Introduction
Pelvic organ prolapse (POP) is a common disease
whose treatment is costly and which affects millions of women
worldwide. Research shows that ~30% of elderly women suffer from
prolapse to a certain degree (1).
Regarding the consequences of POP, it can negatively impair the
quality of the patients' life and cause serious inconveniences to
the patients and their families.
Pelvic organs are supported by levator ani muscles
and connective tissue (endopelvic fascia and ligaments) attached to
the pelvic organs. Therefore, the disruption or dysfunction of
these supportive components may lead to a loss of support and
eventually to POP (2). Gestation
and delivery can increase intra-abdominal pressure and damage the
muscles and fascia in the pelvic floor, which is a well-known risk
factor for POP. However, the underlying mechanisms of the effects
of pelvic mechanical forces on POP has remained elusive.
Cells of the human body are equipped with an
anti-oxidant system. Upon excessive production of reactive oxygen
species (ROS) and the presence of excess levels of metabolites, the
redox balance is disturbed, which activates anti-oxidant systems
and triggers an oxidative stress reaction (3,4).
It has been suggested that mechanical forces can
activate oxidative stress signaling pathways. This process is
caused by a redox imbalance, which further leads to vascular
remodeling (5). Cyclic mechanical
forces were shown to significantly increase the levels of
intracellular ROS in myoblasts in a dose-dependent manner (6).
Furthermore, a close correlation between POP and
oxidative stress in the human body has been evidenced. The content
of isoprostanes, which are oxidative stress-associated factors, was
significantly increased in the cardinal ligament of the uterus and
in the urine of patients with uterine prolapse (7). The expression of the
anti-oxidant-associated gene DSCR-1 decreased by 5.1 times in
patients with POP (8). In skin
fibroblasts, oxidative stress disturbs collagen synthesis and gene
expression (9). Therefore, the
present study hypothesized that the oxidative stress pathway is
involved in the development process of POP.
Mitochondria, as the center of cellular energy
metabolism and redox reactions, are the main cellular site of ROS
generation. At the same time mitochondria are important target
organelles of oxidative damage (10). Oxidative stress changes the
mitochondrial membrane permeability, decreases the mitochondrial
membrane potential (ΔΨm) and suppresses the functions of the
mitochondria. Consequently, caspase-3 is activated by pro-apoptotic
molecules such as cytochrome C from mitochondria, which
eventually leads to cell apoptosis (10). A previous study found that the
apoptotic rate was significantly increased in the supportive tissue
of the pelvic floor of POP patients (11). Caspase-3 and -9 expression was
significantly elevated in the sacral ligament of patients with POP,
which confirmed the involvement of mitochondrial apoptosis in the
pathogenesis of POP (10).
The aim of the present study was to determine the
influence of mechanical strain on human parametrial ligament
fibroblasts (HPLFs), as well as to investigate the underlying
mechanisms of the effects. The levels of ROS, changes in the
mitochondrial membrane potential and the apoptotic rate were
determined in order to explore the possible mechanisms of
mechanical factors in the pathogenesis of POP.
Materials and methods
Ethics statement
The present study was performed on human subjects
and was approved by the Ethics Committee of Renmin Hospital of
Wuhan University (Wuhan, China). Written informed consent was
obtained from all patients prior to participation in the study.
Participants
A total of 10 patients who underwent hysterectomy
surgery for reasons excluding the presence of malignant tumors and
POP, were enrolled in the present study. None of the recruited
women had any connective tissue diseases, pathologically confirmed
endometriosis or estrogen-associated ovarian tumors. Furthermore,
the patients were free from any complications that may lead to
oxidative stress-associated diseases, including coronary heart
disease, diabetes and hyperlipidemia. Patients who received surgery
in the uterosacral ligamental site or had a history of estrogen
application within the past three months were excluded from the
present study.
Primary cell culture
The present study adopted a modified enzyme
digestion method (12) for the
establishment of primary cell cultures. Tissue specimens
(0.5×0.5×0.2 cm3) were obtained from part of the
parametrial ligament during surgery (including sacral ligament and
cardinal ligaments). The samples were washed with
phosphate-buffered saline (PBS) containing 100 KU/ml penicillin G
and 100 mg/ml streptomycin (Jenom, Hangzhou, China), and then
minced into small pieces. The tissues were digested with 1%
collagenase-I (Invitrogen Life Technologies, Inc., Carlsbad, CA,
USA) for 3 h at 37°C in 5% CO2, followed by further
digestion with 0.25% trypsin (Sigma-Aldrich, St. Louis, MO, USA)
for 5 min. 2 ml fetal bovine serum (FBS; Gibco-BRL, Invitrogen Life
Technologies) was used to stop the digestion. Dulbecco's modified
Eagle's medium (DMEM; Jenom, Hangzhou, China) containing 15% FBS
was then slowly added to the culture flask. The medium was replaced
every two days and the primary HPLF cell cultures were grown to
confluence for passage. The HPLFs were used at passage 4–8 for the
subsequent experiments.
Immunocytochemical staining
Cells in the logarithmic growth phase were digested
with 0.25% trypsin, re-suspended and seeded into a six-well plate
containing a pre-placed coverslip, followed by incubation for two
days at a cell density of 105 cells/ml. After cells were
~70% confluent, the cover-slips were removed. Cells were washed
twice in PBS and immersed in 4% paraformaldehyde for 30 min for
fixation. Cells then were washed three times in PBS, immersed in 3%
H2O2 and then incubated in the dark. After
the endogenous peroxidase activity was exhausted, cells were washed
three times in PBS and then incubated with mouse monoclonal
vimentin (cat. no. sc-6260; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA; 200 µg/ml), and mouse monoclonal cyto-keratin-19
(cat. no. sc-6278; Santa Cruz Biotechnology, Inc.; 200
µg/ml) antibodies at 4°C overnight followed by three washes
in PBS. Subsequently, the cells were incubated with secondary
antibodies (k5007; Dako, Glostrup, Denmark) at room temperature in
the dark for 1 h followed by three washes in PBS. The
immunohistochemical stain was evaluated using an upright microscope
(BX51; Olympus, Tokyo, Japan).
