Introduction
Gastric cancer (GC) is one of the most prevalent
types of malignancy worldwide and contributes to the second most
common cause of cancer-associated mortality worldwide (1,2). The
pathogenesis of GC is a multistep process, involving single or
multiple mutations of genes associated with cell proliferation,
invasion and metastasis (3). The
prognosis of patients with GC is poor, with a 5-year overall
survival rate of 28% worldwide (1,2). One
of the key reasons for the poor prognosis of patients with GC is
its character of invasion and metastasis, which has been observed
to occur in >60% of patients at the point of diagnosis (4–6).
Therefore, it's important to understand the molecular basis of GC
invasion and metastasis in order to develop novel therapeutic
strategies for GC (7). The
discovery of microRNAs (miRNAs) is a significant milestone in the
investigation of cancer, including GC (8).
miRNAs are small, non-cording RNAs, 17–25
nucleotides in length, and are involved in the post-transcriptional
regulation of hundreds of target genes. miRNA-protein complexes can
bind to the mRNA of target genes, leading to degradation or
translational inhibition of target genes, which controls a wide
range of biological functions, including cell proliferation,
differentiation and apoptosis (8–10).
miRNAs have been a major focus in studies investigating cancer
(4,7,11,12).
Increasing evidence indicates that miRNAs have paradoxical
functions and act in a cell context-dependent manner (13,14).
The has-miR-34 family is located in Ch1p36, a region
that is frequently deleted in tumors (15). It has been reported that miR-34b/c
is decreased in human non-small cell lung cancer cell lines,
however, miR-34a cannot simply be defined as a tumor suppressor or
oncogene, as paradoxical roles of miR-34a have been observed in
several types of cancer (16). In
human glioma, miR-34a reduces the expression of Notch and acts as a
tumor suppressor (17). The same
effect of miR-34a has been observed in human hepatocellular
carcinoma and GC (18,19). However miR-34a is upregulated in
hepatocellular carcinoma and is associated with
hepatocarcinogenesis (20). A GC
miRNA profile also demonstrates that miR-34a is overexpressed in GC
tissues, compared with normal gastric tissues (21). Therefore, improving current
understanding of miR-34a in GC is important.
The present study investigated miR-34a in clinical
specimens, and in an independent, secondary cohort from The Cancer
Genome Atlas (TCGA), in order to determine the prognostic value of
miR-34a for patients with GC. In an attempt to unravel the
properties of miR-34a, experiments were performed in GC cells.
Furthermore, the downstream target of miR-34a was predicted using a
bioinformatic method, and was verified in GC cells.
Materials and methods
Human tissue samples and cell lines
GC samples were obtained from 40 patients (average
age, 54.5±11.73 years; 25 males and 15 females), with clear
diagnostic information, from the People's Hospital of Guangxi
Zhuang Autonomous Region (Nanning, China). The patients were
recruited between December 2012 and June 2014, and tissue samples
were collected immediately after surgery. Two tissue samples were
obtained from each patient, one sample of GC tissue, and one sample
of adjacent gastric mucosa tissue. Out of the 40, a total of 20
adjacent gastric mucosa tissue samples were identified as normal
gastric mucosa, whereas the other 20 samples were identified as
dysplastic or metaplastic and were excluded from the present study.
Overall, 10 pairs of fresh GC tissues and corresponding adjacent
gastric tissues were used in the present study, due to the good
quality RNA which was extracted from them. All samples were
obtained following the provision of patient consent, and the
experiments were approved by the Ethics Committee of the People's
Hospital of Guangxi Zhuang Autonomous Region, according to the
Declaration of Helsinki.
The SGC7901 GC cell line and normal gastric
epithelial cells were purchased from the Shanghai Institute of
Biochemistry and Cell Biology (Shanghai, China). The cells were
cultured in RPMI-1640 medium (Hyclone, Logan, UT, USA) with 10%
fetal bovine serum (FBS) and incubated at 37°C with 5%
CO2.
