Introduction
2-Methoxyestradiol (2-ME) is a metabolite of the
endogenous estrogen hormone, 17β-estradiol (E2) and the oral
contraceptive agent 17-ethylestradiol, which is produced by
sequential hepatic hydroxylation and methylation from the parent
compounds (1). 2-ME is generated
by catechol-O-methyltransferase, an enzyme expressed in various
mammalian tissues, including the liver, kidneys, brain and red
blood cells (2). 2-ME is the major
catechol estrogen produced by the cytochrome P450 system in the
liver (3). Estrogens act as
important regulators of cell proliferation, cell survival, and
differentiation in a variety of organs and tissues. In addition,
estrogens have been implicated in the etiology of a variety of
malignant cancers and benign tumors (4).
The estrogen metabolite, 2-ME, is an
anti-proliferative and anti-angiogenic molecule that effectively
induces apoptosis in actively proliferating cells in vitro
and in vivo (5,6). 2-ME was found to act as a potent
inhibitor of estrogen-induced proliferation in F344 rat pituitaries
(7). Various mechanisms of 2-ME
activity have been proposed, such as exerting effects on tubulin
polymerization and depolymerization (8,9) or
inducing mitochondrial apoptotic signaling (10). In breast cancer, however, higher
baseline levels of estrogen and certain estrogen metabolites (total
estradiol, bioavailable estradiol, estrone and estrone sulfate)
were associated with higher breast cancer risk in post-menopausal
females, other forms of the estrogen metabolite, 2-ME were also
known as antitumor reagents (11).
Estrogens may cause de novo breast cancer through either
receptor-dependent or independent mechanisms, and through actions
mediated by its receptor, estradiol, which enhances cell
proliferation, a factor causally associated with breast cancer
development (12). Furthermore,
2-ME is recognized for its unique and profound actions on various
tumor cell lines and cancer cells, independent of the hormone
receptor status (13). Regardless
of differences in function, 2-ME has an affinity for estrogen
receptors (ERs) (14,15), however, the underlying mechanisms
of 2-ME via ERs have not been fully elucidated.
Previous studies have shown that CaBP-9k, a
cytosolic calcium binding protein, is expressed in a variety of
mammalian tissues, including the uterus, placenta, the intestine,
kidneys, and pituitary glands (16,17).
In addition, the CaBP-9k gene is well understood in the uterus and
the levels of CaBP-9k mRNA and protein, which are induced by
endocrine disruptors (EDs), were considered to be facilitative
during the screening of environmental estrogenic compounds in an
immature rat model (18). In
addition, CaBP-9k expression was strongly regulated by sex-steroid
hormones in the uterus and pituitary glands of mice (16,19).
Uterine lactoferrin (Ltf), an iron-binding glycoprotein that is
present in the majority of exocrine secretions and in the secondary
granules of leukocytes, is regulated by estrogen in the
reproductive tract (20). In
previous studies, Ltf was localized in uterine epithelial cells and
was shown to fluctuate in a mature mouse, during the estrous cycle,
in response to the rise and fall of estrogen levels (20,21).
The GH3 cell line, a well-established pituitary cell line, is
sensitive to estrogenic stimulation (22). GH3 cells possess an ER that
correlates with cell proliferation (23). Yang et al reported that
treatment with sex-steroids and EDs induces CaBP-9k expression in
GH3 cells (24).
The estrogenic or non-estrogenic activity of
estrogen metabolites and EDs has been evaluated in recent studies
(25,26). However, the residual effect of high
dose 2-ME administered to cancer patients on physiological
conditions remains to be fully elucidated. In the current study, it
was hypothesized that the estrogenicity of 2-ME may increase the
expression of estrogen-mediated genes and contribute to the
induction of endocrine disturbance. Therefore, estrogen response
genes were investigated using CaBP-9k, Ltf, and mRNA expression
levels of steroid hormone receptors in in vitro and in
vivo models.