Compression of human parametrial ligament
fibroblasts
The four-point bending device (Miracle Technology
Co., Ltd., Chengdu, China) is divided into three parts: Mechanical
power systems, host computer and strain-loading dish. The
deformation displacement, loading frequency and loading time were
set via the host computer. An engine was used to generate a
mechanical force, which was used to exert a strain onto a
petri-dish containing cells via a stamping motion. Once the petri
dish was bent, a corresponding force was exerted on the cells in
the petri dish.
Using the four-point bending system for mechanical
loading, the fibroblasts in the exponential growth phase at passage
4–8 derived from 10 patients were subjected to the loading strain.
Parameters were set to a frequency of 0.1 Hz over 4 h, and cells
were subjected to strains of 1,333 µ (1 mm), 2,666 µ
(2 mm) and 5,333 µ (4 mm). The control group samples (0 mm)
were incubated at 37°C without any treatment under the same culture
conditions.
Detection of ROS
The ability of mechanical stress treatment to
increase ROS production in HPLFs was measured by the fluorescent
probe 2′,7′-dichlorodihydrofluorescein diacetate
(H2DCF-DA; Beyotime Institute of Biotechnology,
Shanghai, China). Fibroblasts were subjected to strains of 0,
1,333, 2,666 and 5,333 µ for 4 h using the four-point
bending device. The cells were then washed three times with PBS and
incubated for 40 min at 37°C with 1.5 µl H2DCF-DA
in serum-free medium. Cells were again washed three times with PBS,
and fresh serum-free DMEM was added. Images of cells with
ROS-associated fluorescence were captured using an inverted
fluorescence microscope (CKX31; Olympus, Tokyo, Japan), and images
were analyzed using Image J version 1.46 software (National
Institutes of Health, Bethesda, MD, USA).
JC-1 staining
JC-1 (Beyotime Institute of Biotechnology) is a
sensitive fluorescent probe which can be utilized to measure the
ΔΨm. In healthy cells with high mitochondrial ΔΨm, JC-1
spontaneously forms complexes known as JC-1 aggregates with intense
red fluorescence. By contrast, in apoptotic or unhealthy cells with
low ΔΨm, JC-1 remains in the monomeric form, which shows only green
fluorescence. After the fibroblasts had been subjected to the
respective strains for 4 h, they were rinsed twice with PBS and
incubated with a mixture of 2 µl JC-1 staining solution and
2 ml serum-free medium in the dark at 37°C for 30 min according to
the manufacturer's instructions. The cells were then washed with
JC-1 staining buffer twice, and 2 ml serum-free DMEM was added to
each specimen. The JC-1 fluorescence was observed under the
inverted fluorescence microscope. Image J software was employed to
analyze the red and green fluorescence intensity. The ratio of red
to green fluorescence was calculated, which was indicative of the
ΔΨm.
Detection of apoptosis by Annexin
V/propidium iodide (PI)
The HPLFs were subjected to strains as mentioned
above. Subsequently, the apoptotic rate was determined by Annexin
V/PI (Beyotime Institute of Biotechnology) double staining
according to the manufacturer's instructions. Briefly, cells from
different groups were harvested, washed with ice-cold PBS twice and
re-suspended in 400 µl binding buffer. 5 µl
fluorescein isothiocyanate-conjugated Annexin V and 10 µl PI
were added, followed by incubation for 20 min in the dark at room
temperature. The apoptotic rate was analyzed by flow cytometry (BD
LSR II; BD Biosciences, Franklin Lakes, NJ, USA) using Flow Jo
software 7.6 (BD Biosciences). The cells which stained positive for
Annexin V and negative for PI were considered to be early
apoptotic, while those which were positive for both were identified
as late apoptotic cells. The apoptotic rates were expressed as the
percentage of the total cell population.
Statistical analysis
Statistical analyses were performed using SPSS 16.0
(SPSS Inc., Chicago, IL USA). Image J software-generated
fluorescence values and cell ratios in Flow Jo software 7.6 format
were imported to SPSS, and the significance of differences between
groups was tested by analysis of variance. P<0.05 was considered
to indicate a statistically significant difference between
values.