Immunohistochemistry (IHC)
All the GC specimens were sectioned at 4 µm
for IHC staining, according to the manufacturer's instructions,
using a DAKO REAL EnVision Detection system (Dako, Glostrup,
Denmark). The slides were retrieved in boiling water for 3 min and
cooled at room temperature. Primary rabbit anti-human polyclonal
MET antibody (1:200; cat. no. sc-161; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) was used to detect the expression of MET in
the GC tissues. A semi-quantitative method was used to evaluate the
MET staining in the GC tissues independently by two pathologists,
according to the staining intensity and the percentage of positive
cells.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total miRNA was extracted from the human tissue
samples and the GC cells using RNAiso for Small RNA (Takara, Bio,
Inc., Otsu, Japan), according to the manufacturer's instructions. A
Taqman MicroRNA Reverse Transcription kit (Applied Biosystems Life
Technologies, Foster City, CA, USA) was used to synthesize cDNA,
and the expression of miR-34a was detected using a Taqman MicroRNA
assay (Applied Biosystems Life Technologies), according to the
manufacturer's instructions. U6 small nuclear RNA was used as an
internal control.
Total mRNA was extracted from the cell lines and
fresh GC tissue samples and was detected using a Nanodrop ND 2000
(Thermo Fisher Scientific, Inc., Wilmington, DE, USA). qPCR was
performed to assess the expression of MET using an RT-PCR kit
(Takara, Bio, Inc.). GAPDH was set as an internal control. All the
reactions were performed at least three times using a CFX96 RT-PCR
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Briefly 2 µl cDNA samples were subjected to PCR, using SYBR
Premix Ex Taq (Takara, Bio, Inc.). The following primers
were used: MET, forward GTAAGTGCCCGAAGTGTA, reverse
TTTCTTGCCATCATTGTC; and GAPDH, forward TGTGGGCATCAATGGATTTGG, and
reverse ACACCATGTATTCCGGGTCAAT (Invitrogen Life Technologies,
Carlsbad, CA, USA). The PCR cycling conditions were as follows:
95°C for 30 sec, 40 cycles at 95°C for 5 sec and 60°C for 30 sec,
and final dissociation at 95°C for 15 sec, 60°C for 30 sec and 95°C
for 15 sec. The mRNA expression levels were quantified using the
2−ΔΔCt method (22).
Transfection of miRNA reagents
The miRNA mimic and non-specific control were
purchased from Guangzhou Ribobio Co., Ltd. (Guangzhou, China),
which were transfected into the SGC7901 GC cells at a concentration
of 100 nmol/l using Lipofectamine 2000 (Invitrogen Life
Technologies), according to the manufacturer's instructions. The
culture medium was replaced 6 h after transfection at 37°C.
Cell proliferation assay
The SGC7901 GC cells (1×104 cells/well)
were seeded into a 96-well plate and continuously cultured for 0,
24, 48 and 72 h at 37°C. At each time interval, 20 µl Cell
Counting Kit-8 (CCK8; Beyotime Institute of Biotechnology, Haimen,
China) was added and the cells were incubated at 37°C for 2 h,
prior to detecting the absorbance at 450 nm (Multiskan; Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Colony formation assay
Single SGC7901 cells (300 cells/well) were seeded
into a 6-well plate and cultured for 10 days. The colonies formed
by the single SGC7901 cells were then fixed in 4% paraformaldehyde
for 20 min and stained with 1% crystal violet for 10 min at room
temperature. The numbers of colonies were manually counted and
statistically analyzed.
Cell invasion assay
To evaluate the invasiveness of the GC cells, a
Transwell invasion assay was performed. The upper surface of the
chamber was pre-coated with a 10 µl mixture of Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA) and serum free medium (1:1,
v/v). The cells (1×104 cells) were suspended in 200
µl serum-free medium and added to the upper chamber of the
Transwell (EMD Millipore, Billerica, MA, USA), which was inserted
into a 24-well plate. In the lower chamber, 500 µl RPMI-1640
with 10% FBS was added. Following incubation for 24 h at 37°C, the
cells on the upper chamber were removed and the cells on the lower
chamber were fixed and stained with crystal violet. Images of the
invaded cells were captured in five randomly-selected fields using
a microscope (CX31; Olympus Corporation, Tokyo, Japan) and counted
manually.
Bioinformatic analysis
The TCGA database (https://tcga-data.nci.nih.gov/tcga/tcgaHome2.jsp)
was used to investigate the association between the expression of
miR-34a and clinicopathological features, and the prognosis of
patients with GC. A designed web tool (http://www.cbioportal.org/public-portal/) was used for
examining the TCGA database (23,24).
