Introduction
Lung cancer has been and remains the most common
malignancy in the world, with an estimated 1.6 million novel cases
per year (1). Despite the great
progress made in several areas of oncology, the treatment and
outcome of lung cancer have not significantly improved (2,3). Its
high mortality rate is attributed to a high incidence of
metastases, thereby making systemic therapies the mainstay for
treatment. As chemotherapy against metastatic lung cancer has yet
to be shown effective (4,5), molecular targets are required to be
established to design appropriate pharmacologic agents to provide
novel treatment modalities. In recent years, targeted therapies,
including those directed towards epidermal growth factor receptor,
anaplastic lymphoma kinase, mesenchymal-epithelial transition
factor and angiogenesis, have been increasingly used (6–9).
However, other pathways or molecular biomarkers may be identified
in lung cancer.
Tyrosylprotein sulfotransferase (TPST) is a 54-to
50-kDa integral membrane glycoprotein of the trans-Golgi network
found in essentially all tissues investigated, catalyzing the
tyrosine O-sulfation of soluble and membrane proteins passing
through this compartment (10).
Two different TPSTs (TPST-1 and TPST-2) have been identified
(11,12) and are broadly co-expressed in human
tissues (13,14). The levels of TPST-1 and TPST-2
expression vary among different tissues, which may imply distinct
physiological functions of TPST-1 and TPST-2 (14). Several studies have found that
TPST-1 is highly expressed in breast cacinoma (15), oral squamous cell carcinoma
(16) and soft-tissue sarcoma
(17) compared with expression
levels in normal tissues. In addition, a recent study of human
nasopharyngeal carcinoma (NPC) found that the expression of TPST-1
was directly and clinically correlated with NPC and was associated
with metastasis (18).
To the best of our knowledge, little has been
uncovered regarding the involvement of TPST genes in lung cancer.
TPST expression may be associated with treatment efficacy or
prognosis in patients with lung cancer; however, the knowledge
concerning TPST expression in lung cancer is currently
insufficient. Overexpression of c-Met has been described in lung
cancer and a multitude of other malignant human neoplasms (19–21).
The present study was designed to clarify the TPST-1 expression in
lung cancer by using a number of consecutive cases of primary
tumors with complete histopathologic and clinical data.
Materials and methods
Patients and tumors
The present study was approved by The Third Xiangya
Hospital Institutional Review Board of Central South University
(Changsha, China). All patients with stage I–IV lung cancer who
were undergoing a tumor resection or biospy procedure at the Third
Xiangya Hospital between March 2010 and October 2012 were included.
None of the patients received pre-operative chemotherapy, and all
were treated with routine chemotherapy after the operation. Fixed
in formaldehyde and embedded in paraffin, the specimens from 50
patients (16 women, 34 men) who were pathologically diagnosed with
lung cancer were available for the present study. Clinical data of
patients were obtained through a retrospective analysis of the
reports. Clinical staging was based on the 7th edition of the
tumor-node-metastasis (TNM) classification for lung cancer
(22). In addition, surgically
removed non-neoplastic tissues 5 cm from the cancer tissues were
used as controls. Written informed consent was obtained from all
the participants involved in the present study.
Antibodies
A rabbit polyclonal antibody against human TPST-1
(cat no. SAB1300286) and a mouse monoclonal antibody against human
c-Met (cat. no. SAB4501869) were obtained from Sigma-Aldrich (St.
Louis, MO, USA).