Results
Primary culture and identification of
HPLFs
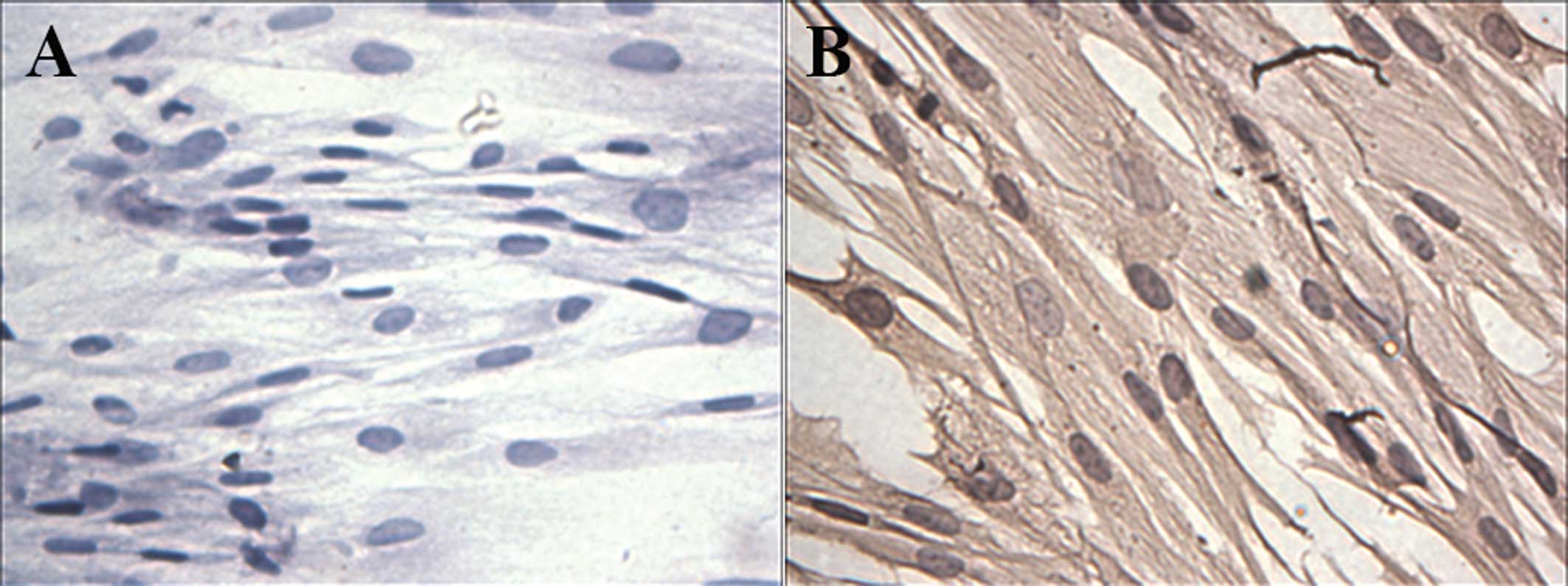
The fibroblasts mainly appeared as long spindles,
but also as irregular triangles, polygons and other shapes as
observed by light microscopy (Fig.
1). Cells were connected to each other to form a network
structure. Immunohistochemical staining showed that cells were
negative for cytokeratin (Fig 1A)
and positive for vimentin (Fig.
1B).
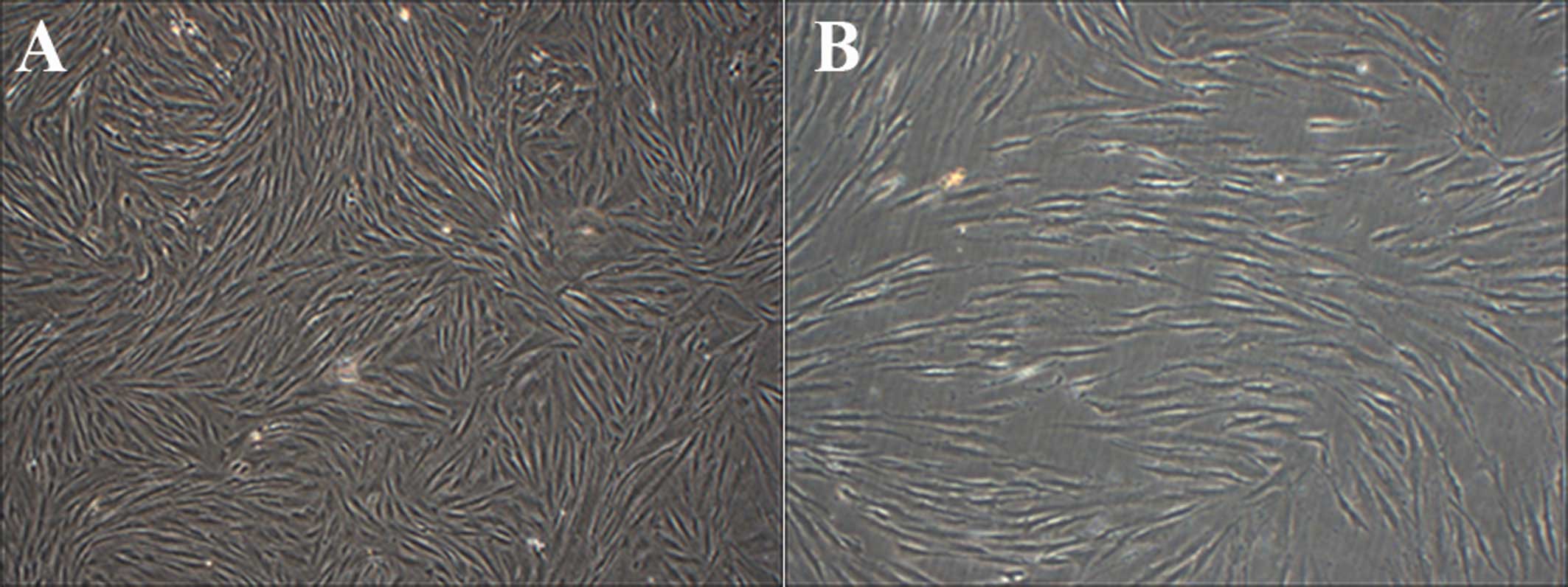
Mechanical stress loading leads to
morphological changes of HPLFs
Under all mechanical stress loading conditions, cell
morphology was atrophied, cell junctions appeared loose and
weakened. Sections of the fibroblasts suffered a deformation from
long spindles to round bodies. A 4-mm strain increased the rate of
cell disruption and cell shedding (Fig. 2).