Materials and methods
Chemicals
E2, progesterone (P4) and mifepristone (RU486) were
obtained from Sigma-Aldrich (St. Louis, MO, USA). 2-ME and ICI
182,780 were purchased from Tocris Bioscience (Bristol, UK).
Cell culture
GH3 cells were obtained from The Korean Cell Line
Bank (Seoul, Republic of Korea). The cells were grown as a
monolayer culture in Dulbecco's modified Eagle's medium (DMEM;
Gibco Life Technologies, Carlsbad, CA, USA), supplemented with 10%
fetal bovine serum (FBS; Gibco Life Technologies), 100 IU/ml
penicillin and 100 µg/ml streptomycin (Gibco Life
Technologies) at 37°C in a humidified atmosphere of 5%
CO2. The GH3 cells were plated on 6-well plastic tissue
culture dishes (NUNC, Roskilde, Denmark) at a density of
3×105 cells/well and grown until 70–80% confluence was
reached. The media was replaced with phenol red-free DMEM
supplemented with 5% charcoal/dextran (CD)-stripped FBS and 100
U/ml penicillin-streptomycin for seven days to ensure depletion of
the steroid hormones in the cells. The cells used in the present
study were grown normally throughout the investigation. After seven
days, the cells were exposed to a single dose of E2
(10−9 M), P4 (10−6 M), 2-ME (10−8,
10−7 or 10−6 M). Each chemical was dissolved
in 100% dimethyl sulfoxide (DMSO) and added to phenol red-free
DMEM-5% CD-FBS (starvation media) with a final DMSO concentration
of 0.1%. The starvation media with 10−9 M E2 served as a
positive control and starvation media alone served as the negative
control [vehicle (VE)]. The GH3 cells were harvested 24 h after
treatment for measurement of the mRNA levels. To examine the
mechanism of CaBP-9k induction by these chemicals, the cells were
pre-treated with 10−7 M ICI 182, 780 and 10−6
M RU486 for 30 min prior to exposure to E2 and 2-ME (27,28).
Following treatment with ICI 182,780 and RU486, the cells were
treated with E2 (10−9 M) and 2-ME (at 10−8,
10−7 or 10−6 M). After 24 h, whole cells were
harvested for mRNA analysis. All of the experiments were performed
in duplicate.
Animals and treatments
Female Institute for Cancer Research mice (postnatal
day 14) were obtained from Koatech Co., Ltd. (Gyeonggi, Republic of
Korea) and maintained in the animal facility at the College of
Veterinary Medicine, Chungbuk National University (Chungbuk,
Republic of Korea). The use of the animals and the experimental
procedures were approved by the Institutional Animal Care and Use
Committee of Chungbuk National University. All of the animals were
housed in polycarbonate cages and used after acclimation to an
environmentally controlled room (temperature, 23±2°C; relative
humidity, 50±10%; frequent ventilation and a 12:12 h light-dark
cycle). The mice were fed soy-free pellet food (Dyets Inc.,
Bethlehem, PA, USA) and had access to water ad libitum
throughout the study. The 35 mice were divided into seven groups
(n=5 per group), and each group was administered subcutaneously
with 24% DMSO, 38% ethanol and 38% sterile saline (VE), 40
µg/kg body weight (BW) E2 (a physiological dose level), or
4, 40 or 80 mg/kg BW 2-ME for three days. The mice were euthanized
24 h after the final injection. To investigate the effect of
antagonism, 10 rats were injected subcutaneously with 10 mg/kg BW
ICI 182,780 and 10 mg/kg BW RU486 30 min prior to injection with 40
mg/kg BW 2-ME for three days. The rats were euthanized 24 h after
the final injection.
Uterine wet weight
Mice were sacrificed by cervical dislocation. The
uterus was excised completely and trimmed free of fat. The body of
the uterus was cut just above the junction with the cervix. The wet
weight of the mouse uterus was recorded.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRI reagent (Ambion
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions and the concentration of total RNA was
determined by measuring the absorbance at 260 nm. Total RNA (1
µg) was reverse transcribed into first-strand cDNA using
M-MLV Reverse Transcriptase (Invitrogen Life Technologies,
Carlsbad, CA, USA) and 9-mer random primers (Takara Bio, Inc.,
Otsu, Japan).