Immunohistochemistry
Immunohistochemistry staining was performed using
the two-step EnVision™ method (Dako, Glostrup, Denmark). Briefly,
5-μm tissue sections were cut from each of the selected 50
paraffin-embedded tumor and control specimens, and they were dried
at 65°C for 30 min. The sections were de-paraffinized with xylene
and re-hydrated with a graded ethanol series. Endogenous peroxide
blocking was performed with 3% hydrogen peroxide (Sinopharm
Chemical Reagent Co. Ltd., Shanghai, China) for 10 min, and antigen
retrieval was performed at 100°C for 30 min in a citrate buffer (10
mmol/1; pH 6.0; Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China). After the sections were washed three times
with phosphate-buffered saline (PBS; Sigma-Aldrich) for 3 min each,
they were incubated with 10% normal goat serum (Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.) to block the non-specific
binding. Then, the sections were incubated with the anti-human
TPST-1 (1:150 dilution), and c-Met (1:500 dilution) monoclonal
antibodies at 4°C overnight. After the sections were washed with
PBS, the secondary antibody, peroxidase-conjugated goat
anti-rabbit/mouse immunoglobulin G (no. K5007; Bottle A; Dako REAL™
EnVision™; Dako, Glostrup, Denmark) was applied for 15 min. The
peroxidase reaction was developed for 3 min at room temperature
with 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich) with
0.03% hydrogen peroxide. Counterstaining was performed with Mayer's
hematoxylin. The negative control was prepared with omission of the
primary antibody and the use of normal serum instead of the primary
antibody.
Evaluation of immunostaining
Two independent pathologists who were blinded to the
clinical data evaluated the staining results. Any cases where
inter-observer discrepancy occurred were reviewed at the
double-head microscope (Olympus, Tokyo, Japan), and an agreement
was reached. Immunochemical scoring was evaluated in a
semi-quantitative fashion according to the similar method described
by Jiang et al (23). It
was based on the percentage of positive cells and the intensity of
staining in their cytoplasm in five randomly visual fields under
the optical microscope. The intensity of staining was scored as
follows: 0, without stain; 1, straw yellow; 2, brown; and 3, dark
brown. According to the percentage of tumor cells stained positive,
the extent of staining was scored as follows: 0, ≤5%; 1, 6–25%; 2,
26–50%; 3, 51–75%; and 4, >75%. The product of the intensity and