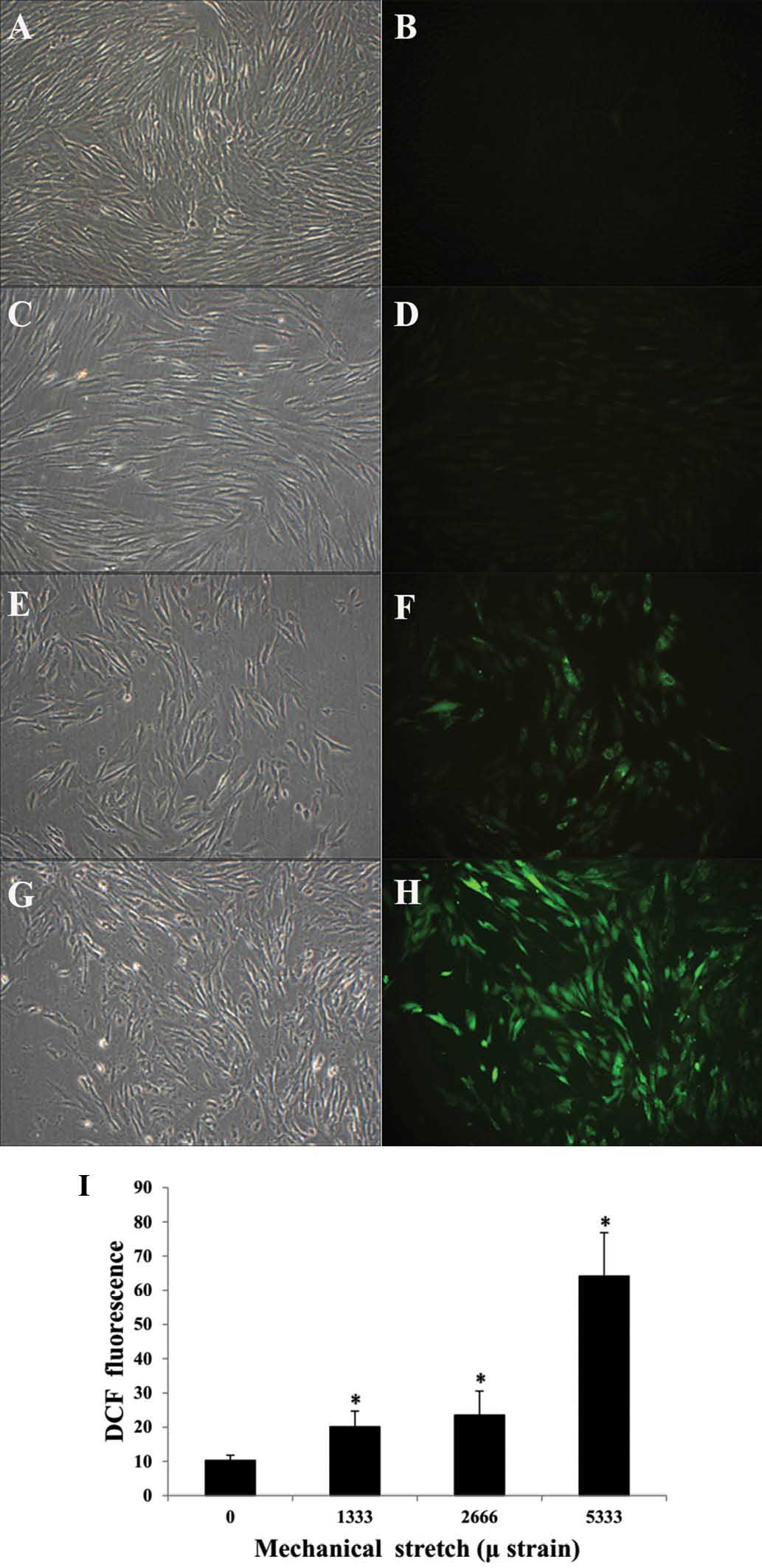
Mechanical stress loading increases
intracellular ROS levels in HPLFs
In the control group, the ROS-associated
fluorescence was weak (Fig. 3A and
B), while in the experimental groups, ROS fluorescence was
enhanced after mechanical stress loading (Fig 3C-H). The quantified fluorescence
intensity following exposure to strains of 0, 1,333, 2,666 and
5,333 µ was 10.27±1.53, 20.13±4.55, 23.55±6.99 and
64.15±12.68, respectively, indicating that ROS production was
significantly increased in the experimental groups compared with
that in the control group (Fig.
3I).
Mechanical stress loading decreases the
ΔΨm in HPLFs
In the control cells, the JC-1 dye emitted a red
fluorescence, indicating an intact ΔΨm (Fig. 4A–C). After mechanical stress
loading, the red fluorescence of the JC-1 dye was weakened, and
green fluorescence was enhanced (Fig.
4D–I). Quantitative analysis of the fluorescent microscopy
images with Image J software showed that the ratio of red to green
fluorescence of cells subjected to strains of 0, 1,333, 2,666 and
5,333 µ was 5.26±0.62, 1.03±0.014, 1.19±0.03 and 0.44±0.01,
respectively (Fig. 4M). The
decreased red to green fluorescence ratio of JC-1 confirmed the
decline in the ΔΨm.
