Introduction
Biomedical materials, including indwelling
catheters, artificial joint replacements, heart valve replacements
and tracheal intubation tubes, have been widely used in clinical
practice, however, biomaterial-associated infection rates have
increased each year, predominantly due to the formation of
bacterial biofilm (BF) on the surface of the biomaterial (1,2).
Bacterial BF is a complex membrane structure comprising bacterial
adhering to a solid surface. The BF can release planktonic bacteria
constantly, resulting in repeated infections and novel BF
formation. Following the formation of extracellular polymer
secretion, drug resistance becomes 10-1,000 times higher, compared
with that of free-floating bacteria (3). Several human bacterial infections are
associated with BF, and antimicrobial therapy is often ineffective
against BF (4). Therefore, it is
essential to identify safe and effective agents for use against BF
formation.
Escherichia coli (E. coli) is a common
pathogen of urinary, respiratory and digestive system infections,
and a frequent conditional pathogen of nosocomial infections
(5). E. coli can attach to
medical devices, including catheters or endotracheal intubation
tubes, to cause associated chronic infections, and can also form
BFs in bladder epithelial cells, resulting in persistent infection
of the urinary tract (6,7). Therefore, how the formation of E.
coli BFs can be inhibited and the associated infections reduced
is an urgent clinical problem, which requires resolution. Previous
studies have revealed that numerous active sulfhydryl-containing
compounds can inhibit BF formation. For example,
N-acetylcysteine exhibits high potency in suppressing the BF
formation of Pseudomonas aeruginosa, E. coli and
Staphylococcus epidermidis (8–10),
and dithiothreitol and β-mercaptoethanol can inhibit the BF
formation of Staphylococcus aureus (11). In addition, certain widely used
clinical mucolytic agents exhibit BF inhibitory capabilities. For
example, our previous studies demonstrated that ambroxol, a
secretolytic agent used in the treatment of respiratory diseases,
effectively inhibits the BF formation of Pseudomonas
aeruginosa (12,13). The synthetic, small molecule
compound, 2-mercaptoethane sulfonate (MESNA) is commonly used as a
chemotherapeutic drug for hemorrhagic cystitis caused by
cyclophosphamide and ifosfamide (14). MESNA is considered to be a
clinically important mucolytic (15) and reducing agent (16). Its active sulfhydryl group (-SH)
can form two disulfide polypeptide chains during sputum
glycoprotein fracture, causing the decomposition and liquefaction
of mucus glycoprotein, and DNA breakage. In addition, the mucolytic
effect of MESNA has been reported to be more marked, compared with
than that of N-acetylcysteine (17). Although certain antibacterial
peptides and nanomaterials are also potent inhibitors of BF
formation (18,19), the feasibility of their use in the
majority of countries, particularly in developing countries, is
limited due to its high cost, compared with MESNA (20). MESNA is used as a safe medicinal
drug in the urinary system (14,16),
with high concentrations in the urine via either intravenous
injection or oral administration (21). It may be a potential reagent for
the treatment of urinary infection with E. coli BF, however,
the effects of MESNA on the inhibition of E. coli BF
formation remains to be elucidated.
Extracellular polysaccharides (EPS) and
extracellular proteins are the major components of E. coli
extracellular polymers, which can affect bacterial adhesion and are
important in maintaining the conformational space of biological
membranes (22). E. coli
adhesion protein-associated genes include fimH, flu
and papC, and EPS-associated genes include glmS,
glmU, msbB and lpxA. The present study
hypothesized that MESNA may be a potent agent for the inhibition of
E. coli BF formation, therefore, the present study was
designed to systematically investigate the anti-biofilm
capabilities of MESNA at different stages in vitro,
including BF early adhesion, production of EPS and extracellular
proteins, BF formation and destruction, downregulation of the
expression of EPS-associated genes and adhesion protein-associated
genes following MESNA treatment. The findings of these
investigations may support the exploitation of MESNA as a promising
and potent inhibitor against E. coli BF formation.
Materials and methods
Bacterial strains and reagents
E. coli ATCC 25922 was purchased from the
National Institute for Food and Drug Control (Beijing, China). The
bacteria were streaked out on Luria-Bertani agar (Sigma-Aldrich,
St. Louis, MO, USA) from frozen stocks and subsequently inoculated
into Luria-Bertani liquid medium (Sigma-Aldrich, St. Louis, MO,
USA) for growth overnight at 37°C with agitation (250 x g). MESNA
was purchased from Sigma-Aldrich. A LIVE/DEAD® BacLight™
Staining kit containing SYTO®9 and propidium iodide was
purchased from Molecular Probes Life Technologies (Carlsbad, CA,
USA). Phosphate-buffered saline (PBS; pH 7.4) was prepared in our
molecular biology laboratory.
Detection of minimum inhibitory
concentration (MIC)
The MIC of MESNA was determined using a broth
microdilution assay, according to Clinical and Laboratory Standards
Institute Guidelines (23).
Briefly, each well of a 96-well microtiter plate, containing a 100
µl series of MESNA diluted with Mueller-Hintor broth
(Beijing Land Bridge Technology Co., Beijing, China), was
inoculated with 100 µl E. coli ATCC 25922. The final
inoculum size was 5×105 CFU/ml, and the final
concentrations of MESNA were 0.078, 0.156, 0.313, 0.625, 1.250,
2.500, 5.000, 10.000, 20.000 and 40.000 mg/ml. Following incubation
in air at 35°C for 20 h, the wells were inspected for microbial
growth and the MIC was defined as the lowest concentration to
inhibit the growth of bacteria. Positive (bacterial suspension) and
negative (broth) controls were also included.
Early bacterial adhesion assay
When concentration of overnight cultured E.
coli ATCC 25922 reached an optical density (OD)600
of 0.05, measured using a spectrophotometer (UV-1700; Shimadzu
Scientific Instruments, Suzhou, China), 100 µl liquid
bacteria was mixed with 100 µl MESNA at concentrations of
0.0, 0.5, 2.0 or 5.0 mg/ml in each well of sterilized medical
polyvinyl chloride (PVC) plates (Yangzhou Kaier Chemical Co., Ltd.,
Jiangsu, China). Each sample was incubated for 2, 4, 6 or 8 h at
37°C. The PVC plates were rinsed with sterile PBS five times and
sonicated for 20 min to allow the adhesive bacteria to shed.
Suspensions of 0-, 10-, 100-, 1,000-, 10,000-, or 100,000-fold
dilutions were smeared onto solid Luria-Broth medium. Colonies were
counted by visual observation following culture at 37°C for 48 h.
All experiments were repeated six times in duplicate.