extent of staining yielded final scores: ≤1, negative; 2–3, weakly
positive (1+); 4–5, moderately positive (2+); and ≥6, strongly
positive (3+).
Statistical analyses
The data were processed and statistically analyzed
using SPSS for Windows XP (Version 13.0; SPSS, Inc., Chicago, IL,
USA). The significance of the association between
immunohistochemical expression and clinical variables was evaluated
by using the χ2 test or Fisher's exact test, as
appropriate. Spearman's rank correlation analysis was used to
analyze the association between TPST-1, and c-Met expression
levels. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients′ demographic data
A total of 50 patients (16 women, 34men) with
stageI–IV lung cancer were enrolledin the present study. The mean
age of the patients was 59.84±9.59 years (mean ± standard
deviation; range, 18–77 years). Among the 50 patients with lung
cancer, 9 (18%) were stage I, 11 (22%) were stage II, 23 (46%) were
stage III and 7 (14%) were stage IV All patient characteristics are
summarized in Table I.
 | Table IDemographic characteristicsof the lung
cancer patients. |
Table I
Demographic characteristicsof the lung
cancer patients.
| Parameter | Patients (n) | % |
|---|
| Mean age (years) | 50 | 59.84±9.59 |
| Gender | | |
| Female | 16 | 32 |
| Male | 34 | 68 |
| Histological
type | | |
| Squamous | 20 | 40 |
| Adeno | 25 | 50 |
| Other | 5 | 10 |
| Differentiation | | |
| Well | 10 | 20 |
| Moderate | 27 | 54 |
| Poor or
undifferentiated | 13 | 26 |
| TNM stage | | |
| I | 9 | 18 |
| II | 11 | 22 |
| III | 23 | 46 |
| IV | 7 | 14 |
| Lymph node
metastasis | | |
| Yes | 27 | 54 |
| No | 23 | 46 |
| Surgery | | |
|
Lobectomy/pneumonectomy | 43 | 86 |
| Biospy | 7 | 14 |
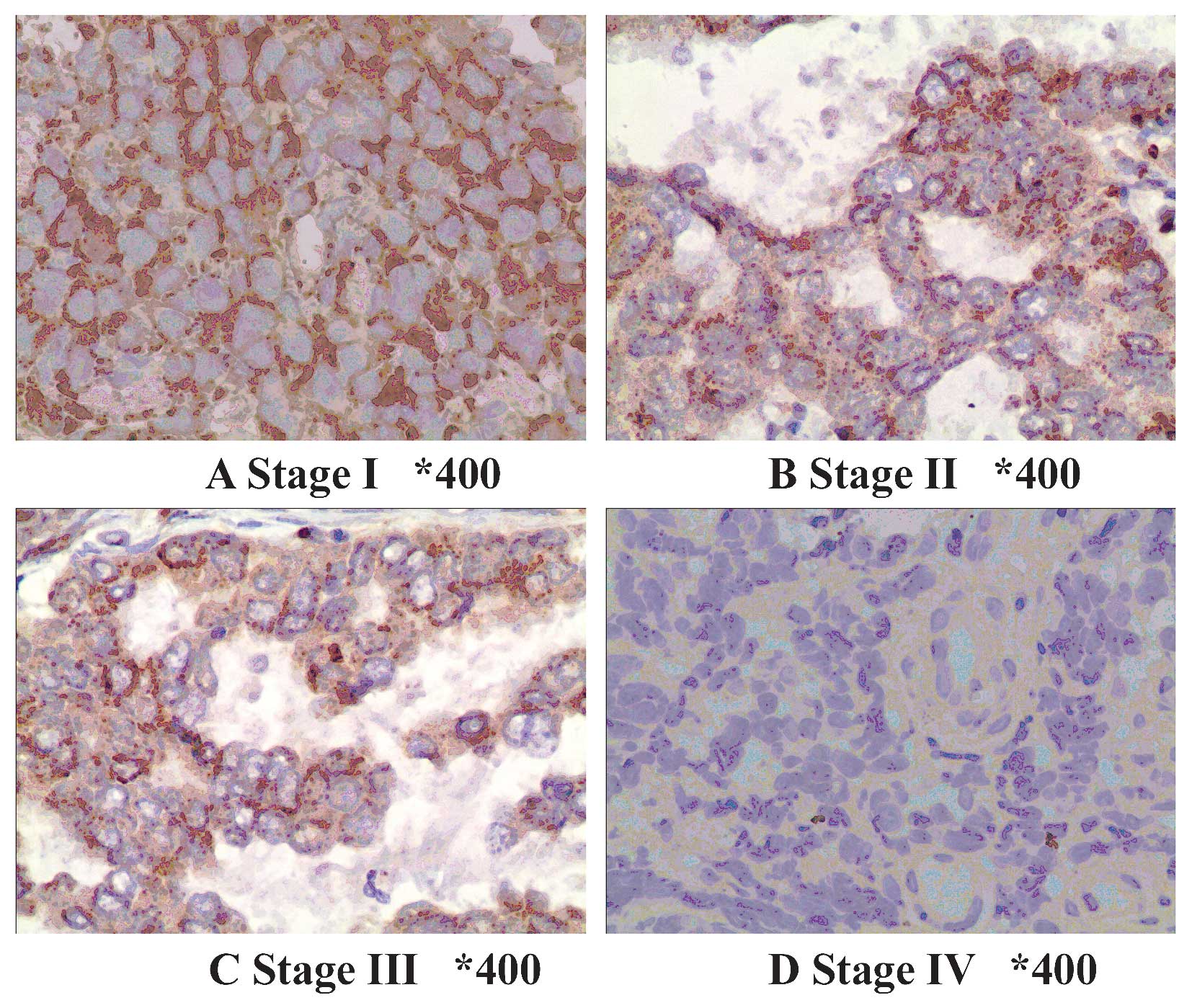
Expression of TPST-1 is decreased in lung
cancer tissues
Positive expression of TPST-1 was identified as a
brownish yellow stain in the cytoplasm of lung cancer cells
(Fig. 1). Immunohistochemical
analysis demonstrated that TPST-1 was expressed in all matched
control lung tissues (100%) and in 30 out of 50 (60%) tumor
tissues. The expression of TPST-1 in tumor tissues appeared to be
significantly lower than that in matched control lung tissues
(P=0.001). Table II shows the
results of the expression of TPST-1 in the tumor group and the
control group.
 | Table IIExpression of tyrosylprotein
sulfotransferase in the tumor group and in the control group. |
Table II
Expression of tyrosylprotein
sulfotransferase in the tumor group and in the control group.
