Introduction
Asthma is a chronic bronchial disease characterized
by bronchial hyper-responsiveness (AHR) and airway inflammation on
exposure to various stimuli. House dust mites (HDM) are one of the
most common and most potent allergens that trigger asthma, and
~60–100% of asthmatics are sensitized to HDM allergens (1); therefore, HDM allergens are
considered one of the most important asthma-inducing allergens.
The c-kit proto-oncogene is a member of the tyrosine
kinase receptor family, which is encoded at the white spotting
locus and binds to the ligand, stem cell factor (SCF). c-kit is
critical for the proliferation, survival and differentiation of
hematopoietic stem cells and progenitor cells (2). Previous studies have focused on the
c-kit-induced differentiation of mast cells and the production of
inflammatory cytokines, including histamine and leukotriene
(3,4).
Immune responses against invading pathogens are
initiated by Toll-like receptors (TLRs), which recognize distinct,
structurally conserved, pathogenic components. The HDM,
Dermatophagoides pteronyssinus, is a common source of indoor
allergens, and ~10% of individuals with asthma suffer from
HDM-mediated allergy (5,6). TLR-dependent activation of
antigen-presenting cells (APCs) leads to the processing and
presentation of antigens to CD4+ T-cells, steering the
inflammatory response towards a T helper (Th)2-mediated response
pathway (7). Dendritic cells (DCs)
are efficient APCs, which are important in the pathogenesis of
allergic asthma. DCs present the HDM allergens, which are
subsequently taken up and processed by Th2 cells, leading to Th2
cell activation. Activated Th2 cells produce various cytokines,
including interleukin (IL)-4, IL-5 and IL-13, which are involved in
the recruitment of eosinophils, goblet cell hyperplasia and in AHR
(8). A previous study demonstrated
that the activation of TLR2 evokes a Th2 immune response and
promotes experimental asthma, indicating that TLR2 can induce an
asthmatic inflammatory response (9). In addition, HDM can promote cell
surface expression of c-kit when bound to its ligand on DCs, which
can prime naive CD4+ T-cells toward Th2 and Th17
responses (10). Based on these
previous findings, the present study aimed to investigate whether
the c-kit receptor is activated by HDM via TLR2 in DCs.
Materials and methods
Animals
Specific-pathogen-free (SPF)-grade male C57BL/6 mice
(6–8 week-old; 20–25 g), were purchased from the Laboratory Animal
Center of the Fourth Military Medical University (Xi'an, China).
The experimental procedures were approved by the ethics committee
of the Affiliated Hospital of Xi'an Medical University (Xi'an,
China).
Generation and culture of
monocyte-derived DCs
The DCs were prepared from bone marrow progenitors,
as described in previous studies (11,12).
The C57BL/6 mice were housed in a temperature (22±2°C) and humidity
(60±5%) controlled environment, under a 12 h light/dark cycle, with
24 h ad libitum access to standard Purina (5001) rodent chow
(autoclaved) and tap water, which was heated to boiling point for
20 min and cooled to room temperature before use. The mice were
anesthetized intraperitoneally with 3 ml/kg-1 chloral
hydrate (Sinochem Qingdao Co., Ltd., Qingdao, China), and
sacrificed by exsanguination from the abdominal aorta after 24 h.
The femurs and tibiae of the male C57BL/6 mice (8–12 weeks old)
were removed and purified from the surrounding muscle tissues by
rubbing with paper tissues. The bone marrow cells were flushed from
the femurs and tibiae of the mice, washed and cultured in 6-well
plates (2×106 cells/ml) containing 4 ml complete medium
(RPMI 1640; Invitrogen Life Technologies; Carlsbad, CA, USA). The
medium was supplemented with 2 mM L-glutamine, 100 U/ml penicillin,
100 µg streptomycin, 50 µM 2-medroxyestradiol, and
10% fetal calf serum (FCS), obtained from Invitrogen Life
Technologies, which contained recombinant granulocyte-macrophage
colony-stimulating factor (GM-CSF; 10 ng/ml; R&D Systems, Inc.,
Minneapolis, MN, USA) and recombinant mouse IL-4 (10 ng/ml; R&D
systems). All cell cultures were incubated at 37°C in a humidified
atmosphere containing 5% CO2.
TLR2-specific silencing in vitro
The DCs were cultured in specific conditions, as
described above. On day 6, the immature DCs were harvested,
suspended in 200 µl serum-free RPMI 1640 and aliquoted into
a 24-well plate (Invitrogen Life Technologies). A total of 1
µg of each TLR2 small interfering (si)RNA (Qiagen, Hilden,
Germany; sense 5′-GACUUAUCCUAUAAUUACUTT-3′ and antisense
5′-AGUAAUUAUAGGAUAAGUCTA-3′) and negative control (scrambled) siRNA
(sense 5′-UAGGCGCAGCUCCGGAUCGDTT-3′ and antisense
5′-CGAUCCGGAGCUGCGCCUADTT-3′) were incubated separately, with 5
µl Lipofectamine (Genlantis, Inc., San Diego, CA, USA) in
100 µl serum-free RPMI 1640 at room temperature for 5 min.
The TLR2-specific and control siRNA mixtures were then added to
respective 200 µl DC cell cultures. Following 4 h incubation
at 37°C, an equal volume of 200 µl RPMI 1640 supplemented
with 20% FCS was added to the cells. On day 7, the DCs were
stimulated with the HDM allergens (10 mg/ml) and incubated for 24
h. The expression of CD117 in the DCs was determined using flow
cytometry on day 8. The siRNA transfection and silencing of the
expression of TLR2 in DCs was performed according to the methods
described in a previous study (13).