Extraction and determination of EPS and
extracellular proteins
The production of extracellular polymeric substances
from the bacterial surfaces was measured, as described previously
(24) following incubation of the
bacteria for 8 h at 37°C, to reach the middle log phase, in MESNA
at concentrations of 0.0, 0.5, 2.0 or 5.0 mg/ml. 10 ml of cultured
cells were centrifuged at 4,000 xg and 4°C for 15 min to obtain a
microbial floc, which was resuspended with distilled water to a
total volume of 10 ml. Subsequently, 12 µl formaldehyde
(37%) was added to the microbial floc, which was maintained at 4°C
for 1 h, followed by the addition of 0.8 ml 1 N NaOH at 4°C for 3
h. The microbial floc solution was centrifuged again at 13.200 xg
at 4°C for 20 min to remove suspended solids prior to chemical
component analysis. The concentrations of EPS and extracellular
proteins in the extracted extracellular polymeric substances were
determined according to phenol-sulfuric acid standard
chromatography and the Bradford reagent method. The polysaccharide
concentration was determined according to the phenol-sulfuric acid
method with glucose as a standard by using chromatography at 490 nm
(25). The protein concentration
was determined using the Bradford reagent with bovine serum albumin
as a standard, for which the dye had an absorption maximum at 595
nm (26).
BF formation assay
To quantify the BF formation of E. coli in
the absence and presence of MESNA, a micro-plate-based assay was
performed, according to a previosly described method (27). Briefly, when the concentration of
overnight-cultured E. coli ATCC 25922 reached a OD600 of
0.05, measured using a spectrophotometer, 2.0 ml liquid bacteria
were added to a 24-well plate with a 1×1 cm silica gel plate
(Shenzhen Hua Feng Li Plastic Products Co., Ltd., Guandong, China).
Subsequently 2.0 ml saline and MESNA solution at concentration of
0.5, 2.0 or 5.0 mg/ml were added to the 24-well plate. All mixtures
were cultured at 37°C, with the medium replaced every 12 h. The
morphology of BF was observed using a S-3400N scanning electron
microscope (SEM; Hitachi, Ltd., Tokyo, Japan) following culture of
the mixtures for 48 h. The numbers of bacteria were counted,
according to the bacterial colony counting method, described
above.
BF destruction assay
Normal saline (0.5 ml) and 2.0 or 5.0 mg/ml MESNA
were added to the E. coli BF in six replicates. Planktonic
bacteria were removed with PBS following incubation of the BF at
37°C for 6 h. Then BF was then stained with SYTO propidium iodide
LIVE/DEAD BacLight 9-bacterial stain, according to the
manufacturer's instructions. The stained BFs were observed under a
confocal laser scanning microscope (LSM 510 META; Zeiss, Wetzlan,
Germany). Signals were recorded using green (excitation 488 nm;
emission 515/30 nm) and red (excitation 568 nm; emission 600/50 nm)
channels. Images were obtained using a layer-by-layer scan along
the Z-axis, with three repeated images for each group, three random
visions for each image and 10–25 layers for each vision.
Three-dimensional reconstruction of the images was then performed
using Amira 5.2.1 software (Visage Imaging, San Diego, CA, USA) to
observe morphological changes in the E. coli BF. Images were
introduced to Image Structure Analyzer (ISA) 3D software, which was
developed by Beyenal et al (28) to estimate structural parameters,
including thickness, textual entropy (TE), areal porosity (AP;
defined as the ratio of void area to the total area) and average
diffusion distance (ADD; defined as the average of the minimum
distance from each cluster pixel to the nearest void pixel over all
clusters).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The overnight-cultured bacteria were diluted in
Luria-Broth medium (1:100) and allowed to grow to an
OD600 of 0.05. MESNA was added to the bacteria at a
final concentration of 0.0, 0.5, 2.0 or 5.0 mg/ml and incubated at
37°C for 2 h. Total RNA was extracted from the bacterial pellet of
5 ml cultured bacteria using A TRIzol® Plus RNA Kit
(Biogenesis, Ltd., Poole, UK), according to manufacturer's
instructions. RT-qPCR was performed using a qRT-PCR kit (Toyobo
Co., Ltd., Tokyo, Japan), according to the manufacturer's
instructions, in a CFX96 Real Time system (Bio-Rad Laboratories,
Hercules, CA, USA) and primers were synthesized by Jie Rui
Industrial, Co., Ltd. (Shanghai, China). The reaction system (20
µl) contained 2X SYBR Green Master Mix (10 µl),
primer solution (0.3 µl), cDNA (2 µl) and
ddH2O (7.4 µl). The reaction procedure involved
incubation at 94°C for 5 min and 40 cycles of 94°C for 30 sec, 57°C
for 30 sec and 72°C for 30 sec. The PCR products were run on a 1.0%
agarose gel. Expression levels of the fimH, flu,
papC, glmS, glmU, msbB and lpxA
genes were detected using RT-qPCR in triplicate. Quantitative
data analysis was completed using an Eppendorf Mastercycler
Real-time PCR system (Biometra GmbH, Goettingen, Germany). The Δ
cycle threshold (CT) was defined as the CT values of each group -
CT values of the corresponding glyceraldehyde 3-phospate
dehydrogenase (Gapdh). The variance of gene expression was
determined using the 2-ΔΔCT equation. The primer
sequences used are listed in Table
I.
 | Table IPrimer sequences used in the reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences used in the reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence
(5′-3′) | Product length
(bp) |
|---|
| Flu | F:
5′-GGCGTTCACCAGGTTCAGGAT-3′ | 148 |
| R:
5′-GGCAACCCTGAAAGTGAAAAACC-3′ | |
| FimH | F:
5′-GGCGGGGTGGCGATTAAAGC-3′ | 188 |
| R:
5′-GAACCAGGGTAGTCCGGCAGAGT-3′ | |
| PapC | F: 5′
-CCGGCCTTTTTCACTGGTTACAC-3′ | 267 |
| R: 5′
-GGCGGGCAGGTGGTGACAAAT-3′ | |
| GlmS | F:
5′-CTGGCTGGCCTGCGTCTGTC-3′ | 186 |
| R:
5′-CGCCACCAGCATCAACAGCACA-3′ | |
| GlmU | F:
5′-TTTGCGTCCTGGTGCTGAGTTG-3′ | 268 |
| R:
5′-GGTCGCGCCTTTGCCTACTGTTA-3′ | |
| MsbB | F:
5′-TGGCCGAATTTCAATAGTCAGGA-3′ | 254 |
| R:
5′-TCGGGGGGCGCTTACATG-3′ | |
| IpxA | F:
5′-GCGAACCGACCCGTGTGG-3′ | 173 |
| R:
5′-GGCGAGAATACAGCGGTTACCT-3′ | |
| GAPDH | F:
5′-ACGCCAGCGACAAACTAATCA-3′ | 192 |
| R:
5′-CCCCCGCTGCTCTCGATTTAC-3′ | |
Statistical analysis
All data were analyzed using SPSS v.17.0 (SPSS,
Inc., Chicago, IL, USA). The data are expressed as the mean ±
standard deviation. Comparisons between different groups was
performed using one-way analysis of variance, and multiple
comparisons were performed using homogeneity of variance analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MIC of MESNA towards E. coli
To determinate the MIC of MESNA towards E.
coli, the E. coli cells were incubated with a gradient
of increasing concentrations of MESNA between 0.078 and 40.000
mg/ml (0.078, 0.156, 0.313, 0.625, 1.250, 2.500, 5.000, 10.000,
20.000 and 40.000 mg/ml). The results revealed that MESNA at 10.000
mg/ml completely inhibited the growth of E. coli, indicating
that the MIC value of MESNA towards E. coli ATCC 25922 was
10.000 mg/ml. Therefore, the concentration of MESNA used for
investigating the inhibitory of MESNA in BF formation was <10
mg/ml, as this was sublethal to E. coli.
