibody and
incubated for 30 min at room temperature. The sections were
developed using HistoFine® Simple Stain
3,3′-diaminobenzidine (Nichirei Corporation), followed by
counterstaining with hematoxylin (Muto Pure Chemicals Co., Ltd.,
Tokyo, Japan). Images of the stained sections were captured using
an Axioskop 2 plus (Carl Zeiss, Jena, Germany).
Statistical analysis
For all statistical analyses the program Statcel
version 3 (OMS, Tokyo, Japan) was utilized. All data are presented
as the mean ± standard deviation, unless otherwise stated. In all
cases, P-values were calculated using a two-tailed Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
mRNA expression levels of TPH1 in RA- or
OA-FLS
The present study initially aimed to detect the mRNA
expression levels of TPH1 in RA- and OA-FLS. RT-qPCR
analysis demonstrated that TPH1 mRNA was expressed in both
RA- and OA-FLS (Fig. 1).
Disease specificity of downregulation the
mRNA expression levels of TPH1 in RA-FLS by DcR3
The mRNA expression levels of TPH1 in RA- and
OA-FLS stimulated with DcR3-Fc were compared with the expression
levels in cells incubated with control IgG1. The mRNA expression
levels of TPH1 were significantly decreased (0.77-fold) in
RA-FLS following treatment with DcR3-Fc (Fig. 2A), whereas the expression of
TPH1 in OA-FLS was not influenced following treatment with
DcR3-Fc (Fig. 2B).
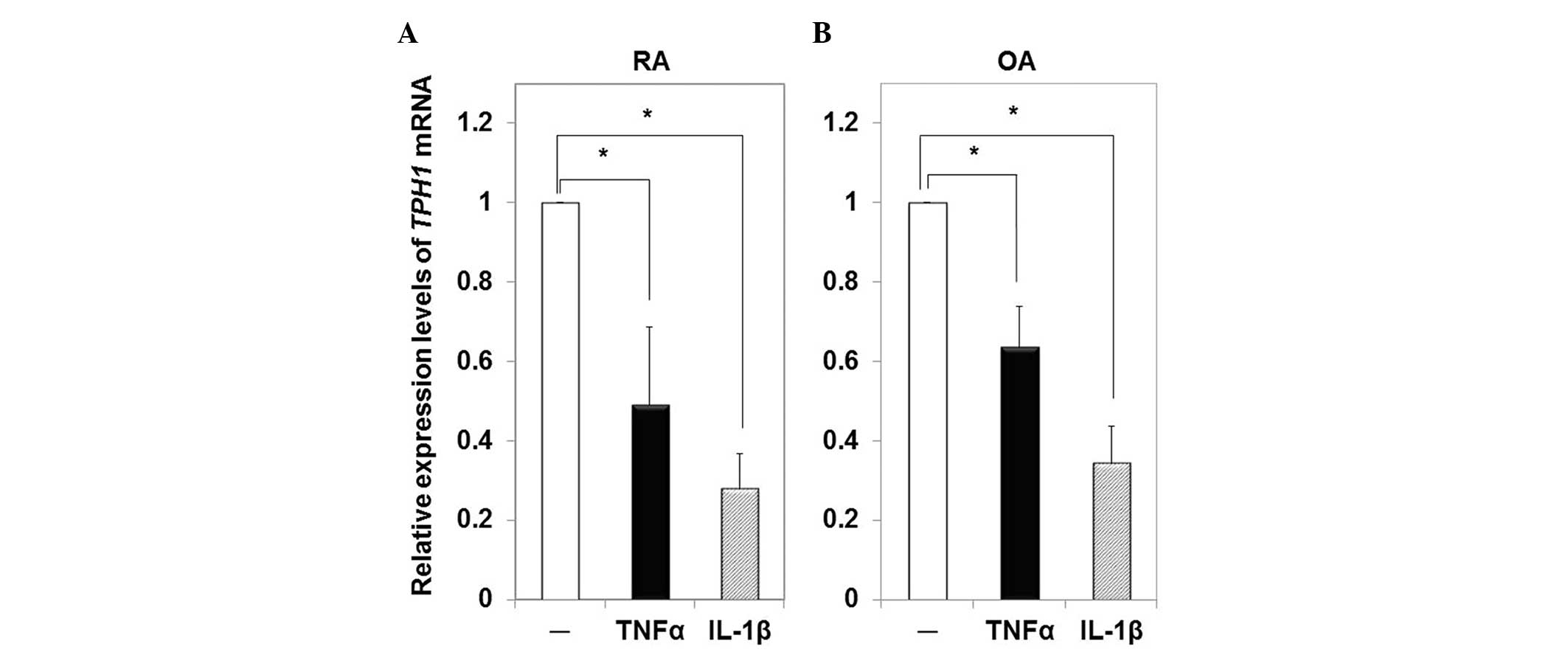
Effects of inflammatory cytokines on the
mRNA expression levels of TPH1 in RA- and OA-FLS
The mRNA expression levels of TPH1 in RA- and
OA-FLS stimulated with TNFα or IL-1β were compared with the
expression levels in the cells incubated with serum-free Opti-MEM
only. Both TNFα and IL-1β suppressed the mRNA expression levels of
TPH1 in RA-FLS (0.49-fold and 0.28-fold, respectively;
Fig. 3A), as well as in OA-FLS
(0.63-fold and 0.34-fold, respectively; Fig. 3B).
Immunohistochemical analysis of serotonin
in RA and OA synovial tissue
To confirm that the serotonin synthesis pathway was
intact in the RA- and OA-FLS, the expression and localization of
serotonin was detected in RA and OA synovial tissue by
immunohistochemical analysis. Immunohistochemical analysis