Sensitizing DCs with HDM
A purified antigen of HDM extract from the D.
pteronyssinus allergen (Peking Union Laboratories, Beijing,
China), containing a known concentration of Der p 1, was used in
the experiments of the present study. The doses of HDM correspond
to the quantity of Der p 1 used. The DCs were collected and
cultured, as described above. They were then treated with HDM (10
µg/ml) and co-cultured for 72 h at 37°C.
Flow cytometry
Flow cytometric analysis was performed to examine
the variation in the expression of CD117 in the DCs, for
verification of the effects of TLR2-specific siRNA on the
expression of c-kit. The cells were filtered through 20 µm
nylon mesh (BD Biosciences, San Jose, CA, USA), and 106
cells were incubated with monoclonal antibodies targeting CD117
(cat. no. MAB332; R&D Systems, Inc.). Following staining, the
cells were analyzed by flow cytometry using a FACSCalibur with
CellQuest version 5.1 software (BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the mature DCs, which
were collected 72 h following siRNA-TRL2 transfection, using RNeasy
Mini kits (Qiagen, Valencia, CA, USA) containing TRIzol reagent for
extraction (Gibco Life Technologies, Carlsbad, CA, USA). The total
RNA was then reverse transcribed into cDNA using a reverse
transcriptase kit at 50°C for 30 min. The cDNA was amplified using
a Promega PCR Single-Step kit (Promega Corporation, Madison, WI,
USA), according to the manufacturer's instructions. The following
primers (Invitrogen Life Technologies) were used: TLR2, sense
5′-ATCAGCAGGAACAGAGCACA-3′ and antisense 5′-AGGAGCAGCAAGCAC-3′;
c-kit, sense 5′-ACCCACAGGTGTCCAATTATTC-3′, antisense
5′-TGGCGTTCATAATTGAAGTCAC-3′; β-actin, sense
5′-CCTCATGAAGATCCTGACCG-3′, and antisense
5′-ACCGCTCATTGCCGATAGTG-3′. β-actin served as the internal control.
Quantification of PCR products was carried out using the FTC-2000
(Shanghai Funglyn Biotech Co., Ltd., Shanghai, China). The
threshold cycle (Ct) of each sample was recorded as a quantitative
measure of the amount of PCR product in the sample. Relative
changes in expression levels were calculated using the
2−ΔΔCT method (14).
Western blotting
The DCs were collected 72 h following siRNA-TRL2
transfection. Total protein was extracted using ice-cold
radioimmunoprecipitation lysis buffer (Santa Cruz, Biotechnology,
Inc., Dallas, TX, USA). The protein concentrations were quantified
using the Bradford method (Quick Start™; Bio-Rad, Hercules, CA,
USA). Cytoplasmic protein samples (20 µg) were separated by
15% SDS-PAGE and semi-dry transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked for 1 h at room temperature with 5% bovine
serum albumin (Santa Cruz, Biotechnology, Inc.). The blots were
then incubated at 4°C overnight with the following primary
antibodies: Anti-TLR2 (sc-12507), anti-c-kit (sc-1494) and
anti-GAPDH (2118S) (Santa Cruz Biotechnology, Inc.), at a 1:1,000
dilution in Tris-buffered saline with Tween 20 (TBST). GAPDH was
used as a loading control. The blots were washed in TBST and then
incubated with an anti-rabbit immunoglobulin G-horseradish
peroxidase-conjugated secondary antibody (1:20,000 dilution in
TBST; Pierce Biotechnology, Inc., Rockford, IL, USA) for 1 h at
room temperature. After further washing with TBST the blots were
visualized using enhanced chemiluminescence (GE Healthcare Life
Sciences, Little Chalfont, UK) and densitometrically quantified
using a Western Blotting Detection system (GE Healthcare Life
Sciences). All results were normalized to GAPDH. Heat-induced
epitope retrieval was conducted.
ELISA
The levels of inflammatory cytokines in the DC
culture supernatants were analyzed using ELISA, with ELISA kits for
IL-6 and IL-12 (R&D Systems, Inc.), according to the
manufacturer's instructions. A total of five mice were used for
each group per experiment.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS Inc., Chicago, IL, USA). Data were analyzed using
one-way analysis of variance, followed by a least significant
difference test. P<0.05 (two-tailed test) was considered to
indicate a statistically significant difference. The results are
expressed as the mean ± standard deviation, unless otherwise
stated.