The cut-off value for miR-34a in the GC of the TCGA cohort was set
using X tile software (version 3.6.1; Yale University, New Haven,
CT, USA) (25). For miRNA target
prediction, three popular public databases [PicTar (http://pictar.mdc-berlin.de/) (26), DIANA Tools (http://diana.imis.athena-innovation.gr/DianaTools/index.php)
(27) and miRTarBase (http://mirtar-base.mbc.nctu.edu.tw/) (28)] were scanned. The selected
Novoseek-inferred disease associations for MET was reviewed using
the integrated gene database, GeneCards (http://www.genecards.org/).
Western blotting
Western blotting was performed to detect the protein
level of MET in different samples. The GC cells were harvested 72 h
after transfection. Total protein was extracted, according to the
manufacturer's instructions of the protein extraction kit (Beyotime
Institute of Biotechnology) and concentrations were determined
using an Enhanced BCA Protein Assay kit (Beyotime Institute of
Biotechnology). A total of 50 µg protein was loaded into
each lane and separated on a 15% gel, which was then wet
transferred onto a poly-vinylidene difluoride membrane (EMD
Millipore). Following blocking with 5% milk powder, the membranes
were incubated with rabbit anti-human polyclonal MET antibody (cat.
no. sc-161; Santa Cruz Biotechnology, Inc.) and mouse anti-human
monoclonal GAPDH antibody (cat. no. sc-322233) Santa Cruz
Biotechnology, Inc.), respectively, overnight at 4°C. Subsequently,
horseradish peroxidase (HRP)-labeled secondary antibody (Beyotime
Institute of Biotechnology) was added and incubated at room
temperature for 2 h. All primary antibodies were used at a
concentration of 1:500 and the second antibody was used at a
dilution of 1:2,000. The proteins were visualized using a Pierce
ECL chemiluminescent substrates (Thermo Fisher Scientific, Inc.)
and analyzed. GAPDH was used as an internal control.
Statistical analyses
All statistical analyses were performed using SPSS
version 20.0 software (IBM, Armonk, NY, USA). Student's t-test was
used for normally distributed data. Bonferron's-corrected
Mann-Whitney U test was used to assess the expression of miR-34a in
the different GC specimens. Correlation between the expression of
miR-663 and the clinicopathological features of the patients was
determined using Pearson's χ2 test. The prognostic value
of miR-34a was estimated using a Kaplan-Meier survive curve. Data
are presented as the mean ± standard deviation. P<0.05
(*) and P<0.01 (**) were considered to
indicate a statistically significant difference. All experiments
were performed at least three times using triplicate samples.
Results
miR-34a is negatively correlated with
invasion/metastasis and the prognosis of patients with GC
On comparing the levels of miR-34a in GC tissues and
normal gastric epithelial, miR-34a was expressed at lower levels in
the GC tissues, compared with the normal gastric tissues
(12.00±0.47, vs. 7.06±0.33; P<0.01; Fig. 1A). The correlation between miR-34a
and the invasion and metastasis of the GC cells was also evaluated
in the GC specimens. The results indicated that miR-34a was
significantly lower in cases exhibiting serosa invasion and
significantly higher in cases exhibiting musocal invasion
(P<0.01; Fig. 1B). Low
expression levels of miR-34a were detected in the GC specimens
exhibiting lymph node metastasis (27/40; P<0.01; Fig. 1C). These results indicated that
miR-34a was negatively correlated with the depth of invasion and
lymph node metastasis in the GC specimens. These results were also
confirmed in an independent cohort from the TCGA database, which
was used to investigate the association between miR-34a and
clinicopathological features and the prognosis of patients with GC.
Among the TCGA cohort (n=352) with diagnostic information, 157
cases had complete follow-up information. The results demonstrated
that the levels of miR-34a were significantly lower in T3 and T4,
compared with T1 (P<0.05; Fig.