| Group | Positive, n
(%) | Negative, n
(%) |
|---|
| Tumor | 30 (60.0) | 20 (40.0) |
| Control | 50 (100.0) | 0 (0.0) |
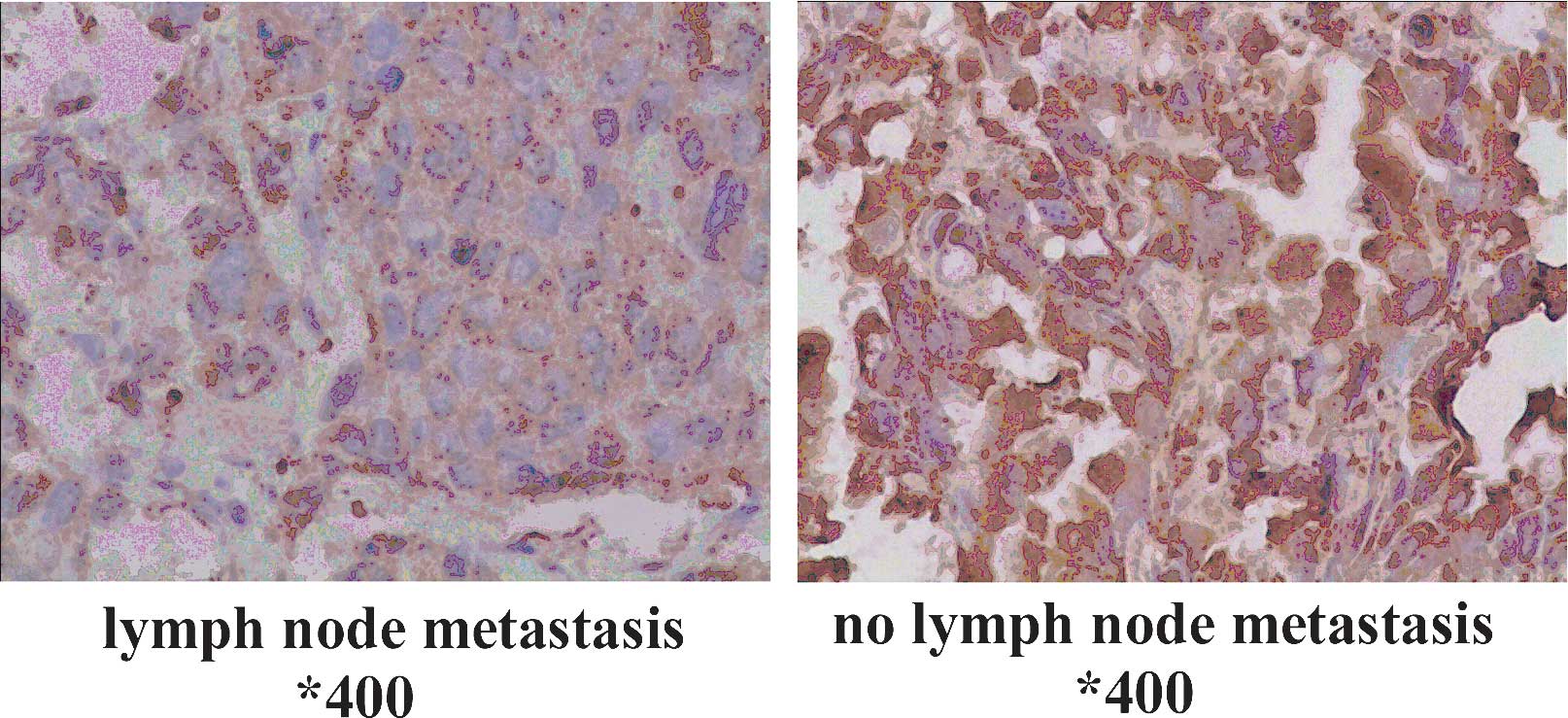
TPST-1 expression is associated with
clinical tumor stage, TNM stage and lymph node metastasis
The association of clinicopathologic characteristics
with TPST-1 expression in lung cancer tissues is summarized in
Table III. The expression of
TPST-1 in clinical stage IV cases was lower than that in clinical
stage I–II cases (Fig. 2). In
addition, TPST-1 expression was highly associated with the TNM
stage (P=0.002) and lymph node metastasis (P<0.001) (Fig. 3). However, statistical analysis
revealed no significant correlations between the expression of
TPST-1 and the histological type or tumor differentiation.