Results
siRNA effectively inhibits the expression
of TLR2
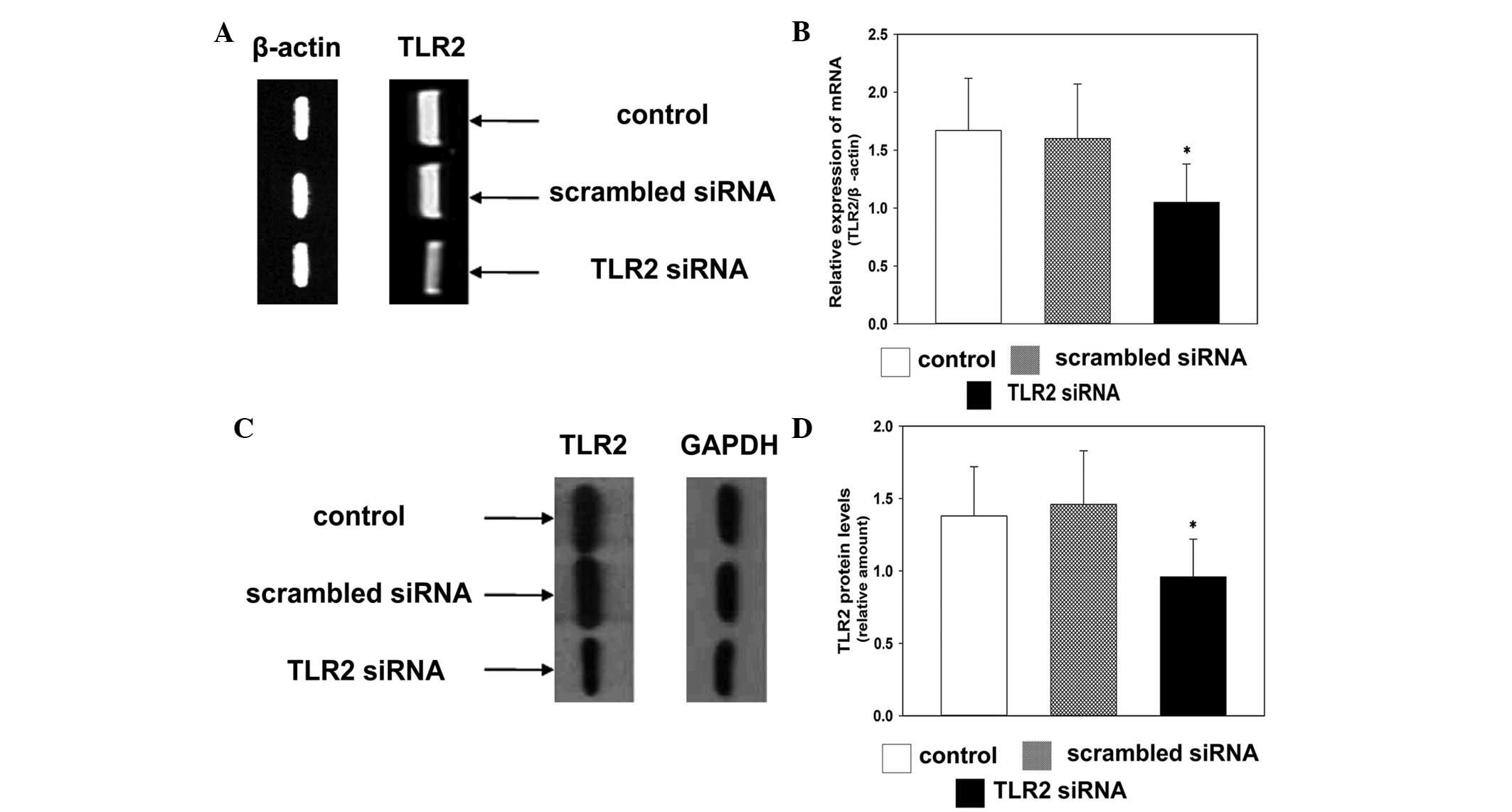
To investigate whether TLR2-specific siRNA silenced
the expression of TLR2 in the present study, the mRNA expression
levels of TLR2 were detected using RT-qPCR. The mRNA expression
levels of TLR2 were downregulated in the TLR2-specific siRNA group
72 h post-transfection, compared with the control group (Fig. 1A and B). This indicated that
TLR2-specific siRNA effectively inhibited the gene expression of
TLR2. Western blot analysis demonstrated that TLR2-specific siRNA
effectively inhibited the protein expression levels of TLR2 72 h
post-transfection in the TLR2 siRNA group, compared with the
control group (Fig. 1C and D).
These results suggested a significant reduction in the expression
of TLR2 in the TLR2-siRNA group, compared with the other
groups.
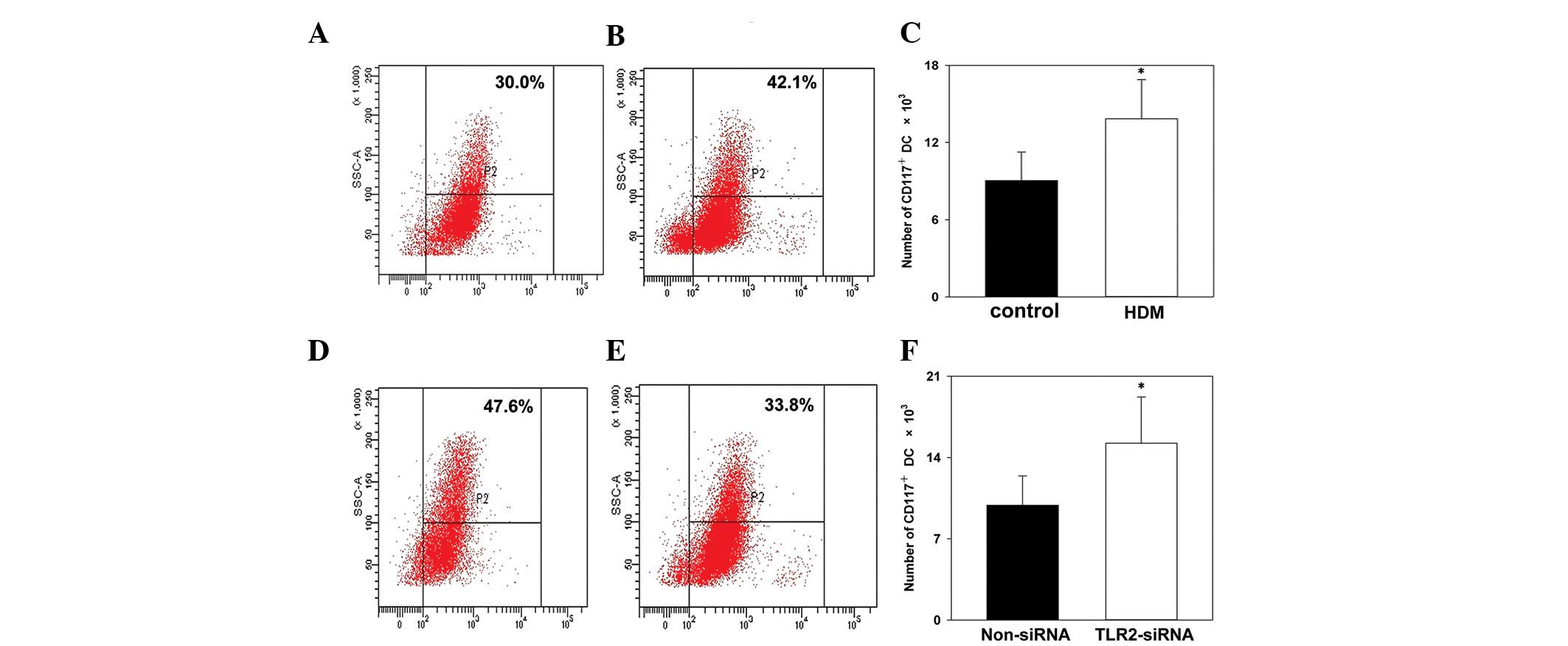
HDM upregulates the expression of CD117
in DCs
To investigate whether HDM activated the expression
of c-kit in DCs in the present study, CD117 surface molecules were