1D). A Kaplan-Meier survival curve was used to analyze the
predictive value of miR-34a GC samples. The results indicated that
patients with high expression levels of miR-34a had a significantly
longer duration of survival following surgery, compared with those
with low expression levels of miR-34a (P<0.05; Fig. 1E). These results indicated that low
levels of miR-34a in GC suggested frequent invasion and metastasis
and predicted poorer prognosis in patients.
miR-34a suppresses the proliferation,
colony formation and invasion of GC cells
GES1 is a normal gastric epithelial cell line, which
was used in the present study as a normal control. The levels of
miR-34a were significantly lower in the SGC7901 GC cell line,
compared with its level in the GES1 line (P<0.01; Fig. 2A), which confirmed the findings of
the expression of miR-34a in the clinical specimens. In order to
determine the effect of miR-34a on the proliferation, colony
formation and invasion abilities of the GC cells, an miR-34a mimic
was used. The OD450 value was signifi-cantly decreased in the group
transfected with the miR-34a mimic, compared with the control group
(P<0.01; Fig. 2B). A colony
formation assay was used as a representative assay to assess the
self-renewal ability of the cancer cells. Compared with the
control, the SGC7901 transfected with the miR-34a mimic exhibited a
reduced colony number (P<0.01; Fig.
2C and D). A cell invasion assay was used to assess the impact
of miR-34a on the invasiveness of GC. The SGC7901 cells transfected
with the miR-34a mimic exhibited a reduced number of invaded cells,
compared with the control (P<0.01; Fig. 2E and F). These data demonstrated
that miR-34a attenuated the proliferation, colony formation and
invasion of the GC cells.
miR-34a inhibits the expression of MET in
GC
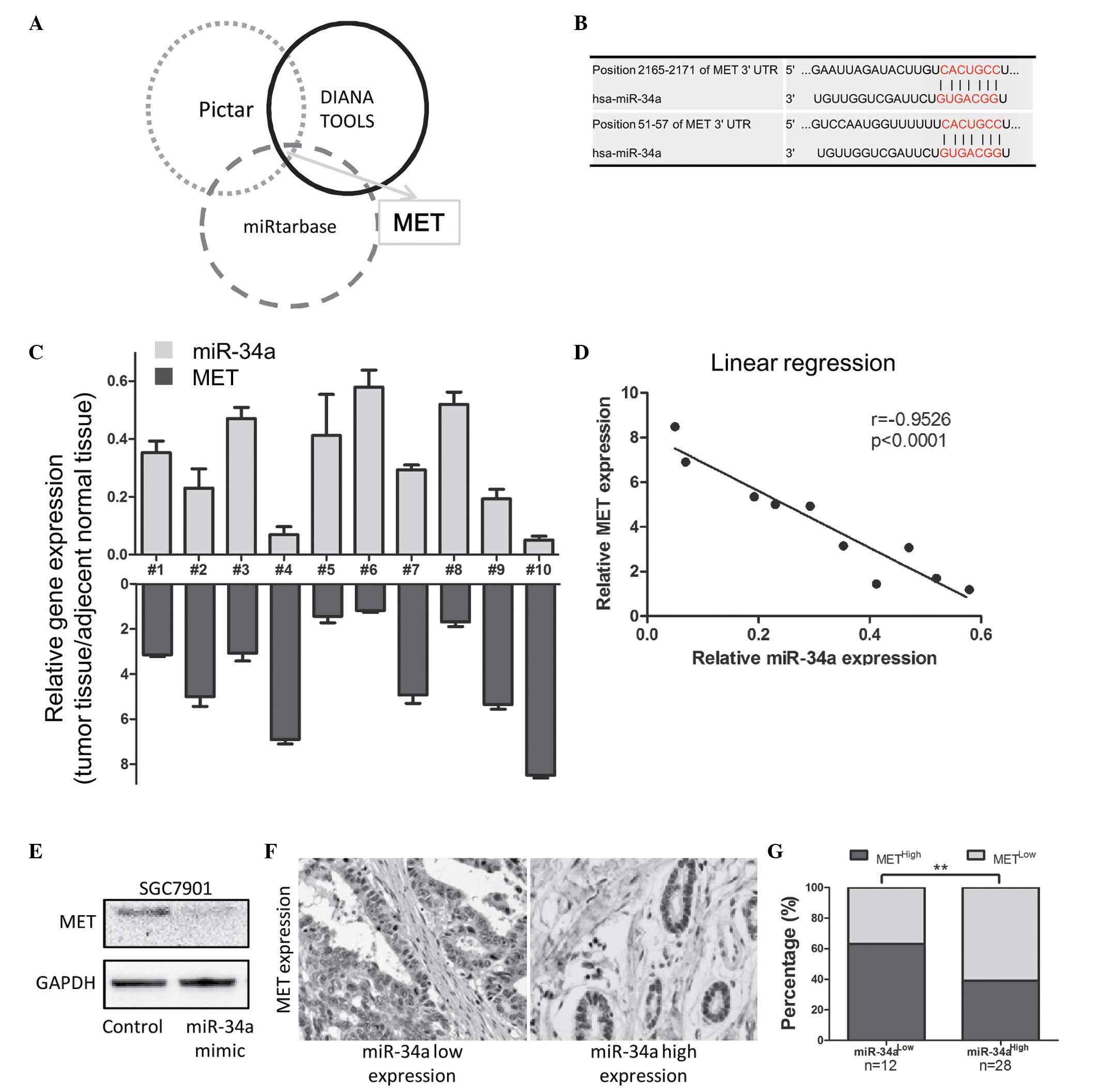
The present study predicted the candidate downstream
targets of miR-34a in the PicTar, DIANA Tools and miRTarBase miR
databases. Among the top 10 candidate genes predicted by these
databases, MET was the only common gene (Fig. 3A and Table I). The predicted binding sites of
MET are listed in Fig. 3B. The