 | Table IIIAssociation between TPST-1 expression
and clinicopathologic variables. |
Table III
Association between TPST-1 expression
and clinicopathologic variables.
| Variable | Patients, n
(%) | TPST-1 expression
| P-value |
|---|
| Positive, n
(%) | Negative, n
(%) |
|---|
| Histological
type | | | | |
| Squamous | 20 (40) | 13 (65.0) | 7 (35.0) | |
| Adeno | 25 (50) | 14 (56.0) | 11 (44.0) | 0.913 |
| Other | 5(10) | 3 (60.0) | 2 (40.0) | |
|
Differentiation | | | | |
| Well | 10 (20) | 6 (60.0) | 4 (40.0) | |
| Moderate | 27 (54) | 17 (63.0) | 10 (37.0) | 0.925 |
| Poor or
undifferentiated | 13 (26) | 7 (53.8) | 6 (46.2) | |
| TNM stage | | | | |
| I | 9(18) | 7 (77.8) | 2 (22.2) | |
| II | 11 (22) | 9(81.8) | 2 (18.2) | 0.002 |
| III | 23 (46) | 14 (60.9) | 9(39.1) | |
| IV | 7(14) | 0(0) | 7 (100) | |
| Lymph node
metastasis | | | | |
| Yes | 27 (54) | 10 (37.0) | 17 (63.0) | <0.001 |
| No | 23 (46) | 20 (87.0) | 3 (13.0) | |
TPST-1 expression is inversely correlated
with c-Met expression in lung cancer tissues
The present study further investigated the
association between TPST-1 and c-Met expression in tumor tissues by
immunohistochemical scoring. A significant association between
TPST-1 and c-Met expression levels was identified (r=-0.470,
P=0.001) (Fig. 4). Table IV shows the negative association
between TPST-1 expression and c-Met expression in tumor
tissues.
 | Table IVAssociation of TPST-1 expression with
c-Met expression in patients with lung cancer [n (%)]. |
Table IV
Association of TPST-1 expression with
c-Met expression in patients with lung cancer [n (%)].
| c-Met
expression | TPST-1 expression
| r | P-value |
|---|
| Negative | Positive (1+) | Positive (2+) | Positive (3+) |
|---|
| Negative | 2 (16.7) | 1 (8.3) | 2 (16.7) | 7 (58.3) | -0.470 | 0.001 |
| Positive (1+) | 5(41.7) | 1 (8.3) | 2 (16.7) | 4 (33.3) | | |
| Positive (2+) | 5 (45.5) | 3 (27.3) | 2 (18.2) | 1 (9.1) | | |
| Positive (3+) | 8 (53.3) | 6 (40.0) | 1 (6.7) | 0 (0.0) | | |
Discussion
Individual patients with lung cancer respond
differently to chemotherapy and have different survival rates. This
variability is associated with the histological lung cancer
sub-type and its individual biological characteristics (24). Therefore, it is important to
enhance the current knowledge of the pathophysiology and molecular
profiles of the different histological types of lung cancer, thus
allowing for personalization of the available therapies.
TPST is an enzyme responsible for protein tyrosine
sulfation (25), which enhances
protein-protein interactions, thereby having an important
functional role. The present study hypothesized that a change in
tyrosine sulfation of human trypsinogens may alter the risk for
numerous types of disease. This notion was based on the observation
that human trypsinogens undergo post-translational sulfation
modification in peptides and proteins synthesized through the
secretory pathway of most eukaryotes (13). In fact, several studies have proved
that TPST-1 deficiency results in a series of dysfunction. Among
these studies, Westmuckett et al (26) found that TPST deficiency results in
early post-natal pulmonary failure in mice. Another study found
that a loss-of-function mutant of the Arabidopsis TPST displayed a
markedly abnormal phenotype including severely stunted growth and
early senescence (27). In
addition, double knockout of TPST-1 and TPST-2 was found to
severely disrupt the integrity of the retina, resulting in abnormal
disc morphology (28). In summary,
the previous studies demonstrated that protein-tyrosine sulfation
is a key determinant in the development and maintenance of tissue
function.