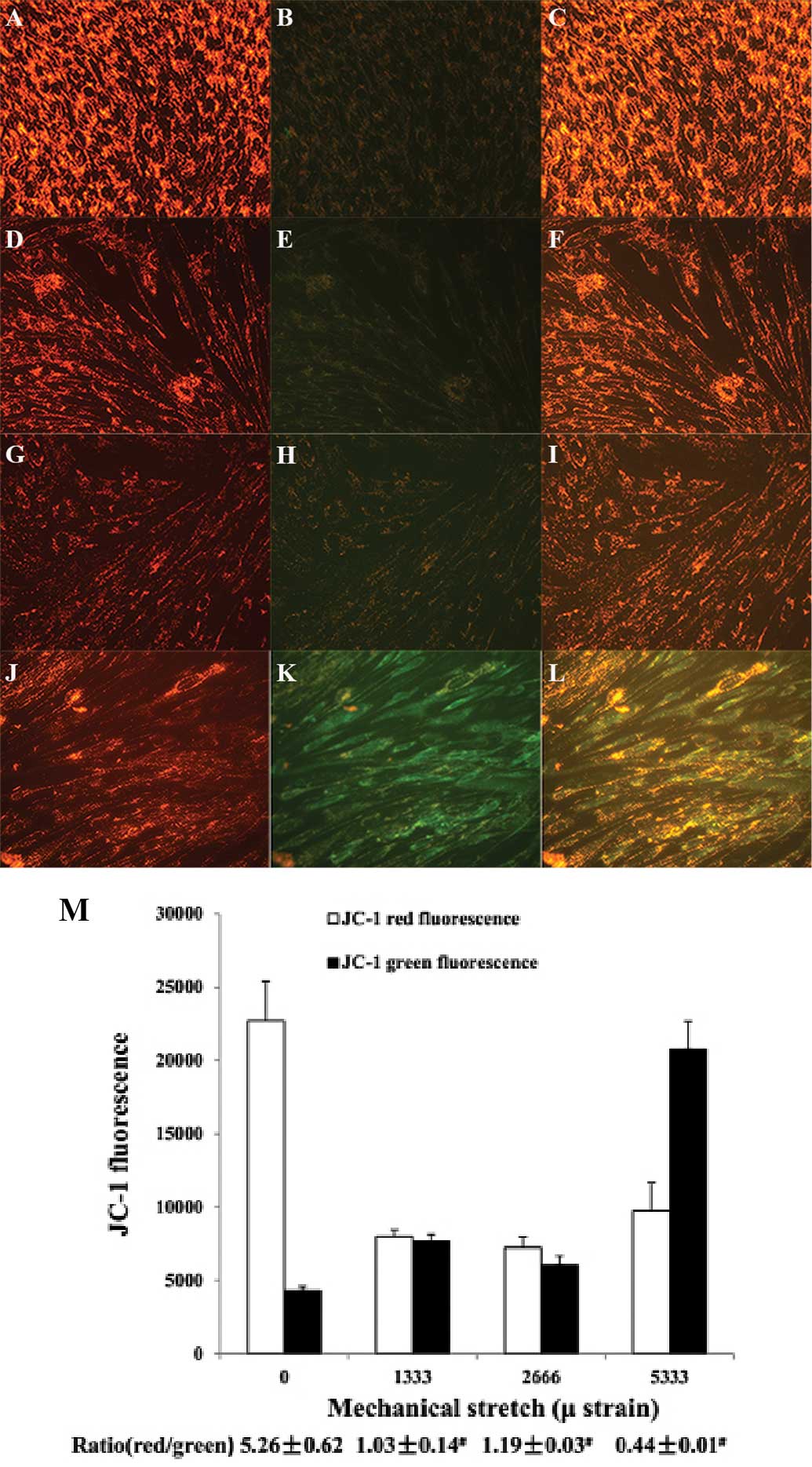 | Figure 4Assessment of the mitochondrial
membrane potential by JC-1 staining. Representative fluorescence
photomicrographs of in-situ JC-1-stained cells in various treatment
groups (magnification, ×200). (A–C), Control; (D–F), 1,333-µ
strain; (G–I), 2,666-µ strain; (J–L), 5,333-µ strain.
Images on the left show red fluorescence, indicating a high
mitochondrial membrane potential; the middle panel shows green
fluorescence, indicative of a loss of mitochondrial membrane
potential. The right-hand panel shows merged red and green
fluorescence. (M) Red and green fluorescence intensity was
quantified by Image J software. The ratio of red to green
fluorescence intensity is indicative of the mitochondrial membrane
potential. Values are expressed as the mean fluorescence intensity
± standard deviation of five fields of view per group. |
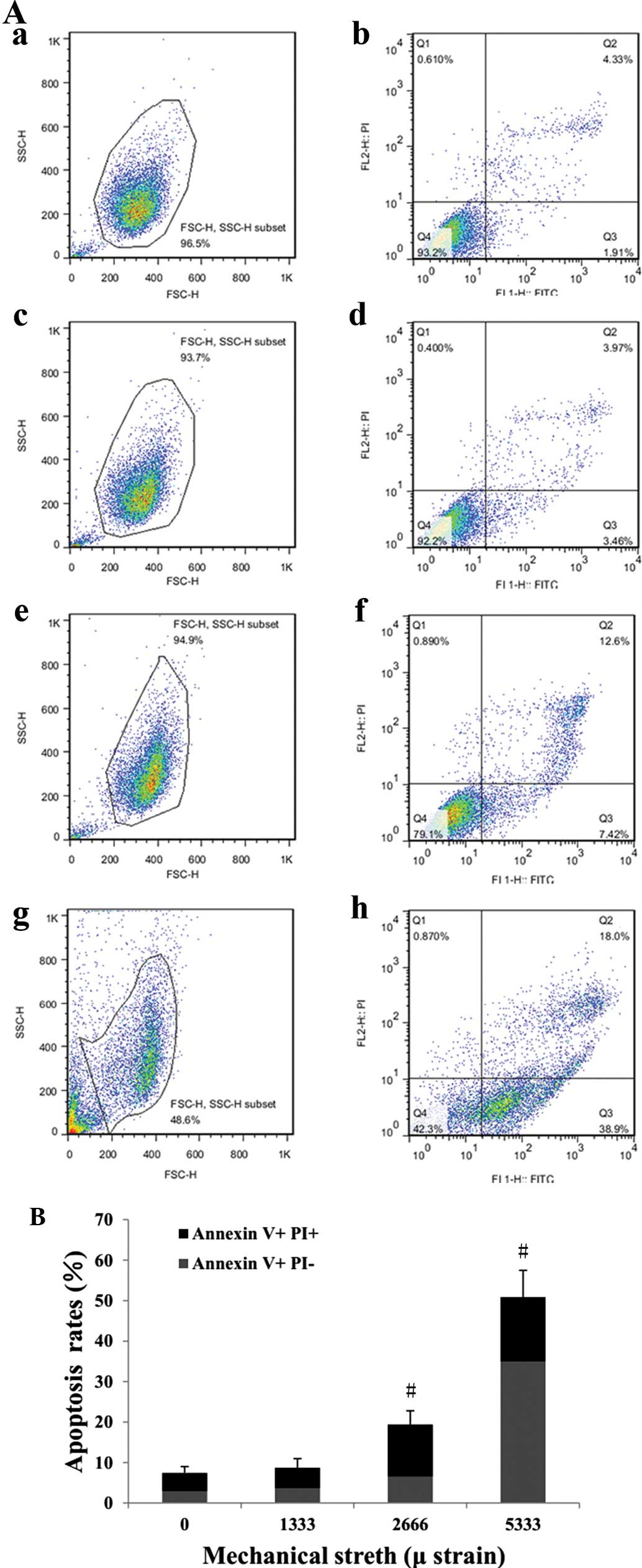
Mechanical stress loading increases the
apoptotic rate of HPLFs
Apoptosis was assessed using Annexin V/PI
double-staining and flow cytometric analysis (Fig. 5A). The quantified results showed
that a strain of 1,333 µ did not markedly affect the
apoptotic rate, while strains of 2,666 and 5,333 µ
significantly enhanced the apoptotic rate compared with that of the
control group (Fig. 5B). In
particular, a strain of 5,333 µ had a marked apoptotic
effect, leading to an apoptotic rate of ~50% among non-necrotic
cells (~50% of total population) with a large amount of cell debris
(Fig. 5Ag and h).
Discussion
Over a woman's lifetime, her pelvic floor tissues
bear various mechanical stresses, including pregnancy, childbirth,
defecation, cough and normal gravity, which contribute to an
increased intra-abdominal pressure (13). POP is thought to be caused by a
decrease in the biomechanical properties of pelvic supports
(14). Uterine cardinal ligaments
and utero-sacral ligaments surrounding the cervix are the major
ligaments to bear pressures and maintain the uterus' normal
position, and patients with POP who suffer an uterine prolapse
attack present with a decreased strength of these ligaments
(15). The decreasing
biomechanical properties are linked with tissue components,
including cells and extracellular matrix (ECM); the ECM is
synthesized and secreted by fibroblasts in response to strain.
Therefore, the present study focused on the mechanisms of the
effects of mechanical strain in the pathogenesis of POP. A
four-point bending device was used to exert cyclic tensions on
fibroblasts of uterus ligaments in order to mimic the
intra-abdominal mechanical strain. The present study aimed at
exploring whether the cytological changes of POP, including
oxidative stress injury, mitochondrial apoptosis and cellular