demonstrated that serotonin was specifically expressed in the
multi-layered lining synovial cells of RA synovial tissue (Fig. 4A). Serotonin was also primarily
present in the outer layer lining cells of OA synovial tissue
(Fig. 4B). Conversely, serotonin
was present at lower levels in the sublining layer of both RA and
OA synovial tissue (Fig. 4B).
Discussion
The present study demonstrated that TPH1 was
expressed in RA-FLS and its expression was downregulated by DcR3.
In the serotonin synthesis pathway, L-tryptophan is hydroxylated
into L-5-hydroxytryptophan by TPH, which is then decarboxylated
into serotonin by an L-amino acid decarboxylase (22). Since TPH is the rate-limiting
enzyme of serotonin synthesis (22), these data suggest that DcR3 may
regulate serotonin synthesis in synovial cells, and thereby
regulate peripheral serotonin levels. The present study
demonstrated that serotonin is synthesized in RA-FLS. Previous
studies have suggested that peripheral serotonin, which is
synthesized through TPH1 activity, has pleiotropic effects,
including functions in the immune system (23), vasoconstriction (24), modulating inflammatory pain
(25) and bone physiology
(24,25). In addition, serotonin regulates
osteoblast proliferation and bone formation (25), and osteoclast differentiation and
activity (26). The regulation of
the expression of TPH1 and its presumed subsequent effects on
serotonin levels by DcR3 in RA-FLS may therefore, have important
consequences for RA.
The results of the present study demonstrated that
serotonin was highly expressed in the disease-specific
multi-layered synovial lining cells of RA, and in the outer layer
lining cells of OA, however, it was weakly expressed in the
sublining cells. These results suggested that serotonin may have a
certain role in synovial lining cells, particularly in
over-proliferating RA synovial cells. Investigation of the synovial
fluid and cartilage may assist in increasing our understanding
regarding the effects of serotonin on arthritis, and on the
destruction of cartilage and bone. Further studies on other tissue
types, including cartilage or synovial fluid, may be required to
elucidate the role of serotonin in bone metabolism.