detected using flow cytometry. HDM upregulated the expression of
CD117 on the surface of DCs, compared with the control group
(Fig. 2A-C). This confirmed that
HDM effectively activated the gene expression of c-kit in the
DCs.
TLR2 siRNA downregulates the expression
of CD117 in DCs
CD117, which is a receptor for SCF, is associated
with the expression of c-kit. To investigate whether TLR2-specific
siRNA inhibited the expression of c-kit, the expression of CD117 on
the surface of the DCs was analyzed using flow cytometry. TLR2
siRNA inhibited the expression of CD117 on the surface of the DCs,
compared with the control group (Fig.
2D-F).
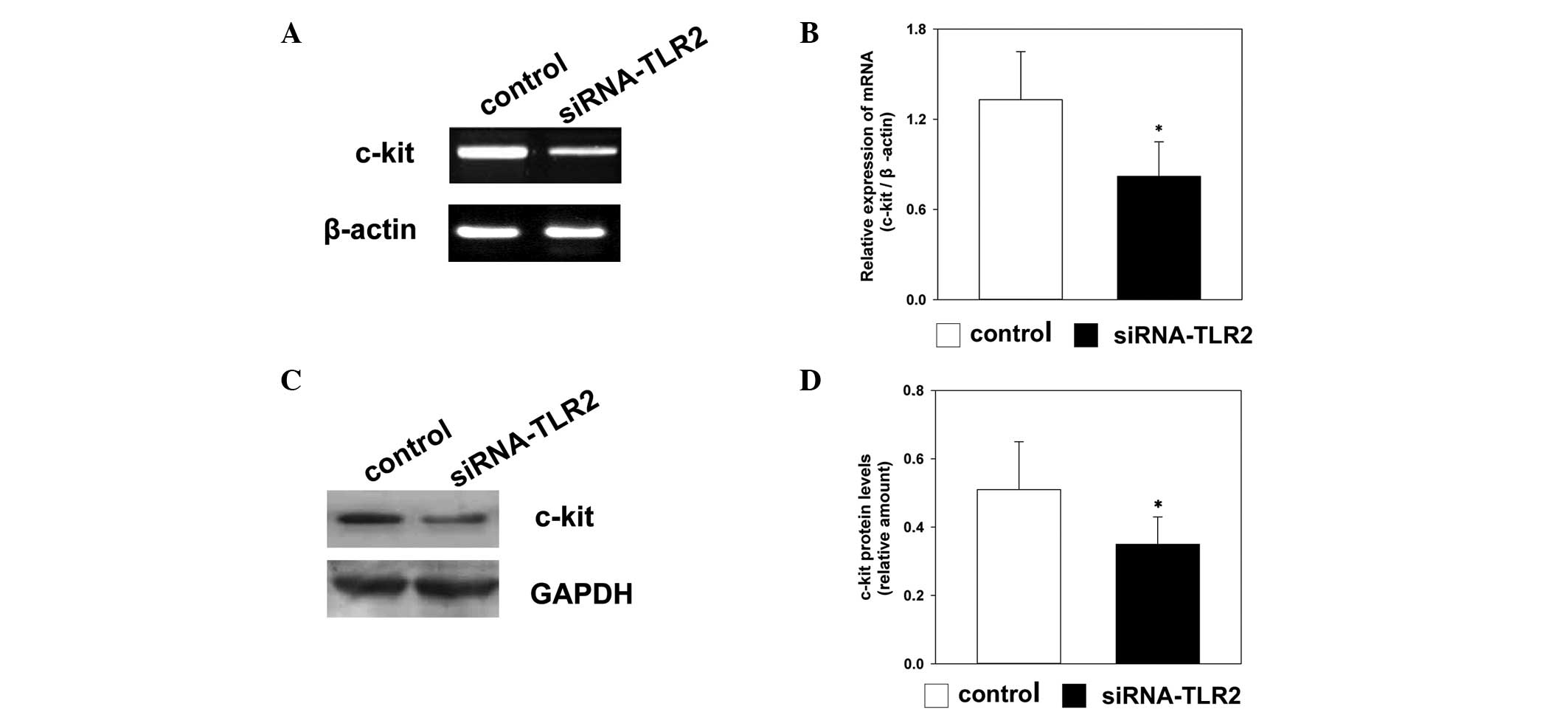
TLR2 siRNA reduces the expression of
c-kit
To investigate whether TLR2-specific siRNA affected
the expression levels of c-kit, the mRNA levels of c-kit were
detected using RT-qPCR, and the protein levels of c-kit were
detected using western blot analysis. The mRNA and protein
expression levels of c-kit were downregulated in the TLR2-siRNA
group 72 h following transfection, compared with the control group.