apoptosis, matched with the mechanical strain loading on the
fibroblasts of uterus ligaments. It has been reported that
four-hour mechanical strain loading on fibroblasts of periodontal
ligaments by a four-point bending device led to obvious changes of
these cells (11). The
experimental conditions of this previous study, combined with the
results of previous preliminary experiments by our group, were used
to select the strains exerted on the uterine ligamental fibroblasts
in the present study (1,333, 2,666 or 5,333-µ strains over
four hours).
In the present study, morphological observation
indicated that part of the fibroblasts suffered a deformation from
long spindles to round bodies under the action of mechanical force.
The cells became easily detached from the petri-dishes, the cell
morphology was atrophied and cell junctions appeared loose, which
was similar to the observations of a previous study (16). It has been reported that the
morphology of fibroblasts undergoes regular changes, and that cells
are oriented perpendicular to the direction of the strain (17). The present study failed to observe
any obvious changes in cell orientation, which may be due to the
short time of mechanical stress loading. A certain amount of time
is required for the cell orientation to change. Future studies by
our group will further investigate this matter.
Furthermore, the production of ROS was assessed in
HPLFs subjected to mechanical stress. ROS-associated fluorescence
in the HPLFs increased with the enhancement of mechanical stress
loading, particularly under the mechanical strain of 5333 µ.
ROS are known to be the main source of oxidative stress, which may
cause oxidative modifications of DNA, lipids and proteins. Under
physiological conditions, modest ROS have positive effects on cells
by scavenging endotoxin and are involved in the regulation of cell
growth, serving as a second messenger (18). However, when the production of ROS
becomes excessive, it may have harmful effects on cells. Similarly,
human pelvic supportive structures continuously inherit
physiological mechanical stress under normal circumstances, which
may also have a beneficial influence on cells. Numerous studies
have shown that mechanical forces can lead to oxidative damage in
variety of non-pelvic floor cell types (5–6,19).
Therefore, the present study hypothesized that these risk factors,
including pregnancy, childbirth, chronic cough and constipation,
increase the abdominal pressure, which exerts excessive mechanical
forces and may induce ROS production, leading to imbalances in the
redox equilibrium of pelvic organs. The results of the present
study demonstrated that low mechanical strain induced a slight
increase in ROS, which was further elevated with the enhancement of
mechanical stress.