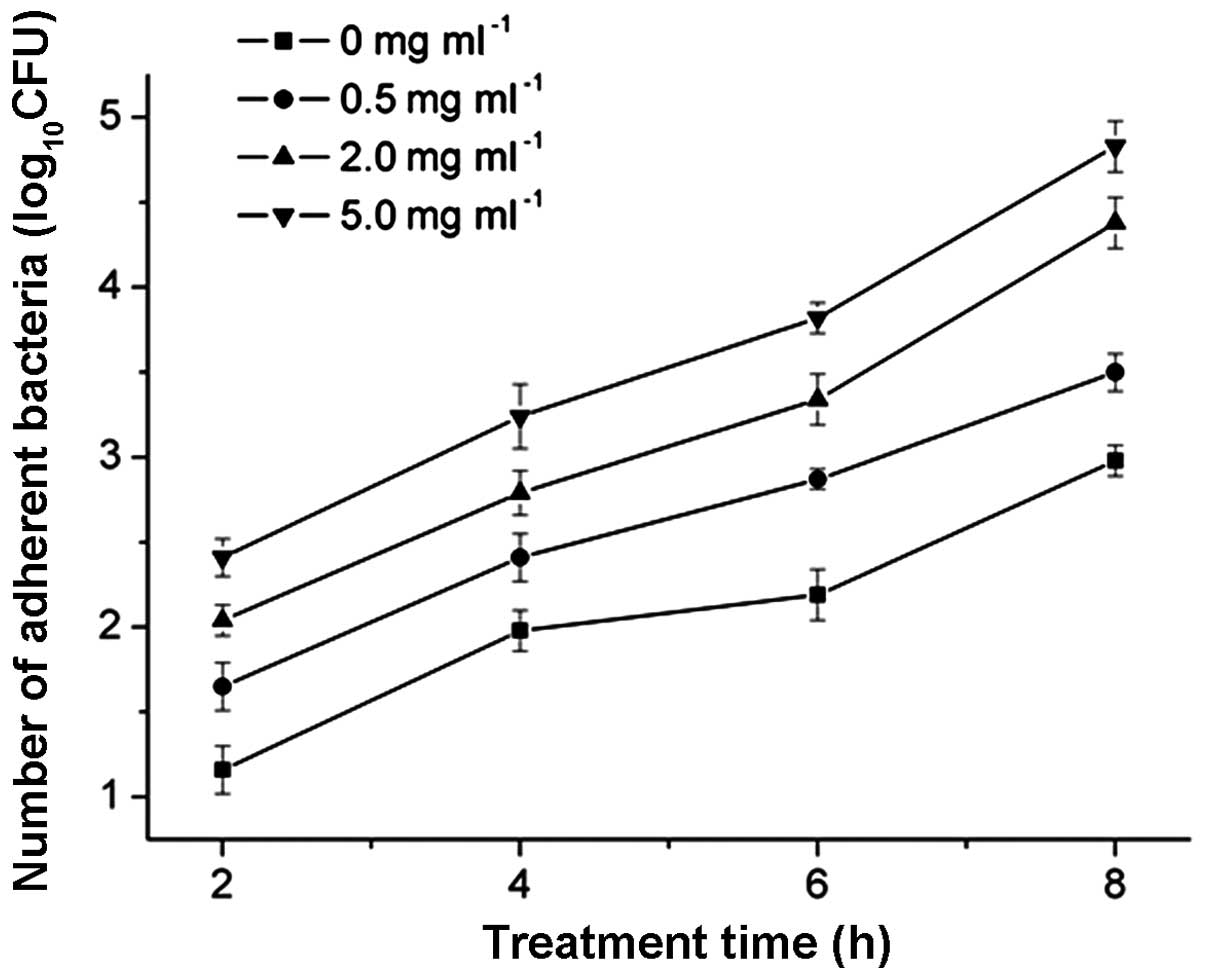
Effects of MESNA on cell adhesion
In the present study, the number of adhering cells
increased with bacterial cell aggregation. MESNA induction caused
cell aggregation in a dose-dependent manner. The inoculum size for
each well was 5×105 CFU/ml. The total quantity of
adhering bacteria treated with 0.5, 2.0 or 5.0 mg/ml of MESNA
increased significantly, compared with the untreated bacteria
(P<0.05; Fig. 1). Compared with
the untreated group, the number of adherent bacteria increased in a
gradients following treatment with 0.5, 2.0 and 5.0 mg/ml MESNA for
2 h (15.35, 31.54 and 51.87%, respectively), 4 h (13.89, 25.62 and
38.89%, respectively) 6 h (12.57, 24.87 and 24.87%, respectively)
and 8 h (9.32, 27.54 and 38.30%, respectively). These results
indicated that the inhibitory effect increased with increasing
concentrations of MESNA.
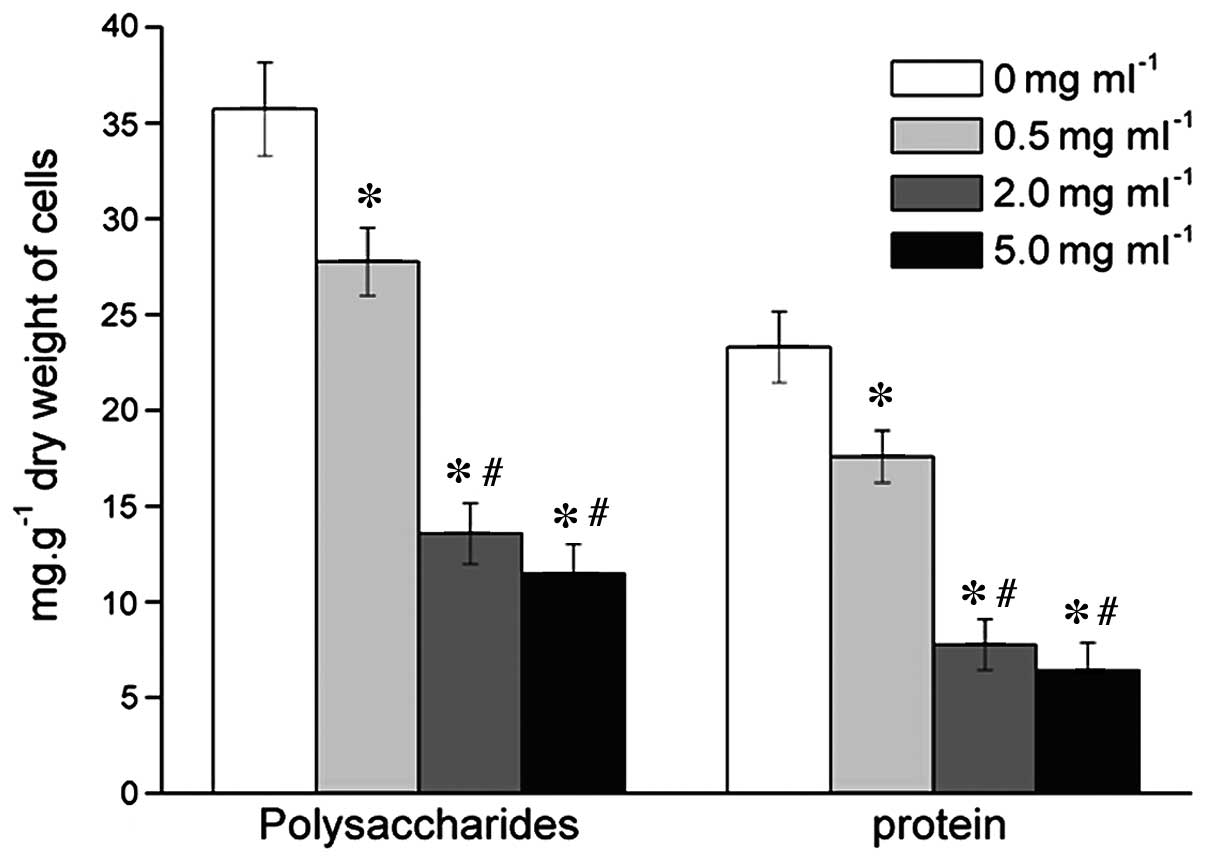
Effects of MESNA on EPS and extracellular
proteins
The EPS of bacteria/bacterial dry weight (g/mg) was
assessed to describe the production of EPS and extracellular
proteins. When the bacteria from the microbial floc were treated
with 0.5, 2.0 or 5.0 mg/ml MESNA, the level of EPS decreased
significantly by 22.28, 62.02 and 67.87%, respectively (Fig. 2); and the level of extracellular
proteins decreased significantly by 24.54, 66.71 and 72.42%,
respectively (Fig. 2), compared
with the untreated group.