There was a significant difference in the expression levels of
c-kit between the TLR2-siRNA group and the control group (Fig. 3).
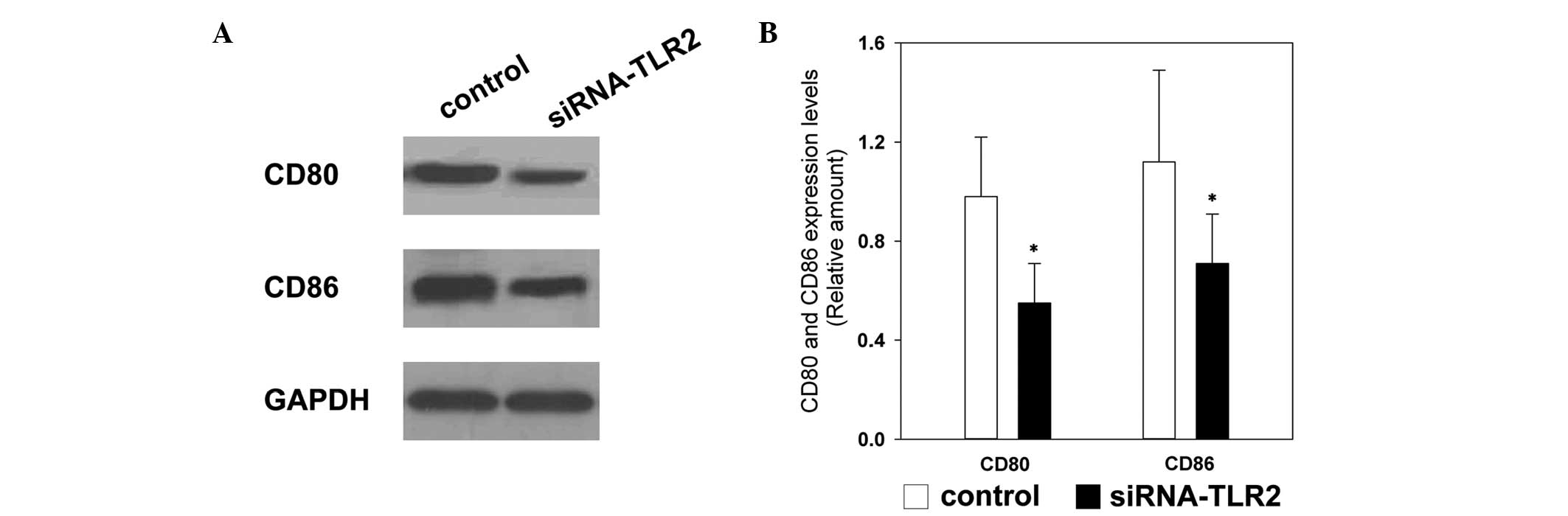
Western blot analysis indicates that
TLR2-siRNA inhibits the expression of CD80 and CD86
CD80/CD86 are important co-stimulatory molecules
between DCs and T-cells, which are important in the differentiation
and activation of Th2 (15). In
the present study, TLR2-siRNA effectively inhibited the expression
levels of CD80 and CD86 72 h following transfection in the
TLR2-siRNA group, compared with the control group (Fig. 4).
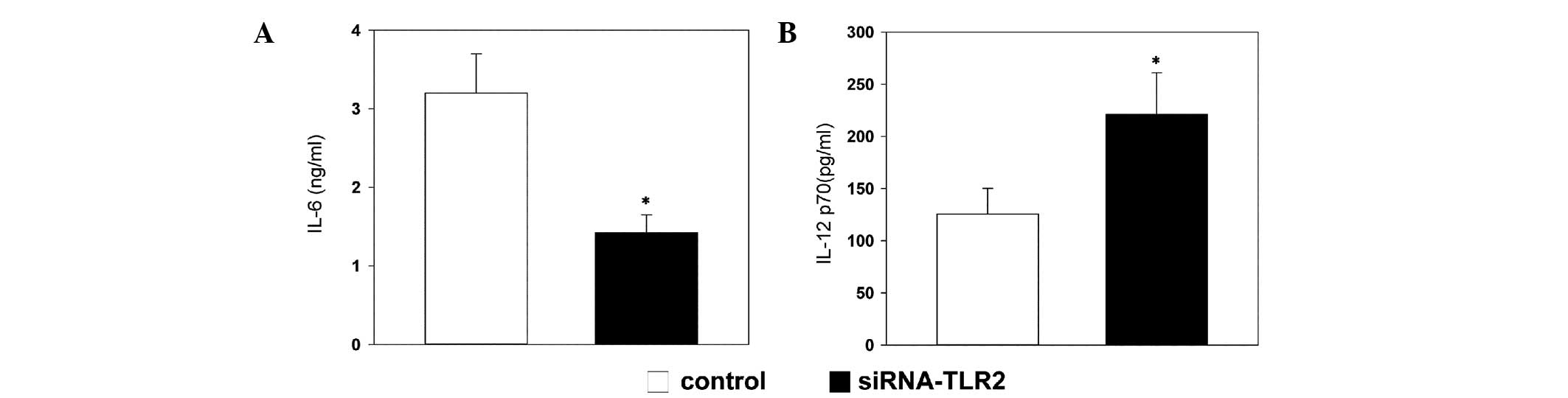
TLR2-siRNA regulates the production of
cytokines
The concentration of IL-6 in the culture
supernatants of the DCs decreased more markedly in the TLR2-siRNA
group, compared with the control group (Fig. 5A). By contrast, the production of
IL-12 was markedly increased in the TLR2-siRNA group, compared with
the control group (Fig. 5B).