mRNA expression of miR-34a and MET were examined in 10 fresh GC
specimens (Fig. 3C). A linear
regression model was used to analyze the underlying association
between the expression of miR-34a and MET. The results indicated
that miR-34a was negatively correlated with MET (P<0.01;
r=−0.9526; Fig. 3D). Following
transfection with the miR-34a mimic, SGC7901 cells exhibited
downregulated protein levels of MET (Fig. 3E). MET was reviewed due to its
significant role in tumor and metastasis (P<0.01; Table II).
 | Table ITop 10 candidate targets of
microRNA-34a, predicted using the PicTar, DIANA Tools and
miRTarBase databases. |
Table I
Top 10 candidate targets of
microRNA-34a, predicted using the PicTar, DIANA Tools and
miRTarBase databases.
| PicTar | DIANA Tools | miRTarBase |
|---|
| DLL1 | CDKN2C | BIRC3 |
| MGC34648 | ACSL4 | GRM7 |
| SYT1 | CDK4 | JAG1 |
| NAV3 | E2F1 | MYC |
| NOTCH1 | MET | MYB |
| RALGPS2 | MYB | MET |
| VAMP2 | ERLIN1 | CDK4 |
| CNTN2 | MMS19 | CCND1 |
| MET | HNF4A | BCL2 |
| ZDHHC23 | DTYMK | NOTCH1 |
 | Table IISelected Novoseek-inferred disease
associations for the MET gene. |
Table II
Selected Novoseek-inferred disease
associations for the MET gene.
| Disease | -log (P-value) | Number of hits |
|---|
| Tumor | 71.6 | 679 |
| Metastasis | 69.6 | 209 |
| Cancer | 65.5 | 337 |
| Hereditary
papillary renal cancer | 61.8 | 3 |
| Gastric
carcinoma | 60.2 | 66 |
| Thyroid papillary
carcinoma | 59.5 | 26 |
| Carcinoma renal
cell | 57.8 | 66 |
| Carcinoma | 57.5 | 102 |
| Hepatocellular
carcinoma | 56.9 | 67 |
| Gastric cancer | 56 | 103 |
| Lymphatic
metastasis | 41.1 | 1 |
| Non-metastatic | 36.1 | 3 |
| Metaplasia | 29.6 | 7 |
| Metastatic
osteosarcoma | 24.9 | 1 |
| Liver
metastases | 11.6 | 20 |
The present study then examined MET staining in
clinical specimens of GC using IHC. The results revealed high
expression levels of MET in the samples with low expression of
miR-34a, however, the expression level was low in the samples with
high expression of miR-34a (Fig.
3F). Statistical analyses revealed that >60% of the GC
samples exhibited low miR-34a/high MET, and high miR-34a/low MET.