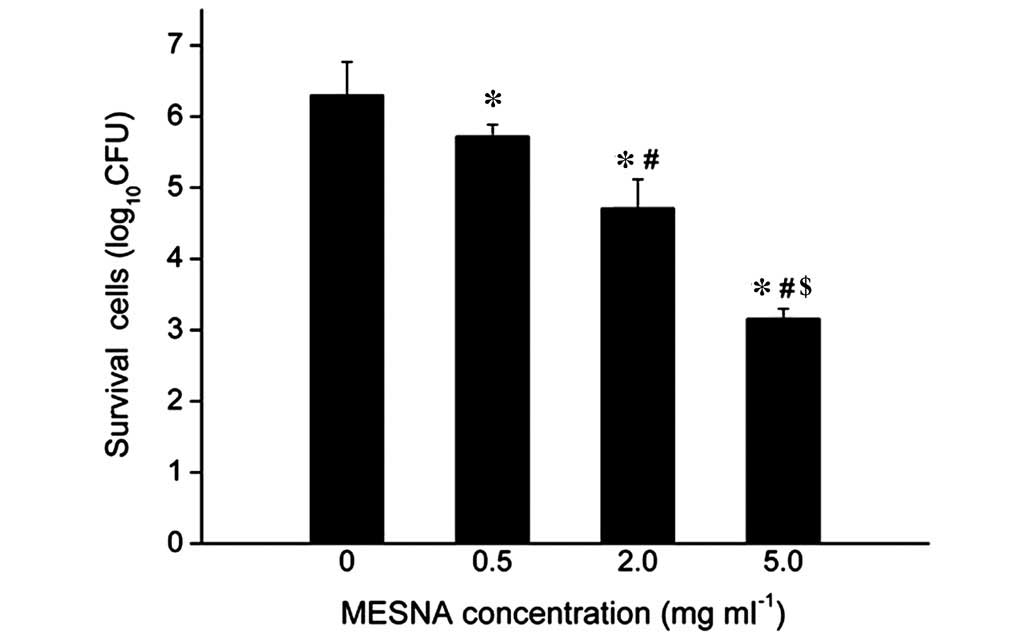
Inhibition of MESNA on E. coli BF
formation
Images of the E. coli BF were captured under
SEM. The BF of the untreated (0.0 mg/ml MESNA) bacteria group was
thicker and denser, with thick and large mucoid materials wrapping
outside (Fig. 3A); the BF of the
0.5 mg/ml MESNA-treated bacteria was thinner and contained fewer
mucus substances (Fig. 3B).
Compared with the untreated or 0.5 mg/ml MESNA-treated groups, the
BF became markedly thinner and the mucus substances were lower in
the 2.0 mg/ml MESNA-treated group (Fig. 3C). The 5.0 mg/ml MESNA-treated
bacteria group was almost scattered with rare myxoid material and
contained no agglomerated bacteria (Fig. 3D). Compared with the untreated
group, the survival of the E. coli decreased by 9.21, 25.24
and 49.84% when treated with 0.5, 2.0 and 5.0 mg/ml MESNA,
respectively (Fig. 4). These
results demonstrated that MESNA exhibited a negative role on E.
coli BF formation, and the survival number of E. coli in
the BF decreased significantly following treatments with increasing