RT-qPCR was performed using a 7300 Real-Time PCR
system (Applied Biosystems Life Technologies, Foster City, CA, USA)
with 1 µl cDNA template added to 10 µl 2X SYBR Premix
Ex Taq (Takara Bio, Inc.) containing specific primers at a
concentration of 10 pM each. The reactions were performed for 40
cycles and the cycling parameters were as follows: Denaturation at
95°C for 30 sec, annealing at 58°C for 30 sec and extension at 72°C
for 30 sec. The fluorescence intensity was measured on termination
of the extension phase of each cycle and the threshold value for
the fluorescence intensity of all samples was set manually. The
reaction cycle at which the PCR products exceeded this fluorescence
intensity threshold in the exponential phase of PCR amplification
was taken as the threshold cycle (CT). The PCR product of
cytochrome c oxidase subunit 1 (1A, a ubiquitously expressed
housekeeping gene) (16) served as
a control for mRNA concentrations in the RT-qPCR reactions. The
relative expression level of each gene was quantified using RQ
study software (Applied Biosystems Life Technologies). The quantity
of transcript present was inversely associated with the observed CT
and, for every two-fold dilution in the quantity of transcript, CT
was expected to increase by one. Relative expression (R) was
calculated using the equation R=2−(ΔCTsample −
ΔCTcontrol). To determine a normalized arbitrary value for
each gene, every data point was normalized to the control gene
(1A), as well as to the respective controls. The primers were as
follows: Sense, 5′-CCAGGG TTTGGAATTATTTC-3′ and antisense,
5′-GAAGATAAACCCTAAGGCTC-3′ for rat and mouse 1A; sense,
5′-AAGAGCATTTTTCAAAAATA-3′ and antisense,
5′-GTCTCAGAATTTGCTTTATT-3′ for rat CaBP-9k; sense,
5′-GACTTGAATCTCCACGATCA-3′ and antisense,
5′-CTTCAAGGTGCTGGATAGAA-3′ for rat ERα; sense,
5′-TGTGTCCAGCTACAAACCAATG-3′ and antisense,
5′-CATCATGCCCACTTCGTAACA-3′ for mouse ERα; sense,
5′-GAAGAGCAAACCTCGAGCAC-3′ and antisense,
5′-AGCAGAAAACCGGGAATCTT-3′ for rat PR; sense,
5′-AATCCCACAGGAGTTTGTCA-3′ and antisense,
5′-GGACAACCCCTTTCTGTCTT-3′ for mouse PR; and sense,
5′-CTTTAAGGTGTCTGGCTGAGAAG-3′ and antisense,
5′-AGTTCTTAGCCTCAGTCACAG GTT-3′ for mouse Ltf.
Statistical analysis
The results are presented as the mean ± standard
error of the mean. P-values were calculated using one-way analysis
of variance, followed by Tukey's test for multiple comparisons of
columns. P<0.05 was considered to indicate a statistically
significant difference.