(P<0.01; Fig. 3G). These
results indicated that miR-34a negatively regulated MET, which
mediated the invasion and proliferation of GC.
Discussion
The dynamic expression and functions of miRNAs are
closely associated with the progression of cancer and malignancy,
which has been confirmed by the detection of miRNAs in clinical
specimens (29). Certain miRNAs
are termed 'oncomiRs' as they exhibit high levels of expression in
cancer and fuel the malignant behavior of cancer cells, examples of
which include miR-10b in breast cancer and miR-21 in glioblastoma
(30,31). Tumor suppressor miRNAs are often
downregulated in cancer and inhibit the carcinogenesis and
progression of cancer, for example, miR-218 has been found to
attenuate the invasion, migration, proliferation and self-renewal
of glioma cells via targeting Bmi (32). B-cell lymphoma (Bcl)2, an
anti-apoptotic gene, has been reported to be negatively regulated
by miR-15a and miR-16-1 (33,34).
Thus, a reduction in the expression levels of these miRNAs lead to
the overexpression of Bcl2 and contributes to the progression of
leukemia and prostate cancer. miR-34a belongs to the miR-34 family,
which have been identified computationally and confirmed as
evolutionarily conserved (35–38).
Human miR-34a is involved in the p53 tumor
suppressor network, thus, its dysregulation is involved in cancer
aggression. While miR-34a is considered to be a multifaceted miRNA
in different types of cancer and has a variety of functions, a
number of which appear opposite, miR-34a predominantly inhibits
proliferation, progression and invasion and has been reported to be
a tumor suppressor in colon cancer and glioblastoma (17,39).
However, miR-34a is overexpressed in hepatocellular carcinoma and
is associated with liver carcinogenesis (20). A previous microRNA profile in human
GC tissue revealed that miR-34a is upregulated, compared with
normal gastric tissue (21). These
paradoxical functions of miR-34a suggest that its effects in GC
require further investigation.
Concerning the association between miR-34a and the
clinicopathological features of GC, the present study examined
level of miR-34a in tissue samples from patients with GC, which
were confirmed in an independent TCGA cohort. The results indicated
that miR-34a was a negative indicator of GC invasion/metastasis and
a valuable predictor for the prognosis of patients with GC,
suggesting that miR-34a may be considered as a tumor suppressor
miRNA in GC.
These clinical data led to the present study
investigating the role of miR-34a through a series of experiments.
Consistent with the clinical data, miR-34a was expressed at a low
level in the SGC7901 GC cell line and at a high level in the normal
GES1 gastric epithelial cell line. Furthermore, miR-34a attenuated
the proliferation, colony formation and invasion of the GC cells
in vitro, which provided further evidence that miR-34a acted
as a tumor suppressor miRNA in the GC cells.
Since miR-34a is an miRNA, the present study
bioinfor-matically analyzed miR-34a in the PicTar, DIANA Tools and
miRTarBase miRNA targets databases. MET was found to be the only
common candidate among the top 10 candidates of each database. MET
was located at Chr7 q31.2, and was observed to be closely
associated with tumors and metastasis. MET is considered to be an
oncogene, activated in several types of cancer (40,41).
In colorectal cancer, the level of c-MET predicts early stage
invasion and regional metastasis (42). Knockdown of c-MET in ovarian cancer
significantly inhibits the extracellular signal-regulated kinase
and phosphoinositide 3-kinase signaling pathways, as well as the
activity of matrix metalloproteinase 2/9 (43). Co-expression of c-Met and
hepatocyte growth factor in colorectal cancer allows identification
of a metastatic phenotype, which correlates with advanced stage and
poor survival rates (44). In
hepatocellular carcinoma, low expression levels of miR-34a have
been demonstrated to contribute to cancer malignancy via targeting
c-Met (19). In the present study,
miR-34a was found to negatively regulate the expression of MET in
GC, suggesting that the tumor suppressing effect of miR-34a may be
mediated by the down-regulation of MET.
Notably, >60% of the samples in the present study
exhibited consistent expression of miR-34a and MET, with
inconsistent expression observed in a minority of the GC samples.