concentrations of MESNA (P<0.05).
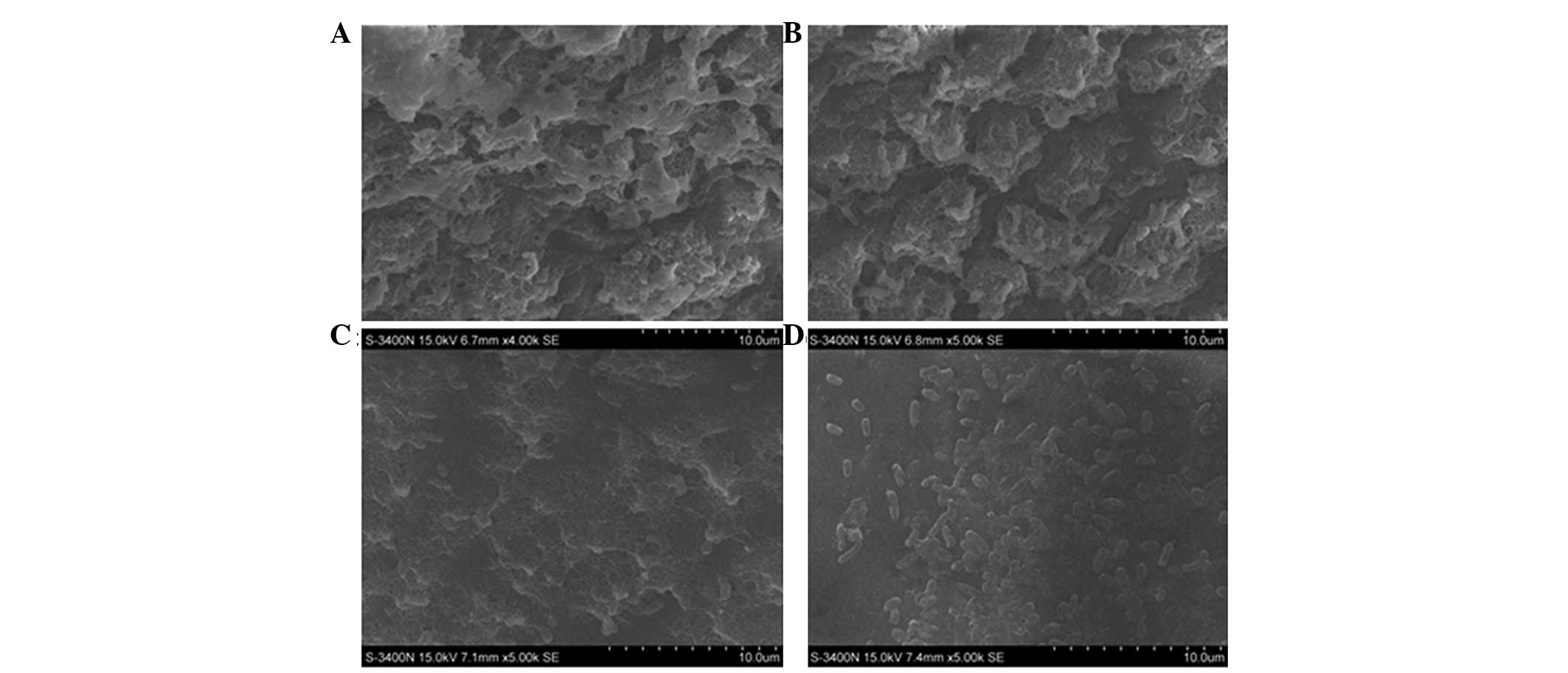 | Figure 3Scanning electron micrograph image of
Escherichia coli ATCC 25922 BF (A) without MESNA
(magnification, x4,000) and with (B) 0.5 mg/ml MESNA
(magnification, x5,000), (C) 2.0 mg/ml MESNA (magnification,
x5,000) and (D) 5.0 mg/ml MESNA (magnification, x5,000). BF,
biofilm; MESNA, 2-mercaptoethane sulfonate. |
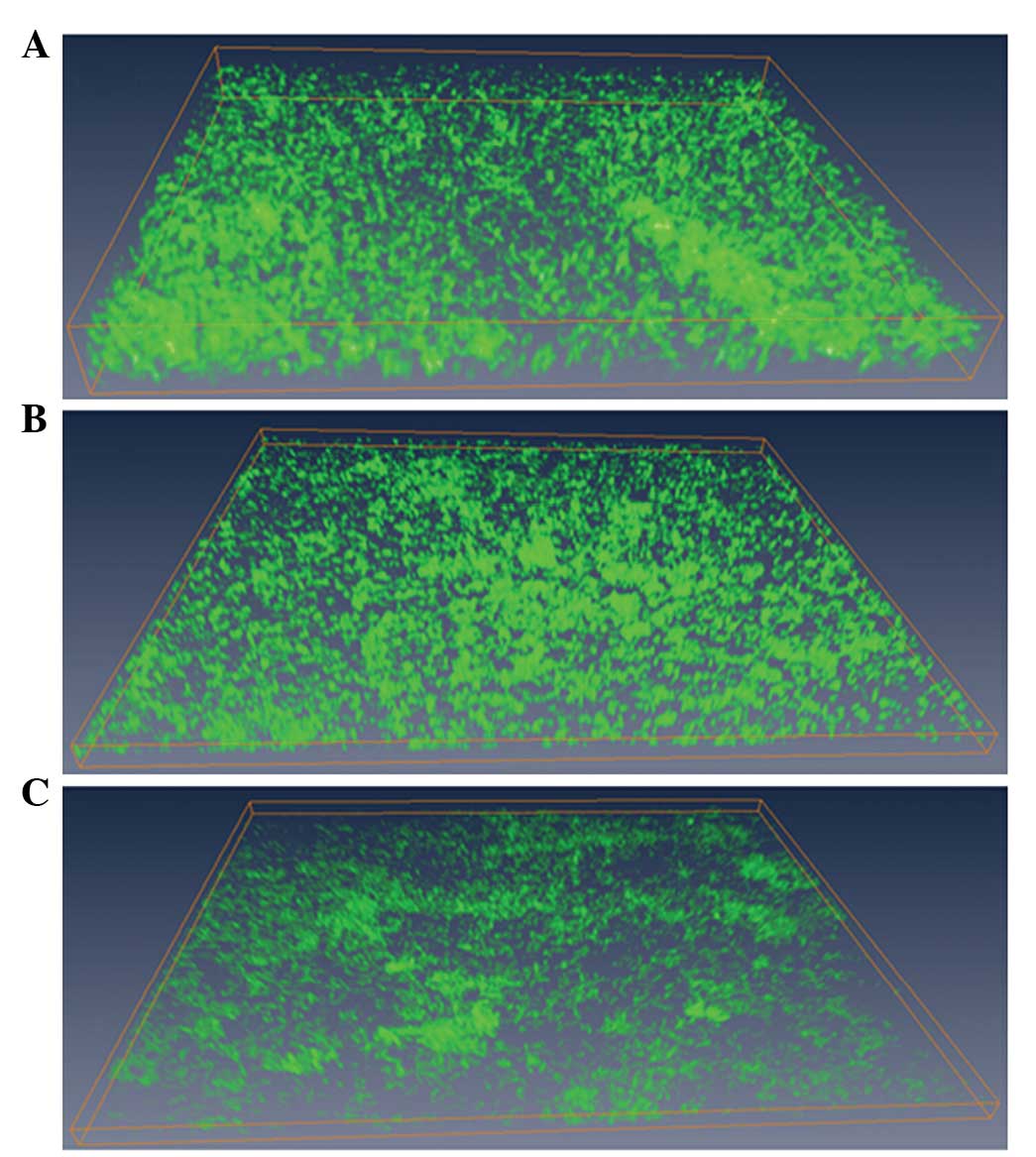
Destructive effect of MESNA on the
matured E. coli BF
The appearance of bacteria in the E. coli BF
were more dense and BF bulges were larger without MESNA treatment
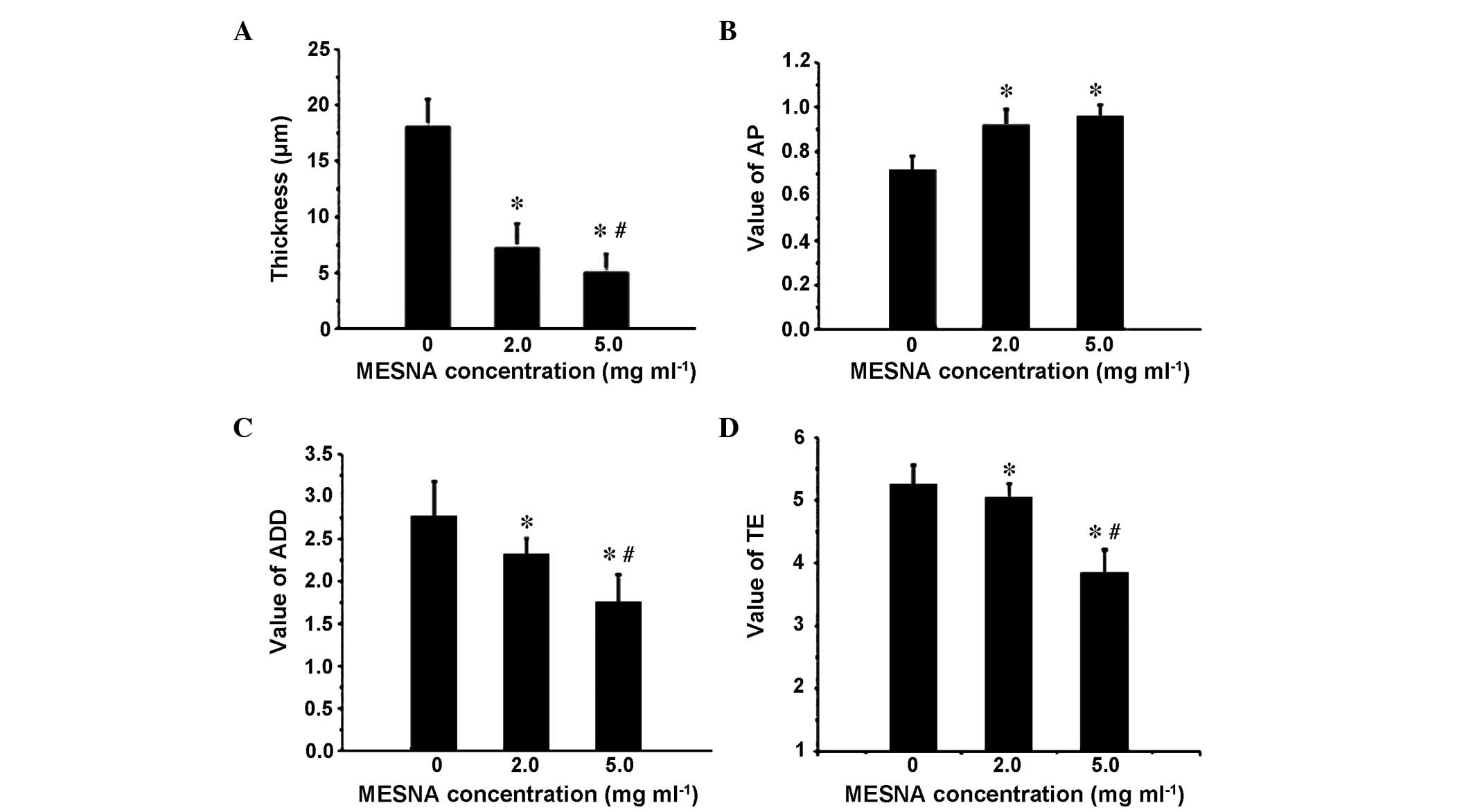
(Fig. 5A). The thickness of the BF
treated with 2.0 mg ml 1 MESNA was decreased (Fig. 5B) and the BF density was more
sparse, compared with the untreated samples. The thickness of the
BF, treated with 5.0 mg/ml MESNA was reduced even further and was
more sparse (Fig. 5C). The ISA
quantitative analysis demonstrated that treatments with either 2.0