To the best of our knowledge, there have been no
previous studies regarding TPST-1 expression in lung cancer tissue
and their possible roles in conjunction with the clinical outcome
of lung cancer patients by means of any modalities. The present
study identified TPST-1 expression in all normal control lung
tissues. These data confirmed once again that TPST-1 is expressed
in normal tissues, in line with a study by Mishiro et al
(14). However, the present study
also observed that TPST-1 was expressed in only 30 out of 50 (60%)
tumor tissue samples. It has not yet been determined why TPST-1
expression is reduced in lung cancer. It is possible that tumors
may be caused by insufficient protein-tyrosine sulfation. Of note,
a recently published study on NPC showed that TPST-1 was
up-regulated at the mRNA as well as the protein level in NPC cells,
and this up-regulation was associated with metastasis (18). This result is contrary to the
observation of the present study. This phenomenon may be explained
by the fact that different tumor types have different signal
transduction pathways and different mechanisms. Another possible
explanation is that the authors of the aforementioned study was
performed using NPC cells cultured in vitro, whereas the
present study assessed tumor samples from patients.
The present study observed a tendency toward a lower
expression rate of TPST-1 when the lung cancer TNM stage was
advanced. Furthermore, TPST-1 expression in patients with lymph
node metastasis was significantly lower than that in patients
without lymph node metastasis. All these results demonstrated that
TPST-1 may be a useful prognostic biomarker. Detection of TPST-1
expression in tumor tissue may provide a more exact prognosis for
patients with lung cancer.
C-Met, a high-affinity receptor for hepatocyte
growth factor, is usually considered as an oncogene (29). Abnormalities regarding C-Met,
including protein overexpression, gene mutation and gene
amplification frequently occur in cancer (30). The overexpression of c-Met has been
detected in several types of human cancer, including liver, lung,
colorectal, stomach, and colon cancer (31–35),
and is usually associated with poor outcome. Consistent with these
studies, the present study also found that c-Met protein was
overexpressed in lung cancer tissues. Furthermore, TPST-1 was
significantly negatively correlated with c-Met expression in lung
cancer. In addition to the other results of the present study, this
result implied that TPST-1 may be a prognostic biomarker in lung
cancer.
It should be noted that the present study has
several limitations. First, the sample size was limited; further
studies with larger samples may provide a more accurate prediction.
Second, the expression of TPST-1 was examined only by
immunohistochemistry, and no molecular studies or RNA analyses of
TPST-1 were conducted. Finally, without a survival analysis, it was
not possible to determine whether TPST-1 was an independent
predictor of survival. Further studies are required to clarify the
impact of TPST-1 expression on the survival of patients with lung
cancer.
In conclusion, the expression of TPST-1 in lung
cancer tumor tissues was significantly reduced compared to that in
matched control lung tissues. TPST-1 expression was associated with
lung cancer TNM stage and lymph node metastasis. The results of the
present study also showed that TPST-1 was significantly negatively
correlated with c-Met expression in lung cancer, suggesting that it
may be a prognostic biomarker for lung cancer.
Abbreviations:
|
TPST
|
tyrosylprotein sulfotransferase
|
Acknowledgments
This work was supported by the National Natural
Scientific Foundation (grant no. 81472774) and the Research
Programme of the Science and Technology Department of Hunan
Province (grant nos. 2012FJ4076 and 2012TT2011).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: Globocan 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamangar F and Dores GM: Defining
priorities to reduce cancer disparities in different geographic
regions of the world. J Clin Oncol. 24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scagliotti GV, De Marinis F, Rinaldi M,
Crinò L, Gridelli C, Ricci S, Matano E, Boni C, Marangolo M, Failla
G, et al: Phase III randomized trial comparing three platinum-based
doublets in advanced non-small-cell lung cancer. J Clin Oncol.