Results
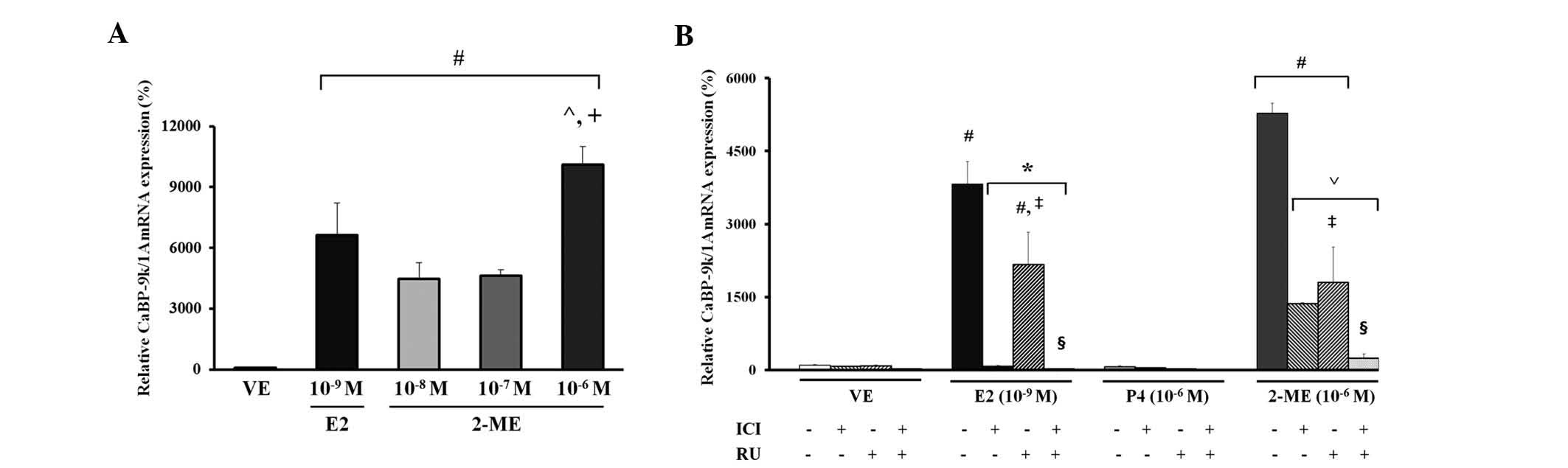
Effects of 2-ME on regulation of CaBP-9k
mRNA expression in GH3 cells
2-ME was applied to GH3 cells at three
concentrations (10−8, 10−7 and
10−6 M). CaBP-9k mRNA levels were then analyzed as an
estrogen marker of rat pituitary GH3 cells using RT-qPCR (25). As shown in Fig. 1A, a significant increase in
expression of CaBP-9k mRNA was observed at concentrations of
10−8, 10−7 and 10−6 M 2-ME, and
10−9 M E2 (P<0.02). Compared with the VE (negative)
controls, the CaBP-9k mRNA level increased by ~66-fold in the
E2-treated (10−9 M) positive control group and by 44.4-
to 100-fold in the 2-ME-treated group. The expression of CaBP-9k
demonstrated a dose-dependent response to 2-ME; treatment with 2-ME
at a dose of 10−6 M resulted in a 2.2-fold increase,
compared with the 10−8 or 10−7 M treatment
group (Fig. 1A). For
identification of the steroid hormone receptor signaling pathway
involved in transcriptional upregulation of CaBP-9k expression by
2-ME, the GH3 cells were pretreated with the ER antagonist, ICI
182,780 or with the PR antagonist, RU486 (28), which determined that co-treatment
with 2-ME (10−6 M) induced the highest expression of
CaBP-9k mRNA among the 10−8, 10−7 and
10−6 M 2-ME doses, and compared with the E2
(10−9 M)-, P4 (10−6 M)- or VE (0.1%
DMSO)-treated groups (Fig.
1B).
CaBP-9k mRNA levels were analyzed using RT-qPCR. As
shown in Fig. 1B, induction of
CaBP-9k mRNA expression by E2 and 2-ME was completely abolished by
co-treatment with ICI 182,780 at a concentration of 10−7
M. Results of co-treatment of the PR antagonist, RU486
(10−6 M) with E2 (10−9 M) and 2-ME
(10−6 M) induced a significant increase in expression of
CaBP-9k mRNA when compared with the VE control (P<0.0001).
Notably, CaBP-9k mRNA expression in the E2- or 2-ME-treated groups
following co-treatment with ICI 182,780 (10−7 M) and
RU486 (10−6 M) was significantly decreased when compared
with co-treatment with RU486 (10−6 M) alone
(P<0.0002; Fig. 1B).