or 5.0 mg/ml decreased the thickness of the E. coli BF
Compared with the untreated group (thickness, 18.11±2.43
µm), when the BF was treated with 2.0 mg/ml and 5.0 mg/ml
MESNA, the thickness reduced significantly by 59.97% (7.25±2.15
µm) and 72.0% (5.07±1.62 µm), respectively
(P<0.05; Fig. 6A), and the AP
increased by 27.78 and 33.33%, respectively (P<0.05; Fig. 6B). When the BF was treated with 5.0
mg/ml MESNA, the ADD and TE decreased by 36.69 and 26.81%,
respectively (P<0.05; Fig. 6C and
D).
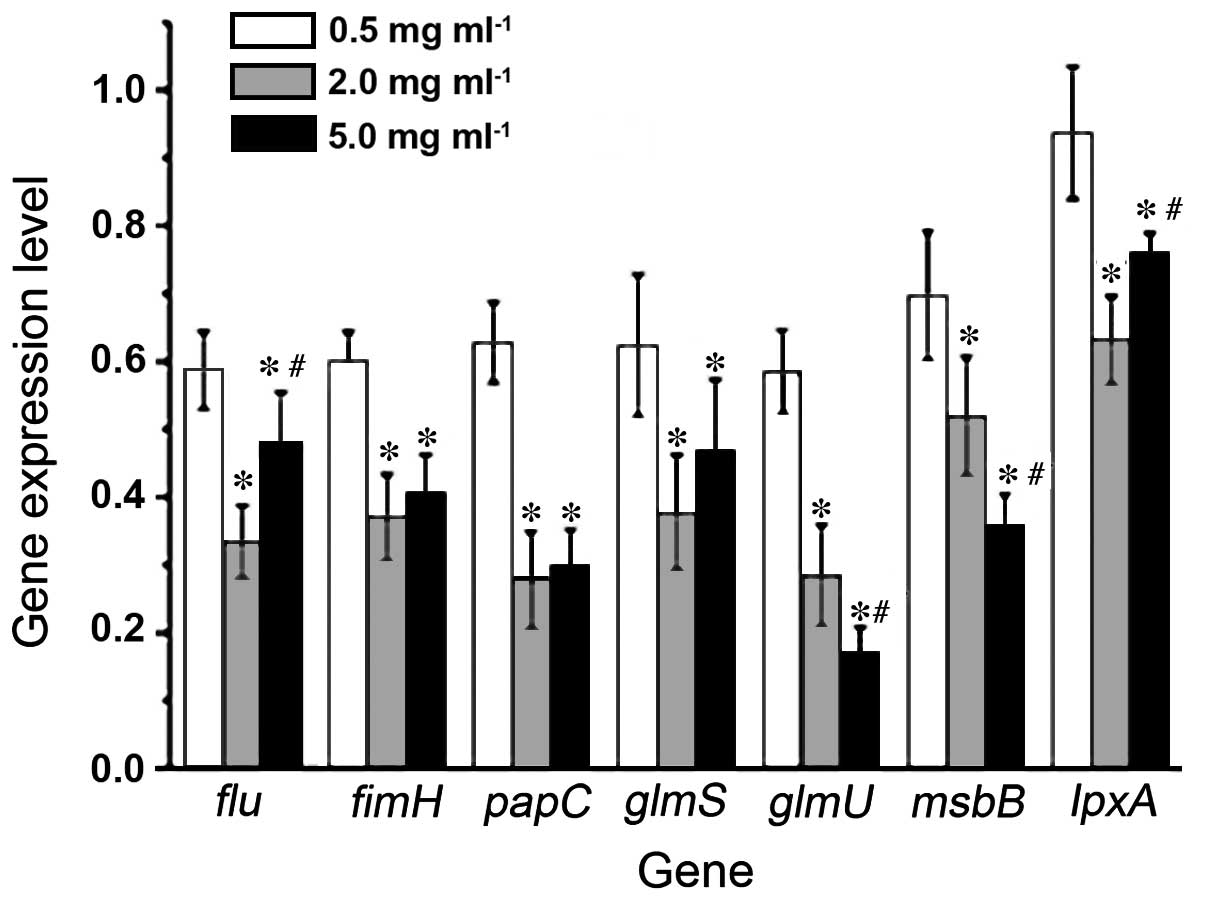
RT-qPCR detection of the expression
levels of FimH, Flu, PapC, GlmS, GlmU, MsbB and LpxA
Following treatment of the E. coli liquid
bacteria with MESNA at concentrations of 0.5, 2.0 and 5.0 mg/ml,
the expression levels of the FimH, Flu, PapC, GlmS, GlmU,
MsbB and LpxA genes, with the exception of lpxA
in the 0.5 mg/ml-treated group, were suppressed (P<0.05),
compared with the mock-treated group. The expression levels of the
majority of genes decreased with MESNA at concentrations of 2.0 and
5.0 mg/ml (Fig. 7).