20:4285–4291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paik PK, Johnson ML, D'Angelo SP, Sima CS,
Ang D, Dogan S, Miller VA, Ladanyi M, Kris MG and Riely GJ: Driver
mutations determine survival in smokers and never-smokers with
stage IIIB/IV lung adenocarcinomas. Cancer. 118:5840–5847. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
An SJ, Chen ZH, Su J, Zhang XC, Zhong WZ,
Yang JJ, Zhou Q, Yang XN, Huang L, Guan JL, et al: Identification
of enriched driver gene alterations in subgroups of non-small cell
lung cancer patients based on histology and smoking status. PLoS
One. 7:e401092012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beau-Faller M, Ruppert AM, Voegeli AC,
Neuville A, Meyer N, Guerin E, Legrain M, Mennecier B, Wihlm JM,
Massard G, et al: MET gene copy number in non-small cell lung
cancer: Molecular analysis in a targeted tyrosine kinase inhibitor
naive cohort. J Thorac Oncol. 3:331–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bearz A, Passalacqua R, Alabiso O, Cinieri
S, Gridelli C, Cravesana C and Crinò L: First-line
bevacizumab-based therapy in advanced non-squamous non-small-cell
lung cancer: Analysis of the Italian patients enrolled in the SAiL
study. Clin Drug Investig. 32:755–760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee RW and Huttner WB: Tyrosine-O-sulfated
proteins of PC12 pheochromocytoma cells and their sulfation by a
tyrosylprotein sulfotransferase. J Biol Chem. 258:11326–11334.
1983.PubMed/NCBI
|
|
11
|
Ouyang YB and Moore KL: Molecular cloning
and expression of human and mouse tyrosylprotein sulfotransferase-2
and a tyrosylprotein sulfotransferase homologue in Caenorhabditis
elegans. J Biol Chem. 273:24770–24774. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beisswanger R, Corbeil D, Vannier C,
Thiele C, Dohrmann U, Kellner R, Ashman K, Niehrs C and Huttner WB:
Existence of distinct tyrosylprotein sulfotransferase genes:
Molecular characterization of tyrosylprotein sulfotransferase-2.
Proc Natl Acad Sci USA. 95:11134–11139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moore KL: The biology and enzymology of
protein tyrosine O-sulfation. J Biol Chem. 278:24243–24246. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mishiro E, Sakakibara Y, Liu MC and Suiko
M: Differential enzymatic characteristics and tissue-specific
expression of human TPST-1 and TPST-2. J Biochem. 140:731–737.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao H, Langerod A, Ji Y, Nowels KW and
Nesland JM: Different gene expression patterns in invasive lobular
and ductal carcinomas of the breast. Mol Biol Cell. 15:2523–2536.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toruner GA, Ulger C, Alkan M, Galante AT,
Rinaggio J, Wilk R, Tian B, Soteropoulos P, Hameed MR, Schwalb MN
and Dermody JJ: Association between gene expression profile and
tumor invasion in oral squamous cell carcinoma. Cancer Genet
Cytogenet. 154:27–35. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barretina J, Taylor BS, Banerji S, Ramos
AH, Lagos-Quintana M, Decarolis PL, Shah K, Socci ND, Weir BA, Ho
A, et al: Subtype-specific genomic alterations define new targets
for soft-tissue sarcoma therapy. Nat Genet. 42:715–721. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu J, Deng X, Tang M, Li L, Xiao L, Yang
L, Zhong J, Bode AM, Dong Z, Tao Y and Cao Y: Tyrosylprotein
sulfotransferase-1 and tyrosine sulfation of chemokine receptor 4
are induced by Epstein-Barr virus encoded latent membrane protein 1
and associated with the metastatic potential of human
nasopharyngeal carcinoma. PLoS One. 8:e561142013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Landi L, Minuti G, D'Incecco A and
Cappuzzo F: Targeting c-MET in the battle against advanced
nonsmall-cell lung cancer. Curr Opin Oncol. 25:130–136. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Renzo MF, Olivero M, Ferro S, Prat M,
Bongarzone I, Pilotti S, Belfiore A, Costantino A, Vigneri R and
Pierotti MA: Overexpression of the c-MET/HGF receptor gene in human
thyroid carcinomas. Oncogene. 7:2549–2553. 1992.PubMed/NCBI
|
|
21
|
Natali PG, Nicotra MR, Di Renzo MF, Prat
M, Bigotti A, Cavaliere R and Comoglio PM: Expression of the
c-Met/HGF receptor in human melanocytic neoplasms: Demonstration of
the relationship to malignant melanoma tumour progression. Br J
Cancer. 68:746–750. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rami-Porta R, Crowley JJ and Goldstraw P:
The revised TNM staging system for lung cancer. Ann Thorac
Cardiovasc Surg. 15:4–9. 2009.PubMed/NCBI
|
|
23
|
Jiang J, Zhu Y, Wu C, Shen Y, Wei W, Chen
L, Zheng X, Sun J, Lu B and Zhang X: Tumor expression of B7-H4
predicts poor survival of patients suffering from gastric cancer.