Discussion
In the present study, specific siRNA was used to
silence the expression of TLR2. Silencing of TLR2 was found to
down-regulate the expression of CD117 on the surface of DCs. In
addition, it decreased the transcription and translation of c-kit,
a proto-oncogene that encodes a tyrosine kinase receptor. CD117 is
the expression product of c-kit and is a member of the
platelet-derived growth factor family of tyrosine kinase receptors,
which binds to the SCF ligand. Numerous studies have been performed
to investigate the role of c-kit in the development of asthma, and
have indicated that c-kit/SCF is closely associated with mast cell
and eosinophil infiltration of the airways, and the promotion of
mast cell degranulation (16,17).
Pathogen recognition is mediated by a set of
germline-encoded receptors, which are termed pattern-recognition
receptors (PRRs). TLRs are mammalian PRRs, which are important in
the recognition of microbial components, and HDM allergens can be
recognized by TLRs. A previous study suggested that c-kit in DCs
may be activated by HDM allergens, regulate T-helper cell
differentiation and induce the inflammatory response in
experimental allergic mice (10).
Redecke et al (18) also
demonstrated that activation of TLR2 induces a Th2 immune response
and promotes the presentation of experimental asthma; however, the
underlying molecular mechanism remains to be elucidated. In the
present study, specific siRNA inhibition of TRL2 resulted in a
significant decrease in the transcription and expression of c-kit
in DCs. In addition, HDM upregulated the expression of CD117 on the
surface of DCs. These results indicated that activation of TLR2 may
be correlated with the functioning of c-kit, therefore it can be
concluded that there is a HDM-TLR2-c-kit pathway, which induces Th2
activation in response to HDM. More TLR2 and TLR4 are expressed on
the surface of DCs, compared with other TLRs (19). Previous studies have reported that
TLR4 signaling in lung DCs is required for the induction of Th1
responses, but is not essential for the induction of Th2 responses
(20–22).
Co-stimulatory molecules between DCs and T-cells,
including CD80 (B7-1)/CD86 (B7-2) and CD28, are crucial in the
differentiation of naive CD4 T-cells, which appears to be one of
the initial steps in airway sensitization, ultimately leading to
the generation of a Th2-type immune response (23,24).
In the present study, a specific siRNA was used to inhibit the
expression of TLR2. Knockdown of TLR2 effectively downregulated the
expression of CD80 and CD86 in the experimental group, compared
with the control group. These results demonstrated that activation
of TRL2 promoted the expression levels of CD80 and CD86 on the
surface of DCs, inducting the activation and differentiation of
naive CD4 T-cells. It can, therefore, be inferred that DCs
initially bind to HDM allergens through TLR2 and present the
allergen to Th2 cells, resulting in Th2 cell activation via
co-stimulatory molecules. Activated Th2 cells produce various
cytokines, including IL-4, IL-5, IL-13 and GM-CSF, which are
essential in the pathogenesis of asthma by promoting the survival
and recruitment of eosinophils and mast cells (25).
In the present study, by inhibiting the expression
of TLR2 through specific siRNA, the activated DCs produced less
IL-6 and more IL-12. Furthermore, these alterations in cytokine
production were associated with the downregulation of c-kit.
Notably, a previous study demonstrated that direct inhibition of
TLR2 results in the production of IL-12 in macrophages by
interfering with JNK (26). In
addition, IL-6 has been associated with Th2 and Th17
differentiation (27), and DC
production of IL-6 has induces Th-cell differentiation toward the
Th2 cell phenotype. By contrast, IL-12 steers CD4+
T-cell responses toward a Th1 phenotype by inducing the production
of interferon-γ in naive Th-cells (28–30).
Several signaling pathways, including the Ras/extracellular
signal-regulated kinase, phosphatidylinositol 3-kinase (PI3K),
phospholipase C and D, Src kinase and janus kinase/signal
transducer and activator of transcription pathways are activated
downstream of c-kit following ligand binding (31,32).
The c-kit/PI3K signaling axis positively regulates the production
of IL-6. In addition, c-kit is associated with Th2 responses
through the Notch ligand Jagged-2 signaling pathway (9,33).
Overexpression of IL-12 in DCs significantly decreases Th2
sensitization to inhaled antigens and eosinophilic airway
inflammation by skewing the response towards strong Th1 immunity
(29,34). Furthermore, previous studies have
demonstrated that IL-12 is more suitable in reducing the
infiltration of inflammatory cells and inhibiting inflammatory
response in allergic asthma (35,36).
However, endogenous IL-12 contributes to the recruitment of
eosinophils into the airways, as observed in asthma, possibly via
enhancement of the expression of vascular cell adhesion molecule-1
(37). Since the complex signal
transduction pathways ultimately result in the same cytokine
performing distinct functions in the development of asthma,
clarification of the pathogenesis of asthma is difficult.