Discussion
To identify an effective agent to inhibit the
formation of E. coli BF, the present study investigated the
MIC of MESNA and then evaluated the effects of subinhibitory
concentrations of MESNA on the E. coli BF through
observation of its effects on bacterial adhesion, and BF formation
and destruction. The mechanism underlying the action of MESNA on
the inhibition of BF formation was also investigated by detecting
the expression levels of the FimH, Flu and
PapC adhesion protein-associated genes and the GlmS,
GlmU, MsbB and LpxA EPS-associated genes. The
results demonstrated that the MIC to E. coli ATCC 25922 was
10.000 mg/ml, and MESNA at subinhibitory concentrations inhibited
the formation of E. coli BF and destroyed the BF. The
mechanism of the inhibitory effects of MESNA may be that MESNA
inhibited the expression of EPS-associated genes and adhesion
protein-associated genes. In addition MESNA may inhibit the
production of EPS and extracellular proteins by breaking down
disulfide bonds. Notably, in the present study, 2 mg/ml MESNA
reduced the levels of the E. coli BF EPS and extracellular
proteins to 62.02 and 66.7%, respectively, while
N-acetylcysteine at the same concentration has been observed
to reduce the exopolysaccharide matrix of the BF of four E.
coli strains to between 25 and 68% (9), suggesting that MESNA can achieve
similar inhibitory effects on BF formation as
N-acetylcysteine.
The adhering and BF-forming abilities of E.
coli decreased following previous treatment with subinhibitory
concentrations of MESNA (Figs. 1,
3 and 4), and MESNA at subinhibitory
concentrations destroyed the formed BF (Figs. 5 and 6). Quantitative and qualitative CLSM and
ISA3D image analysis were also performed to demonstrate the effect
of MESNA on the obstruction of E. coli BF maturation. The
thickness and ADD of the E. coli BF decreased, while the AP
increased. AP and ADD are two parameters, which can directly
describe the characteristics of BF structure and nutrient supply
(28). The results indicated that
the BF became more sparse, the size of the channels among the
colonies increased and the nutrient supplying distance was
shortened following MESNA treatment. Considering that TE is a
reflection of BF homogeneity index (28), and the BF homogeneity and
uniformity were reduced by MESNA treatmen, it can be concluded that
MESNA may have a destructive effect on maturation of E. coli
BFs.
The expression levels of the fimH, flu
and papC adhesion protein-associated genes and the
glmS, glmU, msbB and lpxA
EPS-associated genes were significantly reduced following MESNA
treatment (Fig. 7). Therefore, the
inhibitory effects of MESNA on the E. coli BF may be through
inhibiting the expression of these genes. The Flu gene
encodes Ag43, which is a transcriptional regulation protein that is
involved in E. coli secretion and mediates the adhesion,
invasion, long-term colonization and formation of BF (29,30).
The expression of Ag43 is predominantly regulated by OxyR. Ulett
et al (29) used the
dithiothreitol reducing agent to reduce E. coli BF
formation, and found that the expression of the Ag43-encoding
Flu gene was reduced. MESNA is not only an important mucus
solvent, but is also a reducing agent, and the present study
hypothesized that the inhibitory effect of MESNA on the expression
of the Flu gene expression is associated with the
competitive or non-competitive inhibition of enzymes containing
sulfhydryl in bacteria. FimH exists in the end of I pilus,
which is the predominant adhesion factor in the type I pili of
E. coli. This bacterium can identify the host cell
surface-mannose through the I pilus to attach. FimH not only
mediates the formation of E. coli BF in vitro, but it
also promotes the formation of BF in cells (7,31).
The present study hypothesized that MESNA may inhibit the
expression of the FimH gene, and thus reduces the production
of certain enzymes associated with the formation of BF. The present
study also demonstrated that MESNA inhibited the expression of the
PapC gene. Notably, the PapC gene is an important
gene encoding P fimbriae and an important virulence factor
contributing to the formation of BF (32). Uridine diphosphate
N-acetylglucosamine (UDP-GlcNac) is the basic material of E.
coli BF polysaccharides (33,34).
NsgB and GlmS are important catalytic enzymes of mutual
transformation in sugar amine metabolism of glucose 6-phosphate and
fructose 6-biphosphate, which is the first and rate-limiting step
of hexaose biosynthesis. GlmU exhibits 1-phosphoglucosamine
acetyltransferase and N-acetyl-glucosamine 1-phosphate uridyl
transferase activity, which is the most important enzyme to
catalyze the formation of UDP-GlcNac. The MsbB-encoding
product mediates peptidoglycan production (35), and the LpxA gene-encoding
product mediates endotoxin formation. Therefore, expression of the
downstream MsbB and LpxA genes are also associated
with the formation of UDP-GlcNac (36). Therefore, MESNA inhibited the
production of various adhesion proteins and EPS to affect the
formation of the E. coli BF.
The present study demonstrated that MESNA exhibited
inhibitory effects on the formation of the E. coli BF in
vitro. These findings offer support for further investigations
of MESNA on the formation of E. coli BF in vivo, and
may eventually lead to either an effective clinical treatment or
cure for E. coli infection with BF formation in the
future.
Acknowledgments
The authors would thank to Professor Wang Ning and
Professor Zhao Kecen at the Southwest Hospital of the Third
Military Medical University (Chongqing, China), the Central
Laboratory of the Experimental Medicine Division of the Southwest
Hospital of The Third Military Medical University, and associate
Professor Ju Lu of the Electron Microscopy Laboratory of the Third
Military Medical University for their assistance with the
experiments. The authors would also like to thank Professor Haluk
Beyenal of American Montana State University (Bozeman, MT, USA) for
providing ISA3D software. This study was supported by the National
Natural Science Foundation of China (grant. nos. 30772363 and
30901279).
References
|
1
|
Arciola CR: New concepts and new weapons
in implant infections. Int J Artif Organs. 32:533–536.
2009.PubMed/NCBI
|
|
2
|
Vasilev K, Cook J and Griesser HJ:
Antibacterial surfaces for biomedical devices. Expert Rev Med
Devices. 6:553–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Høiby N, Bjarnsholt T, Givskov M, Molin S
and Ciofu O: Antibiotic resistance of bacterial biofilms. Int J
Antimicrob Agents. 35:322–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lopes SP, Ceri H, Azevedo NF and Pereira
MO: Antibiotic resistance of mixed biofilms in cystic fibrosis:
Impact of emerging microorganisms on treatment of infection. Int J
Antimicrob Agents. 40:260–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tao XB, Qian LH, Li Y, Wu Q, Ruan JJ, Cai
DZ and Peng H: Hospital-acquired infection rate in a tertiary care
teaching hospital in China: A cross-sectional survey involving 2434
inpatients. Int J Infect Dis. 27:7–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anderson GG, Palermo JJ, Schilling JD,
Roth R, Heuser J and Hultgren SJ: Intracellular bacterial
biofilm-like pods in urinary tract infections. Science.