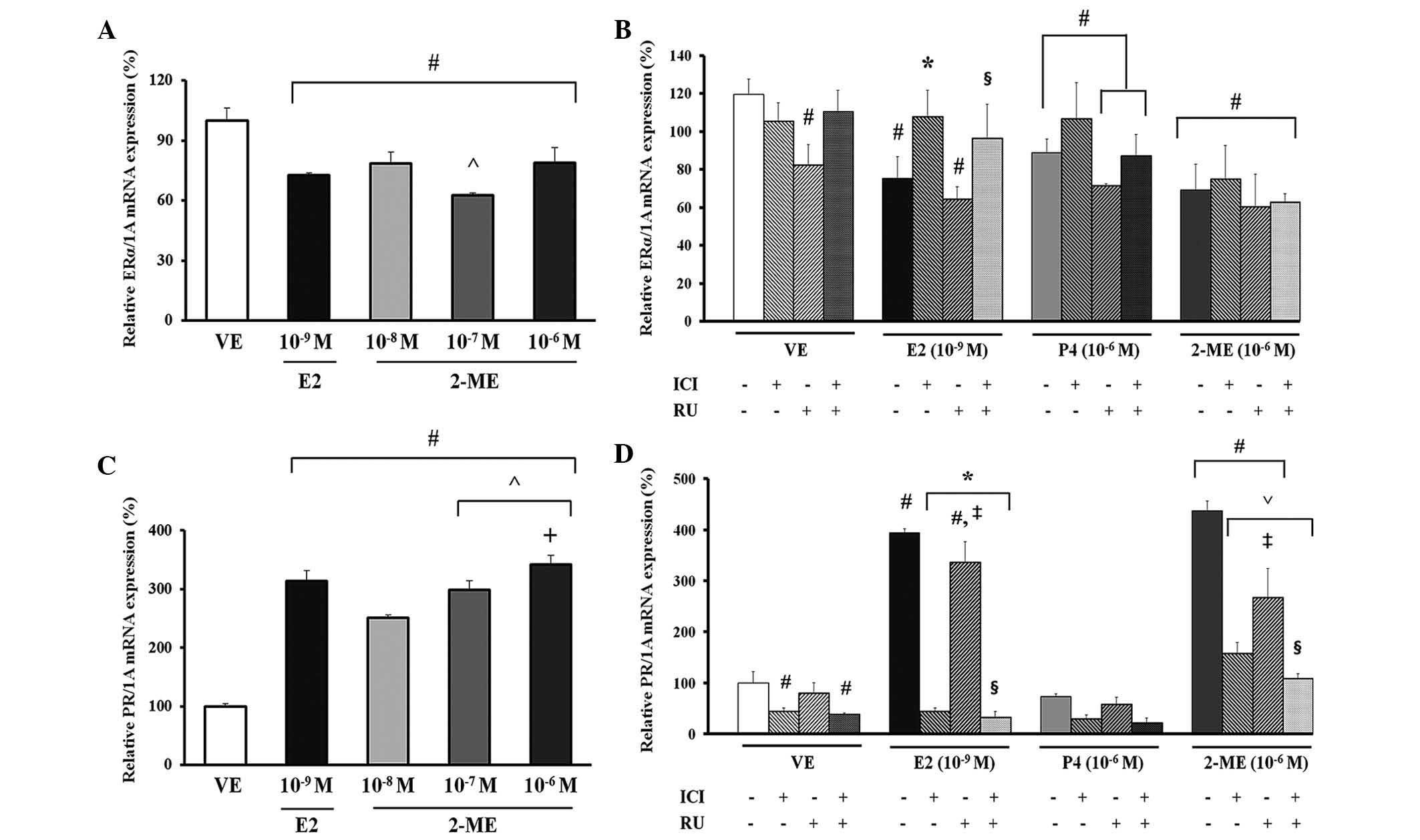
Effect of 2-ME on mRNA expression of
steroid hormone receptors in GH3 cells
2-ME was applied to GH3 cells at three
concentrations (10−8, 10−7 or 10−6
M) for evaluation of the potential impact of 2-ME. In addition, the
mRNA levels of ERα between ERα and PR were measured to evaluate the
estrogenicity of estrogen metabolite, 2-ME (27). GH3 cells were treated with 2-ME
(10−8, 10−7 and 10−6 M), E2
(10−9 M) or the VE (0.1% DMSO). Compared with the VE
control, E2 induced a decrease of ~1.4-fold and treatment with all
doses of 2-ME resulted in decreased expression of ERα mRNA by ~1.2-
to 1.3-fold (Fig. 2A). The
dose-dependent response of 2-ME on ERα gene expression was observed
in the 10−7 M treatment group compared with the
10−8 M 2-ME treatment group. In addition, the 2-ME
treatment group experienced a similar response to that of the E2
treatment group by demonstrating a decrease in ER expression levels
(Fig. 2A). For identification of
the steroid hormone receptor signaling pathway involved in
transcriptional upregulation of CaBP-9k mRNA expression by 2-ME,
the GH3 cells were pretreated with the ER antagonist, ICI 182,780
or with the PR antagonist, RU486 (28). In the case of ERα mRNA expression
compared with the VE control, E2 induced a decrease of ~1.5-fold