In conclusion, the results of the present study
demonstrated that TLR2 is important in the activation of c-kit in
DCs. Inhibiting the expression of TRL2 with specific siRNA resulted
in the downregulation of co-stimulatory molecules (B7), reduced
expression of IL-6 and increased expression of IL-12. These effects
were associated with the development of allergic asthma; however,
the involvement of other TLRs in the activation of c-kit in DCs
remains to be elucidated. Further investigations are required to
understand the role of TLRs in the development of asthma.
Acknowledgments
The present study was supported by grants from The
Nature Science Foundation of The Education Department of Shaanxi
Provincial Government (grant. no. 11JK0662), and the Innovation and
Entrepreneurship Training Project of University Student in Shaanxi
Province (grant no. 20131741).
References
|
1
|
Roche N, Chinet TC and Huchon GJ: Allergic
and nonallergic interactions between house dust mite allergens and
airway mucosa. Eur Respir J. 10:719–726. 1997.PubMed/NCBI
|
|
2
|
Edling CE and Hallberg B: c-Kit - a
hematopoietic cell essential receptor tyrosine kinase. Int J
Biochem Cell Biol. 39:1995–1998. 2007. View Article : Google Scholar
|
|
3
|
Lukacs NW, Strieter RM, Lincoln PM,
Brownell E, Pullen DM, Schock HJ, Chensue SW, Taub DD and Kunkel
SL: Stem cell factor (c-kit ligand) influences eosinophil
recruitment and histamine levels in allergic airway inflammation. J
Immunol. 156:3945–3951. 1996.PubMed/NCBI
|
|
4
|
Lukacs NW, Kunkel SL, Strieter RM, Evanoff
HL, Kunkel RG, Key ML and Taub DD: The role of stem cell factor
(c-kit ligand) and inflammatory cytokines in pulmonary mast cell
activation. Blood. 87:2262–2268. 1996.PubMed/NCBI
|
|
5
|
Platt-Mills TA, Sporik RB, Chapman MD and
Heymann PW: The role of indoor allergens in asthma. Allergy. 50(22
Suppl): 5–12. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Platts-Mills TA, Vervloet D, Thomas WR,
Aalberse RC and Chapman MD: Indoor allergens and asthma: Report of
the Third International Workshop. J Allergy Clin Immunol.
100:S2–S24. 1997. View Article : Google Scholar
|
|
7
|
Redecke V, Häcker H, Datta SK, Fermin A,
Pitha PM, Broide DH and Raz E: Activation of Toll-like receptor 2
induces a Th2 immune response and promotes experimental asthma. J
Immunol. 172:2739–2743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hammad H and Lambrecht BN: Dendritic cells
and epithelial cells: Linking innate and adaptive immunity in
asthma. Nat Rev Immunol. 8:193–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Redecke V, Häcker H, Datta SK, Fermin A,
Pitha PM, Broide DH and Raz E: Cutting edge: Activation of
Toll-like receptor 2 induces a Th2 immune response and promotes
experimental asthma. J Immunol. 172:2739–2743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krishnamoorthy N, Oriss TB, Paglia M, Fei
M, Yarlagadda M, Vanhaesebroeck B, Ray A and Ray P: Activation of
c-Kit in dendritic cells regulates T helper cell differentiation
and allergic asthma. Nat Med. 14:565–573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Min WP, Gorczynski R, Huang XY, Kushida M,
Kim P, Obataki M, Lei J, Suri RM and Cattral MS: Dendritic cells
genetically engineered to express Fas ligand induce donor-specific
hypo-responsiveness and prolong allograft survival. J Immunol.
164:161–167. 2000. View Article : Google Scholar
|
|
12
|
Lutz MB, Kukutsch N, Ogilvie AL, Rössner
S, Koch F, Romani N and Schuler G: An advanced culture method for
generating large quantities of highly pure dendritic cells from
mouse bone marrow. J Immunol Methods. 223:77–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng X, Vladau C, Zhang X, Suzuki M,
Ichim TE, Zhang ZX, Li M, Carrier E, Garcia B, Jevnikar AM and Min
WP: A novel in vivo siRNA delivery system specifically targeting
dendritic cells and silencing CD40 genes for immunomodulation.
Blood. 113:2646–2654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Tsuyuki S, Tsuyuki J, Einsle K, Kopf M and
Coyle AJ: Costimulation through B7-2 (CD86) is required for the
induction of a lung mucosal T helper cell 2 (TH2) immune response
and altered airway responsiveness. J Exp Med. 185:1671–1680. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ito T, Smrž D, Jung MY, Bandara G, Desai
A, Smržová Š, Kuehn HS, Beaven MA, Metcalfe DD and Gilfillan AM:
Stem cell factor programs the mast cell activation phenotype. J
Immunol. 188:5428–5437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Columbo M, Horowitz EM, Botana LM,
MacGlashan DW Jr, Bochner BS, Gillis S, Zsebo KM, Galli SJ and
Lichtenstein LM: The human recombinant c-kit receptor ligand,
rhSCF, induces mediator release from human cutaneous mast cells and
enhances IgE-dependent mediator release from both skin mast cells
and peripheral blood basophils. J Immunol. 149:599–608.
1992.PubMed/NCBI
|
|
18
|
Redecke V, Häcker H, Datta SK, Fermin A,
Pitha PM, Broide DH and Raz E: Cutting edge: Activation of
toll-like receptor 2 induces a Th2 immune response and promotes
experimental asthma. J Immunol. 172:2739–2743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Werling D, Hope JC, Howard CJ and Jungi
TW: Differential production of cytokines, reactive oxygen and
nitrogen by bovine macrophages and dendritic cells stimulated with
Toll-like receptor agonists. Immunology. 111:41–52. 2004.