In the present study, the inflammatory cytokines,
TNFα and IL-1β, suppressed the mRNA expression levels of
TPH1 in RA- and OA-FLS, however, DcR3 only suppressed the
mRNA expression of TPH1 in RA-FLS. These results indicated
that the mRNA expression of TPH1 in RA-FLS was downregulated
by DcR3 in a disease-specific manner. Since the concentration of
DcR3 in the sera and synovial fluid of patients with RA is
significantly higher, as compared with patients with OA (27), the higher levels of DcR3 may lead
to decreased levels of peripheral serotonin via DcR3-mediated
suppression of the mRNA expression of TPH1. The present
study also provided important information regarding the influence
of inflammatory cytokines on the expression levels of TPH1 in RA-
and OA-FLS, and of the expression of serotonin in the synovial
tissue of RA and OA.
In conclusion, the results of the present study
suggested that DcR3 may affect the expression of peripheral
serotonin in rheumatoid synovial tissue by regulating the mRNA
expression of TPH1 in RA-FLS. These results indicated that
DcR3 may be involved in the pathogenesis of RA, particularly in
processes including modulation of inflammatory pain and bone
remodeling. In addition to DcR3, TPH1 may also be a potential
therapeutic target for the treatment of RA.
Acknowledgments
The present study was supported by a grant-in-aid in
the form of a health science research grant from the Japanese
Ministry of Education, Science, and Culture (no. 24592261). The
findings of the present study were presented at the 2013 American
College of Rheumatology/Association of Rheumatology Health
Professionals Annual Meeting, October 25–30, 2013 (San Diego, CA,
USA).
References
|
1
|
Chou CT, Yang JS and Lee MR: Apoptosis in
rheumatoid arthritis - expression of Fas, Fas-L, p53, and Bcl-2 in
rheumatoid synovial tissues. J Pathol. 193:110–116. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tak PP, Zvaifler NJ, Green DR and
Firestein GS: Rheumatoid arthritis and p53: How oxidative stress
might alter the course of inflammatory diseases. Immunol Today.
21:78–82. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamanishi Y, Boyle DL, Rosengren S, Green
DR, Zvaifler NJ and Firestein GS: Regional analysis of p53
mutations in rheumatoid arthritis synovium. Proc Natl Acad Sci USA.
99:10025–10030. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alamanos Y and Drosos AA: Epidemiology of
adult rheumatoid arthritis. Autoimmun Rev. 4:130–136. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu P, Lu N, Shi ZG, Zhou J, Wu ZB, Yang
Y, Ding J and Chen ZN: CD147 overexpression on synoviocytes in
rheumatoid arthritis enhances matrix metalloproteinase production
and invasiveness of synoviocytes. Arthritis Res Ther. 8:R442006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayashi S, Miura Y, Nishiyama T, Mitani M,
Tateishi K, Sakai Y, Hashiramoto A, Kurosaka M, Shiozawa S and
Doita M: Decoy receptor 3 expressed in rheumatoid synovial
fibroblasts protects the cells against Fas-induced apoptosis.
Arthritis Rheum. 56:1067–1075. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pitti RM, Marsters SA, Lawrence DA, Roy M,
Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT,
et al: Genomic amplification of a decoy receptor for Fas ligand in
lung and colon cancer. Nature. 396:699–703. 1998. View Article : Google Scholar
|
|
8
|
Bai C, Connolly B, Metzker ML, Hilliard
CA, Liu X, Sandig V, Soderman A, Galloway SM, Liu Q, Austin CP and
Caskey CT: Overexpression of M68/DcR3 in human gastrointestinal
tract tumors independent of gene amplification and its location in
a four-gene cluster. Proc Natl Acad Sci USA. 97:1230–1235. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang CR, Wang JH, Hsieh SL, Wang SM, Hsu
TL and Lin WW: Decoy receptor 3 (DcR3) induces osteoclast formation
from monocyte/macrophage lineage precursor cells. Cell Death
Differ. 11(Suppl 1): S97–S107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu MJ, Lin WW, Tsao WC, Chang YC, Hsu TL,
Chiu AW, Chio CC and Hsieh SL: Enhanced adhesion of monocytes via
reverse signaling triggered by decoy receptor 3. Exp Cell Res.