and treatment with ICI 182,780 and/or RU486 induced 2-ME resulted
in a decrease of ~1.7- to 1.9-fold (Fig. 2B). Notably, ERα mRNA expression in
the E2 treatment group, as a result of co-treatment with ICI
182,780 (10−7 M) and RU486 (10−6 M), was
markedly increased compared with co-treatment with RU486
(10−6 M) alone (Fig.
2B; P<0.005). In the case of PR mRNA expression, the
positive control, E2, and all doses of 2-ME induced a significant
increase in expression of PR mRNA compared with the VE (Fig. 2C; P<0.0003).
For identification of the steroid hormone receptor
signaling pathway involved in transcriptional regulation of PR mRNA
expression by 2-ME, GH3 cells were pretreated with the ER
antagonist, ICI 182,780 or with the PR antagonist, RU486 (28). Compared with VE, the E2 and 2-ME
treatment groups showed a significantly increased pattern of PR
expression (P<0.0001). Of particular interest, PR mRNA
expression resulting from co-treatment with ICI 182,780
(10−7 M) and RU486 (10−6 M) in the E2 or 2-ME
treatment groups was markedly decreased compared with co-treatment
with RU486 (10−6 M) alone (Fig. 2D; P<0.0001). Thus, the magnitude
and pattern of induction of PR mRNA by 2-ME were very similar to
the enhanced expression of CaBP-9k following treatment with 2-ME
(Fig. 1A and 2C; Fig.
1B and 2D).