The present study assessed the effects of mechanical
stress on the ΔΨm of HPLFs. Mitochondria are among the primary
organelles targeted by oxidative stress, as excessive ROS can
directly harm mitochondria (20).
Low permeability and the electrochemical proton gradient of the
mitochondrial inner membrane are the basis for maintaining the ΔΨm
and essential for normal physiological function. The ΔΨm decreases
in the early stage of apoptosis, triggering a series of biochemical
changes, including the release of cytochrome C and B-cell lymphoma
2 (Bcl-2) as well as the activation of caspase. Cytochrome C and
Bcl-2, as caspase activators, regulate energy metabolism and
cellular apoptosis. Caspases can trigger the cascade reaction of
cellular apoptosis, resulting in cellular death. It has been
reported that mechanical strain can cause a decrease of myocardial
ΔΨm (21,22). The results of the present study
indicated that the ΔΨm of HPLFs decreased following mechanical
stress. When HPLFs were subjected to strains of 1,333 and 2,666
µ, the ΔΨm dropped as indicated by a ratio of red to green
fluorescence of ~1 as opposed to ~5 in the control group. Of note,
a mechanical strain of 5,333 µ caused a distinct increase of
green fluorescence intensity and the ratio of red to green
fluorescence intensity was <0.4. These results proved that
mechanical stress decreased the ΔΨm. The decline of the ΔΨm can
active the mitochondrial apoptotic pathway, which results in
cellular apoptosis.
Mechanical stress, such as tension and fluid shear
stress, and the cellular apoptotic rate are linked, and when cells
are exposed to forces above physical stimulation, the apoptotic
rate increases in proportion with the magnitude of the force
(23,24). However, to date, the underlying
mechanism of mechanical stress-induced cellular apoptosis has
remained elusive. Li et al (25) suggested that mechanical
load-induced apoptosis of articular cartilage may be associated
with the release of a large amount of calcium ions by the
endoplasmic reticulum, leading to the activation of a mitochondrial
apoptotic pathway. Ding et al (26) reported that compression-induced
apoptosis of nucleus pulposus cells was associated with
ROS-mediated mitochondrial apoptosis. A histological study of
tissues from patients with POP also proved that the mitochondrial
apoptotic pathway was activated (10). Thus, the present study hypothesized
that mechanical stress may cause apoptosis of fibroblasts from
uterus ligaments through the activation of the mitochondrial
apoptotic pathway. The present study showed that a mechanical
strains significantly increased the cellular apoptosis rate to
>17 times that of the control cells. Furthermore, the present
study and our preliminary results indicated that high mechanical
strains led to increased cellular detachment from the slide,
significant increases in intracellular ROS production, decreases in
the ΔΨm and a high level of apoptosis, which proceeded via the
mitochondrial pathway. The results of the present study verified
its preliminary hypothesis that mechanical stress initiated
mitochondrial-depended apoptosis in HPLFs via injuring their
mitochondria on the account of oxidative stress. Furthermore,
cellular fragmentation was enhanced by a strain of 5,333 µ,
leading of a population of non-necrotic cells of only 50% out of
which 50% were apoptotic, while a strain of 2,666 µ resulted
in 95% non-necrotic cells. The low amount of necrosis in the
lower-strain groups may be due to the short time (four hours) over
which the strain was applied, and in which time cellular apoptosis
mainly stayed in the early stage. It is possible that the increased
amount of cellular fragments was caused by excessive mechanical
stress, which directly damaged cellular integrity. Therefore, the
strain of 5,333 µ mechanically damaged the fibroblasts of
uterus ligaments and the oxidative stress-induced mitochondrial
pathway may therefore not be the only mechanism leading to cellular
apoptosis under high mechanical stress loading. These remaining
questions will be addressed in further studies by our group.
As one of the most important risk factors involved
in POP pathogenesis, mechanical strain has already drawn the
attention of numerous researchers. Mechanical indicators of POP
patients' pelvic supports and possible intervention methods have
been studied previously (27). At
present, there is no established animal model or fibroblasts cell
line of uterus ligaments to be experimented on, which has
restricted the development of POP research. The present study
clarified the pathogenesis of POP from the angle that mechanical
strain induced oxidative stress, which in turn caused apoptosis of
HPLFs via a mitochondrial pathway, and provided a theory for the
association between oxidative stress-associated cell injury and POP
pathogenesis. However, the present study only provided preliminary
information on the effects of mechanical strain on the development
of POP, which require further in-depth mechanistic study. In
particular, the effects of various intensities and periods of
mechanical strain require to be tested. Further study is also
required regarding the cytomechanical differences between HPLFs
from POP and non-POP patients, and the possible attenuating effects
of anti-oxidative agents on the development of POP may be worth
investigating.
Acknowledgments
This work was supported by the National Natural
Science Foundation of China (no. 81270684) and the Foundation of
Collaborative and Innovation Projects of Wuhan University School of
Medicine (no. 523-266078).
References
|
1
|
Peng P, Zhu L, Lang J, Wang WY and Shi HH:
Unilateral sacrospinous ligament fixation for treatment of genital
prolapse. Chin Med J (Engl). 123:19952010.
|
|
2
|
Jelovsek JE, Maher C and Barber MD: Pelvic
organ prolapse. Lancet. 369:1027–1038. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wondrak GT, Roberts MJ, Jacobson MK and
Jacobson EL: 3-hydroxypyridine chromophores are endogenous
sensitizers of photooxidative stress in human skin cells. J Biol
Chem. 279:30009–30020. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mizutari K, Ono T, Ikeda K, Kayashima K
and Horiuchi S: Photo-enhanced modification of human skin elastin
in actinic elastosis by N(epsilon)-(carboxymethyl) lysine, one of
the glycoxidation products of the maillard reaction. J Invest
Dermatol. 108:797–802. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu D and Kassab GS: Role of shear stress
and stretch in vascular mechanobiology. J Roy Soc Interface.