301:105–107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berry RE, Klumpp DJ and Schaeffer AJ:
Urothelial cultures support intracellular bacterial community
formation by uropathogenic Escherichia coli. Infect Immun.
77:2762–2772. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao T and Liu Y: N-acetylcysteine inhibit
biofilms produced by Pseudomonas aeruginosa. BMC Microbiol.
10:1402010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marchese A, Bozzolasco M, Gualco L, Debbia
EA, Schito GC and Schito AM: Effect of fosfomycin alone and in
combination with N-acetylcysteine on E. coli biofilms. Int J
Antimicrob Agents. 22:95–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leite B, Gomes F, Teixeira P, Souza C,
Pizzolitto E and Oliveira R: Staphylococcus epidermidis biofilms
control by N-acetylcysteine and rifampicin. Am J Ther. 20:322–328.
2013. View Article : Google Scholar
|
|
11
|
Wu X, Wang Y and Tao L: Sulfhydryl
compounds reduce Staphylococcus aureus biofilm formation by
inhibiting PIA biosynthesis. FEMS Microbiol Lett. 316:44–50. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Q, Yu J, Yang X, Wang J, Wang L, Lin Y
and Lin L: Ambroxol interferes with Pseudomonas aeruginosa quorum
sensing. Int J Antimicrob Agents. 36:211–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li F, Wang W, Hu L, Li L and Yu J: Effect
of ambroxol on pneumonia caused by Pseudomonas aeruginosa with
biofilm formation in an endotracheal intubation rat model.
Chemotherapy. 57:173–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kyung YS, Park HY and Lee G: Preservation
of uroplakins by 2-mercaptoethanesulfonate in
cyclophosphamide-induced rat cystitis. Arch Toxicol. 85:51–57.
2011. View Article : Google Scholar
|
|
15
|
Casale M, Di Martino A, Salvinelli F,
Trombetta M and Denaro V: MESNA for chemically assisted tissue
dissection. Expert Opin Investig Drugs. 19:699–707. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ludwig U, Riedel MK, Backes M, Imhof A,
Muche R and Keller F: MESNA (sodium 2-mercaptoethanesulfonate) for
prevention of contrast medium-induced nephrotoxicity-controlled
trial. Clin Nephrol. 75:302–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tekeres M, Horváth A, Bárdosi L and
Kenyeres P: Clinical studies on the mucolytic effect of mesna. Clin
Ther. 4:56–60. 1981.PubMed/NCBI
|
|
18
|
Hou S, Liu Z, Young AW, Mark SL,
Kallenbach NR and Ren D: Effects of Trp- and Arg-containing
antimicrobial-peptide structure on inhibition of Escherichia coli
planktonic growth and biofilm formation. Appl Environ Microbiol.
76:1967–1974. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Evliyaoğlu Y, Kobaner M, Celebi H, Yelsel
K and Doğan A: The efficacy of a novel antibacterial hydroxyapatite
nanoparticle-coated indwelling urinary catheter in preventing
biofilm formation and catheter-associated urinary tract infection
in rabbits. Urol Res. 39:443–449. 2011. View Article : Google Scholar
|
|
20
|
Tran N and Tran PA: Nanomaterial-based
treatments for medical device-associated infections. Chemphyschem.
13:2481–2494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goren MP, Houle JM, Bush DA, Li JT, Newman
CE and Brade WP: Similar bioavailability of single-dose oral and
intravenous mesna in the blood and urine of healthy human subjects.
Clin Cancer Res. 4:2313–2320. 1998.PubMed/NCBI
|
|
22
|
Flemming HC and Wingender J: The biofilm
matrix. Nat Rev Microbiol. 8:623–633. 2010.PubMed/NCBI
|
|
23
|
Wayne PA: Clinical and laboratory
standards institute performance standards for antimicrobial
susceptibility testing: Eighteenth informational supplement. (CLSI
document M100-S18). USA: 2008
|
|
24
|
Wu B, Wang Y, Lee YH, Horst A, Wang Z,
Chen DR, Sureshkumar R and Tang YJ: Comparative eco-toxicities of
nano-ZnO particles under aquatic and aerosol exposure modes.
Environ Sci Technol. 44:1484–1489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saha SK and Brewer CF: Determination of
the concentrations of oligosaccharides, complex type carbohydrates
and glycoproteins using the phenol-sulfuric acid method. Carbohydr
Res. 254:157–167. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Strathmann M, Wingender J and Flemming HC:
Application of fluorescently labelled lectins for the visualization
and biochemical characterization of polysaccharides in biofilms of
Pseudomonas aeruginosa. J Microbiol Methods. 50:237–248. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beyenal H, Donova C, Lewandowski Z and
Harkin G: Three-dimensional biofilm structure quantification. J
Microbiol Methods. 59:395–413. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ulett GC, Valle J, Beloin C, Sherlock O,
Ghigo JM and Schembri MA: Functional analysis of antigen 43 in
uropathogenic Escherichia coli reveals a role in long-term
persistence in the urinary tract. Infect Immun. 75:3233–3244. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Naves P, del Prado G, Huelves L, Gracia M,
Ruiz V, Blanco J, Dahbi G, Blanco M, Ponte Mdel C and Soriano F:
Correlation between virulence factors and in vitro biofilm
formation by Escherichia coli strains. Microb Pathog. 45:86–91.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schembri MA and Klemm P: Biofilm formation
in a hydrodynamic environment by novel fimh variants and
ramifications for virulence. Infect Immun. 69:1322–1328. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tiba MR, Nogueira GP and Leite Dda S:
Study on virulence factors associated with biofilm formation and
phylogenetic groupings in Escherichia coli strains isolated from
patients with cystitis. Rev Soc Bras Med Trop. 42:58–62. 2009.In
Portuguese. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Itoh Y, Rice JD, Goller C, Pannuri A,
Taylor J, Meisner J, Beveridge TJ, Preston JF III and Romeo T:
Roles of pgaABCD genes in synthesis, modification and export of the
Escherichia coli biofilm adhesin polybeta-1,6- N-acetyl-d-gluc
osamine. J Bacteriol. 190:3670–3680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suman E, D'souza SJ, Jacob P, Sushruth MR
and Kotian MS: Anti-biofilm and anti-adherence activity of Glm-U
inhibitors. Indian J Med Sc. 65:387–392. 2011. View Article : Google Scholar
|
|
35
|
Yeom J, Lee Y and Park W: Effects of
non-ionic solute stresses on biofilm formation and
lipopolysaccharide production in Escherichia coli O157H7. Res
Microbiol. 163:258–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ulaganathan V, Buetow L and Hunter WN:
Nucleotide substrate recognition by UDP-N-acetylglucosamine
acyltransferase (LpxA) in the first step of lipid A biosynthesis. J
Mol Biol. 369:305–312. 2007. View Article : Google Scholar : PubMed/NCBI
|