The effects of miR-34a occur in a tissue-specific manner, which
involves several downstream targets. In glioma, miR-34a is reported
to inhibit Notch1 and Notch2, leading to glioma growth suppression
(17). In GC, survivin has been
identified as another downstream target of miR-34a in modulating
proliferation and invasion (18).
Therefore, it is of importance to discern the interaction of
miR-34a and its targets in different types of cancer.
In conclusion, with confirmation using TCGA data,
the results of the present study revealed miR-34a as a tumor
suppressor miRNA, which attenuated proliferation and invasion,
predominantly through targeting MET in GC. These findings provide a
novel perceptive to further understand the underlying molecular
basis of the proliferation and invasion of GC and potential
therapeutic approaches for GC.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Lin CC, Mariotto AB, et al:
Cancer treatment and survivorship statistics, 2014. CA Cancer J
Clin. 64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khalighinejad N, Hariri H, Behnamfar O,
Yousefi A and Momeni A: Adenoviral gene therapy in gastric cancer:
A review. World J Gastroenterol. 14:180–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leja M, Wex T and Malfertheiner P: Markers
for gastric cancer premalignant lesions: Where do we go? Dig Dis.
30:268–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amedei A, Della Bella C, Silvestri E,
Prisco D and D'Elios MM: T cells in gastric cancer: Friends or
foes. Clin Dev Immunol. 2012:6905712012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toiyama Y, Okugawa Y and Goel A: Dna
methylation and microRNA biomarkers for noninvasive detection of
gastric and colorectal cancer. Biochem Biophys Res Commun.
455:43–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Felipe AV, Oliveira J, Chang PY, et al:
RNA interference: a promising therapy for gastric cancer. Asian Pac
J Cancer Prev. 15:5509–5515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pasquinelli AE: MicroRNAs and their
targets: Recognition, regulation and an emerging reciprocal
relationship. Nat Rev Genet. 13:271–282. 2012.PubMed/NCBI
|
|
9
|
Wang Y and Lee CG: MicroRNA and
cancer-focus on apoptosis. J Cell Mol Med. 13:12–23. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bueno MJ, Pérez de Castro I and Malumbres
M: Control of cell proliferation pathways by microRNAs. Cell Cycle.
7:3143–3148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang LH and He XH: Macro-management of
microRNAs in cell cycle progression of tumor cells and its
implications in anti-cancer therapy. Acta Pharmacol Sin.
32:1311–1320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lowery AJ, Miller N, McNeill RE and Kerin
MJ: MicroRNAs as prognostic indicators and therapeutic targets:
Potential effect on breast cancer management. Clin Cancer Res.
14:360–365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nouraee N and Calin GA: MicroRNAs as
cancer biomarkers. Microrna. 2:102–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berindan-Neagoe I, Monroig Pdel C,
Pasculli B and Calin GA: MicroRNAome genome: A treasure for cancer
diagnosis and therapy. CA Cancer J Clin. 64:311–336. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maroof H, Salajegheh A, Smith RA and Lam
AK: Role of microRNA-34 family in cancer with particular reference
to cancer angiogenesis. Exp Mol Pathol. 97:298–304. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balca-Silva J, Sousa Neves S, Goncalves
AC, et al: Effect of miR-34b overexpression on the radiosensitivity
of non-small cell lung cancer cell lines. Anticancer Res.
32:1603–1609. 2012.PubMed/NCBI
|
|
17
|
Li Y, Guessous F, Zhang Y, et al:
MicroRNA-34a inhibits glioblastoma growth by targeting multiple
oncogenes. Cancer Res. 69:7569–7576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao W, Fan R, Wang L, et al: Expression
and regulatory function of miRNA-34a in targeting survivin in
gastric cancer cells. Tumour Biol. 34:963–971. 2013. View Article : Google Scholar
|
|
19
|
Dang Y, Luo D, Rong M and Chen G:
Underexpression of miR-34a in hepatocellular carcinoma and its
contribution towards enhancement of proliferating inhibitory
effects of agents targeting c-MET. PLoS One. 8:e610542013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pineau P, Volinia S, McJunkin K, et al:
miR-221 overexpression contributes to liver tumorigenesis. Proc
Natl Acad Sci USA. 107:264–269. 2010. View Article : Google Scholar :
|
|
21
|
Yao Y, Suo AL, Li ZF, et al: MicroRNA
profiling of human gastric cancer. Mol Med Rep. 2:963–970.