8:1379–1385. 2011. View Article : Google Scholar
|
|
6
|
Tan JL: Cell proliferation,
differentiation, apoptosis under a serial stretches and the
underlying mechanisms. PhD dissertation. The Fourth Military
Medical University; Xi'an: 2010
|
|
7
|
Choy KW, Liu YM, Chu CY, Wang CC, Lui WT,
Lee LL, Pang MW, Rogers MS and Yip SK: High isoprostane level in
cardinal ligament-derived fibroblasts and urine sample of women
with uterine prolapse. BJOG. 115:1179–1183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Visco AG and Yuan L: Differential gene
expression in pubococ-cygeus muscle from patients with pelvic organ
prolapse. Am J Obstet Gynecol. 189:102–112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Makpol S, Azura Jam F, Anum Mohd Yusof Y
and Zurinah Wan Ngah W: Modulation of collagen synthesis and its
gene expression in human skin fibroblasts by tocotrienol-rich
fraction. Arch Med Sci. 7:889–895. 2011. View Article : Google Scholar
|
|
10
|
Kim E J, Chung N, Park SH, Lee KH, Kim SW,
Kim JY, Bai SW and Jeon MJ: Involvement of oxidative stress and
mitochondrial apoptosis in the pathogenesis of pelvic organ
prolapse. J Urol. 189:588–594. 2013. View Article : Google Scholar
|
|
11
|
Wen Y, Ho JY, Polan ML and Chen B:
Expression of apoptotic factors in vaginal tissues from women with
urogenital prolapse. Neurourol and Urodynam. 30:1627–1632. 2011.
View Article : Google Scholar
|
|
12
|
Bao R, Liu X and Zhou J: Primary culture
and identification of human fibroblasts. J XinX Med Coll.
5:0152011.In Chinese.
|
|
13
|
Chow D and Rodríguez LV: Epidemiology and
prevalence of pelvic organ prolapse. Curr Opin Urol. 23:293–298.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jean-Charles C, Rubod C, Brieu M,
Boukerrou M, Fasel J and Cosson M: Biomechanical properties of
prolapsed or non-prolapsed vaginal tissue: impact on genital
prolapse surgery. Int Urogynecol J. 21:1535–1538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lang JH and Zhang XD: Obstetrics and
Gynecology Clinical Anatomy. 2010 edition. Shandong Science and
Technology Press; Jinan: pp. 692010
|
|
16
|
Ewies AA, Elshafie M, Li J, Stanley A,
Thompson J, Styles J, White I and Al-Azzawi F: Changes in
transcription profile and cytoskeleton morphology in pelvic
ligament fibroblasts in response to stretch: The effects of
estradiol and levormeloxifene. Mol Human Reprod. 14:127–135. 2008.
View Article : Google Scholar
|
|
17
|
Li XN: Study on the methods and platform
of image processing for cellular biomechanical experiments. PhD
dissertation. Sichuan University; Chengdu: 2003
|
|
18
|
Fransen M, Nordgren M, Wang B and
Apanasets O: Role of peroxisomes in ROS/RNS-metabolism:
Implications for human disease. Biochim Biophys Acta.
1822:1363–1373. 2012. View Article : Google Scholar
|
|
19
|
Grote K, Flach I, Luchtefeld M, Akin E,
Holland SM, Drexler H and Schieffer B: Mechanical stretch enhances
mRNA expression and proenzyme release of matrix metalloproteinase-2
(MMP-2) via NAD (P) H oxidase-derived reactive oxygen species. Circ
Res. 92:e80–e86. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teshima Y, Takahashi N, Thuc LC, Nishio S,
Nagano-Torigoe Y, Miyazaki H, Ezaki K, Yufu K, Hara M, Nakagawa M
and Saikawa T: High-glucose condition reduces cardioprotective
effects of insulin against mechanical stress-induced cell injury.
Life Sci. 87:154–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choudhary R, Baker KM and Pan J: All-trans
retinoic acid prevents angiotensin II- and mechanical
stretch-induced reactive oxygen species generation and
cardiomyocyte apoptosis. J Cell Physiol. 215:172–181. 2008.
View Article : Google Scholar
|
|
23
|
Meyer T, Meyer U, Stratmann U, Wiesmann HP
and Joos U: Identification of apoptotic cell death in distraction
osteogenesis. Cell Biol Int. 23:439–446. 1999. View Article : Google Scholar
|
|
24
|
Ning QM and Qang XR: Early apoptosis
induced by mechanical stretch in human alveolar typeIIepithelial
cells. Journal of Shanghai Jiao Tong University (Medical Science
Edition). 27:961–964. 2007.
|
|
25
|
Li H, Zhang XY, Wu TJ, Cheng W, Liu X,
Jiang TT, Wen J, Li J, Ma QL and Hua ZC: Endoplasmic reticulum
stress regulates rat mandibular cartilage thinning under
compressive mechanical stress. J Biol Chem. 288:18172–18183. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding F, Shao ZW, Yang SH, Wu Q, Gao F and
Xiong LM: Role of mitochondrial pathway in compression-induced
apoptosis of nucleus pulposus cells. Apoptosis. 17:579–590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miao YL, Hu CH, Wang JL, et al: The
preliminary study on biomechanical properties of posterior vaginal
wall tissue of the patient with rectocele. Chinese J Clin Obstet
Gynecol. 9:180–183. 2008.In Chinese.
|