View Article : Google Scholar
|
|
20
|
Agrawal S, Agrawal A, Doughty B, Gerwitz
A, Blenis J, Van Dyke T and Pulendran B: Cutting edge: Different
Toll-like receptor agonists instruct dendritic cells to induce
distinct Th responses via differential modulation of extracellular
signal-regulated kinase-mitogen-activated protein kinase and c-Fos.
J Immunol. 171:4984–4989. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boonstra A, Asselin-Paturel C, Gilliet M,
Crain C, Trinchieri G, Liu YJ and O'Garra A: Flexibility of mouse
classical and plasmacytoid-derived dendritic cells in directing T
helper type 1 and 2 cell development: Dependency on antigen dose
and differential Toll-like receptor ligation. J Exp Med.
197:101–109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Byun EH, Kim WS, Kim JS, Won CJ, Choi HG,
Kim HJ, Cho SN, Lee K, Zhang T, Hur GM and Shin SJ: Mycobacterium
para-tuberculosis CobT activates dendritic cells via engagement of
Toll-like receptor 4 resulting in Th1 cell expansion. J Biol Chem.
287:38609–38624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haczku A, Takeda K, Redai I, Hamelmann E,
Cieslewicz G, Joetham A, Loader J, Lee JJ, Irvin C and Gelfand EW:
Anti-CD86 (B7.2) treatment abolishes allergic airway
hyperresponsiveness in mice. Am J Respir Crit Care Med.
159:1638–1643. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mathur M, Herrmann K, Qin Y, Gulmen F, Li
X, Krimins R, Weinstock J, Elliott D, Bluestone JA and Padrid P:
CD28 interactions with either CD80 or CD86 are sufficient to induce
allergic airway inflammation in mice. Am J Respir Cell Mol Biol.
21:498–509. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hammad H and Lambrecht BN: Dendritic cells
and epithelial cells: Linking innate and adaptive immunity in
asthma. Nat Rev Immunol. 8:193–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang S, Chen Z, Hu C, Qian F, Cheng Y, Wu
M, Shi B, Chen J, Hu Y and Yuan Z: Hepatitis B virus surface
antigen selectively inhibits TLR2 ligand-Induced IL-12 production
in monocytes/macrophages by interfering with JNK activation. J
Immunol. 190:5142–5151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dodge IL, Carr MW, Cernadas M and Brenner
MB: IL-6 production by pulmonary dendritic cells impedes Th1 immune
responses. J Immunol. 170:4457–4464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuipers H, Hijdra D, De Vries VC, Hammad
H, Prins JB, Coyle AJ, Hoogsteden HC and Lambrecht BN:
Lipopolysaccharide-induced suppression of airway Th2 responses does
not require IL-12 production by dendritic cells. J Immunol.
171:3645–3654. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bilenki L, Gao X, Wang S, Yang J, Fan Y,
Han X, Qiu H and Yang X: Dendritic cells from mycobacteria-infected
mice inhibits established allergic airway inflammatory responses to
ragweed via IL-10- and IL-12-secreting mechanisms. J Immunol.
184:7288–7296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuipers H, Heirman C, Hijdra D, Muskens F,
Willart M, van Meirvenne S, Thielemans K, Hoogsteden HC and
Lambrecht BN: Dendritic cells retrovirally overexpressing IL-12
induce strong Th1 responses to inhaled antigen in the lung but fail
to revert established Th2 sensitization. J Leukoc Biol.
76:1028–1038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dabbagh K, Takeyama K, Lee HM, Ueki IF,
Lausier JA and Nadel JA: IL-4 induces mucin gene expression and
goblet cell meta-plasia in vitro and in vivo. J Immunol.
162:6233–6237. 1999.PubMed/NCBI
|
|
32
|
Lennartsson J, Jelacic T, Linnekin D and
Shivakrupa R: Normal and oncogenic forms of the receptor tyrosine
kinase kit. Stem Cells. 23:16–43. 2005. View Article : Google Scholar
|
|
33
|
Amsen D, Blander JM, Lee GR, Tanigaki K,
Honjo T and Flavell RA: Instruction of distinct CD4 T helper cell
fates by different notch ligands on antigen-presenting cells. Cell.
117:515–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hofstra CL, Van Ark I, Hofman G, Kool M,
Nijkamp FP and Van Oosterhout AJ: Prevention of Th2-like cell
responses by coadministration of IL-12 and IL-18 is associated with
inhibition of antigen-induced airway hyperresponsiveness,
eosinophilia, and serum IgE levels. J Immunol. 161:5054–5060.
1998.PubMed/NCBI
|
|
35
|
Wang S, Fan Y, Han X, Yang J, Bilenki L
and Yang X: IL-12-dependent vascular cell adhesion molecule-1
expression contributes to airway eosinophilic Inflammation in a
mouse model of asthma-like reaction. J Immunol. 166:2741–2749.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van der Pouw Kraan TC, Boeije LC, de Groot
ER, Stapel SO, Snijders A, Kapsenberg ML, van der Zee JS and Aarden
LA: Reduced production of IL-12 and IL-12-dependent IFN-gamma
release in patients with allergic asthma. J Immunol. 158:5560–5565.
1997.PubMed/NCBI
|
|
37
|
Kuipers H, Hijdra D, De Vries VC, Hammad
H, Prins JB, Coyle AJ, Hoogsteden HC and Lambrecht BN:
Lipopolysaccharide-induced suppression of airway Th2 responses does
not require IL-12 production by dendritic cells. J Immunol.
171:3645–3654. 2003. View Article : Google Scholar : PubMed/NCBI
|