2009.PubMed/NCBI
|
|
22
|
Braun CJ, Zhang X, Savelyeva I, Wolff S,
Moll UM, Schepeler T, Ørntoft TF, Andersen CL and Dobbelstein M:
p53-Responsive micrornas 192 and 215 are capable of inducing cell
cycle arrest. Cancer Res. 68:10094–10104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cerami E, Gao J, Dogrusoz U, et al: The
cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao J, Aksoy BA, Dogrusoz U, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBio-Portal. Sci Signal. 6:pl12013. View Article : Google Scholar
|
|
25
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: a new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krek A, Grün D, Poy MN, et al:
Combinatorial microRNA target predictions. Nat Genet. 37:495–500.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Huang J, Yang N, et al: microRNAs
exhibit high frequency genomic alterations in human cancer. Proc
Natl Acad Sci USA. 13:9136–9141. 2006. View Article : Google Scholar
|
|
28
|
Hsu SD, Lin FM, Wu WY, et al: miRTarBase:
A database curates experimentally validated microRNA-target
interactions. Nucleic Acids Res. 39:D163–D169. 2011. View Article : Google Scholar
|
|
29
|
Calin GA, Ferracin M, Cimmino A, et al: A
MicroRNA signature associated with prognosis and progression in
chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parrella P, Barbano R, Pasculli B, et al:
Evaluation of microRNA-10b prognostic significance in a prospective
cohort of breast cancer patients. Mol Cancer. 13:1422014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hermansen SK, Dahlrot RH, Nielsen BS,
Hansen S and Kristensen BW: MiR-21 expression in the tumor cell
compartment holds unfavorable prognostic value in gliomas. J
Neurooncol. 111:71–81. 2013. View Article : Google Scholar
|
|
32
|
Tu Y, Gao X, Li G, et al: MicroRNA-218
inhibits glioma invasion, migration, proliferation and cancer
stem-like cell self-renewal by targeting the polycomb group gene
Bmi1. Cancer Res. 73:6046–6055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aqeilan RI, Calin GA and Croce CM: miR-15a
and miR-16-1 in cancer: Discovery, function and future
perspectives. Cell Death Differ. 17:215–220. 2010. View Article : Google Scholar
|
|
34
|
Bonci D, Coppola V, Musumeci M, et al: The
miR-15a-miR-16-1 cluster controls prostate cancer by targeting
multiple oncogenic activities. Nat Med. 14:1271–1277. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Houbaviy HB, Murray MF and Sharp PA:
Embryonic stem cell-specific MicroRNAs. Dev Cell. 5:351–358. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dostie J, Mourelatos Z, Yang M, Sharma A
and Dreyfuss G: Numerous microRNPs in neuronal cells containing
novel microRNAs. RNA. 9:180–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lim LP, Glasner ME, Yekta S, Burge CB and
Bartel DP: Vertebrate microRNA genes. Science. 299:15402003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He L, He X, Lim LP, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cho WC: OncomiRs: The discovery and
progress of microRNAs in cancers. Mol Cancer. 6:602007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lutterbach B, Zeng Q, Davis LJ, et al:
Lung cancer cell lines harboring MET gene amplification are
dependent on Met for growth and survival. Cancer Res. 67:2081–2088.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Engelman JA, Zejnullahu K, Mitsudomi T, et
al: MET amplifi-cation leads to gefitinib resistance in lung cancer
by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takeuchi H, Bilchik A, Saha S, et al:
c-MET expression level in primary colon cancer: A predictor of
tumor invasion and lymph node metastases. Clin Cancer Res.
9:1480–1488. 2003.PubMed/NCBI
|
|
43
|
Sawada K, Radjabi AR, Shinomiya N, et al:
c-Met overexpression is a prognostic factor in ovarian cancer and
an effective target for inhibition of peritoneal dissemination and
invasion. Cancer Res. 67:1670–1679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kammula US, Kuntz EJ, Francone TD, et al:
Molecular co-expression of the c-Met oncogene and hepatocyte growth
factor in primary colon cancer predicts tumor stage and clinical
outcome. Cancer Lett. 248:219–228. 2007. View Article : Google Scholar
|