292:241–251. 2004. View Article : Google Scholar
|
|
11
|
Tateishi K, Miura Y, Hayashi S, Takahashi
M and Kurosaka M: DcR3 protects THP-1 macrophages from apoptosis by
increasing integrin alpha4. Biochem Biophys Res Commun.
389:593–598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi G, Wu Y, Zhang J and Wu J: Death decoy
receptor TR6/DcR3 inhibits T cell chemotaxis in vitro and in vivo.
J Immunol. 171:3407–3414. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsuji S, Hosotani R, Yonehara S, Masui T,
Tulachan SS, Nakajima S, Kobayashi H, Koizumi M, Toyoda E, Ito D,
et al: Endogenous decoy receptor 3 blocks the growth inhibition
signals mediated by Fas ligand in human pancreatic adenocarcinoma.
Int J Cancer. 106:17–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu KY, Kwon B, Ni J, Zhai Y, Ebner R and
Kwon BS: A newly identified member of tumor necrosis factor
receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J
Biol Chem. 274:13733–13736. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Migone TS, Zhang J, Luo X, Zhuang L, Chen
C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, et al: TL1A is a
TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell
costimulator. Immunity. 16:479–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahashi M, Miura Y, Hayashi S, Tateishi
K, Fukuda K and Kurosaka M: DcR3-TL1A signalling inhibits
cytokine-induced proliferation of rheumatoid synovial fibroblasts.
Int J Mol Med. 28:423–427. 2011.PubMed/NCBI
|
|
17
|
Fukuda K, Miura Y, Maeda T, Takahashi M,
Hayashi S and Kurosaka M: Decoy receptor 3 regulates the expression
of various genes in rheumatoid arthritis synovial fibroblasts. Int
J Mol Med. 32:910–916. 2013.PubMed/NCBI
|
|
18
|
Ducy P and Karsenty G: The two faces of
serotonin in bone biology. J Cell Biol. 191:7–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yadav VK and Ducy P: Lrp5 and bone
formation: A serotonin-dependent pathway. Ann NY Acad Sci.
1192:103–109. 2010. View Article : Google Scholar
|
|
20
|
Voog O, Alstergren P, Leibur E, Kallikorm
R and Kopp S: Immediate effects of the serotonin antagonist
granisetron on temporomandibular joint pain in patients with
systemic inflammatory disorders. Life Sci. 68:591–602. 2000.
View Article : Google Scholar
|
|
21
|
Arnett FC, Edworthy SM, Bloch DA, McShane
DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS,
et al: The American Rheumatism Association 1987 revised criteria
for the classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lovenberg W, Jequier E and Sjoerdsma A:
Tryptophan hydroxylation: Measurement in pineal gland, brainstem,
and carcinoid tumor. Science. 155:217–219. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Geba GP, Ptak W, Anderson GM, Paliwal V,
Ratzlaff RE, Levin J and Askenase PW: Delayed-type hypersensitivity
in mast cell-deficient mice: Dependence on platelets for expression
of contact sensitivity. J Immunol. 157:557–565. 1996.PubMed/NCBI
|
|
24
|
Walther DJ and Bader M: A unique central
tryptophan hydroxylase isoform. Biochem Pharmacol. 66:1673–1680.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karsenty G and Yadav VK: Regulation of
bone mass by serotonin: Molecular biology and therapeutic
implications. Annu Rev Med. 62:323–331. 2011. View Article : Google Scholar
|
|
26
|
Gustafsson BI, Thommesen L, Stunes AK,
Tommeras K, Westbroek I, Waldum HL, Slørdahl K, Tamburstuen MV,
Reseland JE and Syversen U: Serotonin and fluoxetine modulate bone
cell function in vitro. J Cell Biochem. 98:139–151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayashi S, Miura Y, Tateishi K, Takahashi
M and Kurosaka M: Decoy receptor 3 is highly expressed in patients
with rheumatoid arthritis. Mod Rheumatol. 20:63–68. 2010.
View Article : Google Scholar
|