Cancer Immunol Immunother. 59:1707–1714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamamoto J, Soejima K, Yoda S, Naoki K,
Nakayama S, Satomi R, Terai H, Ikemura S, Sato T, Yasuda H, et al:
Identification of microRNAs differentially expressed between lung
squamous cell carcinoma and lung adenocarcinoma. Mol Med Rep.
8:456–462. 2013.PubMed/NCBI
|
|
25
|
Mishiro E, Liu MY, Sakakibara Y, Suiko M
and Liu MC: Zebrafish tyrosylprotein sulfotransferase: Molecular
cloning, expression and functional characterization. Biochem Cell
Biol. 82:295–303. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Westmuckett AD, Hoffhines AJ, Borghei A
and Moore KL: Early postnatal pulmonary failure and primary
hypothyroidism in mice with combined TPST-1 and TPST-2 deficiency.
Gen Comp Endocrinol. 156:145–153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Komori R, Amano Y, Ogawa-Ohnishi M and
Matsubayashi Y: Identification of tyrosylprotein sulfotransferase
in Arabidopsis. Proc Natl Acad Sci USA. 106:15067–15072. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sherry DM: Lack of protein-tyrosine
sulfation disrupts photoreceptor outer segment morphogenesis,
retinal function and retinal anatomy. Eur J Neurosci. 32:1461–1472.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cooper CS, Park M, Blair DG, Tainsky MA,
Huebner K, Croce CM and Vande Woude GF: Molecular cloning of a new
transforming gene from a chemically transformed human cell line.
Nature. 311:29–33. 1984. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamamoto S, Tsuda H, Miyai K, Takano M,
Tamai S and Matsubara O: Gene amplification and protein
overexpression of MET are common events in ovarian clear-cell
adenocarcinoma: Their roles in tumor progression and
prognostication of the patient. Mod Pathol. 24:1146–1155. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun YL, Liu WD, Ma GY, Gao DW, Jiang YZ,
Liu Q and Du JJ: Expression of HGF and Met in human tissues of
colorectal cancers: Biological and clinical implications for
synchronous liver metastasis. Int J Med Sci. 10:548–559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Siemens H, Neumann J, Jackstadt R,
Mansmann U, Horst D, Kirchner T and Hermeking H: Detection of
miR-34a promoter methylation in combination with elevated
expression of c-Met and β-catenin predicts distant metastasis of
colon cancer. Clin Cancer Res. 19:710–720. 2013. View Article : Google Scholar
|
|
33
|
Patil MA, Lee SA, Macias E, Lam ET, Xu C,
Jones KD, Ho C, Rodriguez-Puebla M and Chen X: Role of cyclin DI as
a mediator of c-Met-and beta-catenin-induced hepatocarcinogenesis.
Cancer Res. 69:253–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jardim DL, de Melo Gagliato D, Falchook
GS, Janku F, Zinner R, Wheler JJ, Subbiah V, Piha-Paul SA, Fu S,
Murphy MB, et al: MET aberrations and c-MET inhibitors in patients
with gastric and esophageal cancers in a phase I unit. Oncotarget.
5:1837–1845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun W, Ai T, Gao Y, Zhang Y, Cui J and
Song L: Expression and prognostic relevance of MET and phospho-BAD
in non-small cell lung cancer. Onco Targets. 6:1315–1323. 2013.
|