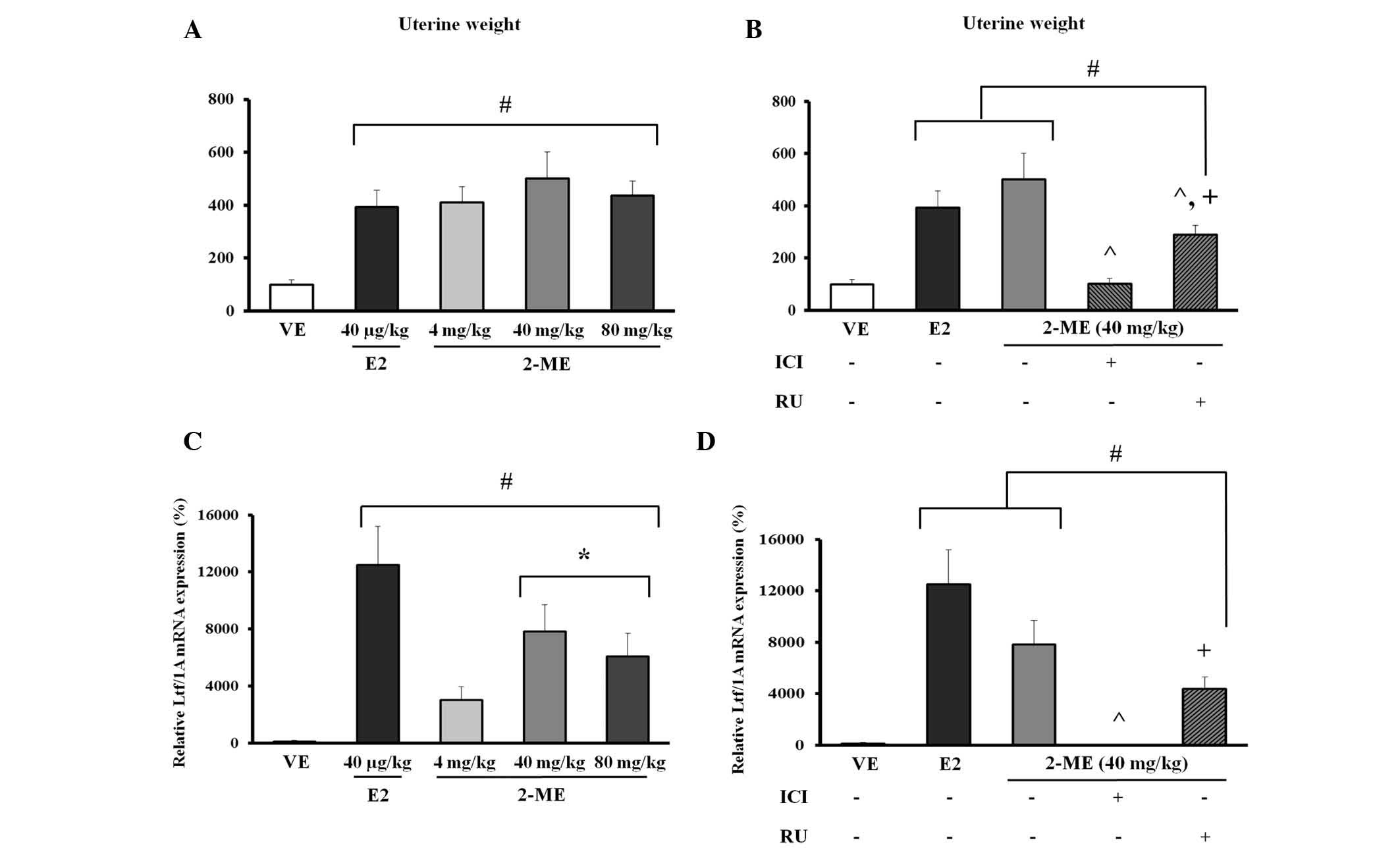
Effects of 2-ME on uterine wet
weight
A uterotrophic assay was used to identify an
estrogen-like effect (26). The
effects were determined by subcutaneous injection with 2-ME (4, 40
or 80 mg/kg BW) or E2 (40 µg/kg BW). ICI 182,780 or RU486
were treated with 2-ME (40 mg/kg BW) for determining the steroid
hormone receptor signaling pathway involved in 2-ME. Compared with
the VE, an ~3.9-fold increase in uterine wet weight was observed
upon treatment with 40 µg/kg BW E2, and ~4.1- to 5.0-fold
increase at 4, 40 and 80 mg/kg BW 2-ME. However, no significant
dose-dependent result was observed for any dose of 2-ME (Fig. 3A; P<0.05). Due to demonstrating
the greatest increase in uterine wet weight among the 4, 40 and 80
mg/kg BW doses of 2-ME, the steroid hormone receptor antagonist
treatment of 40 mg/kg BW 2-ME, became the focus (Fig. 3A). Co-treatment with ICI 182,780
(10 mg/kg BW) on 2-ME (40 mg/kg BW) induced a 5-fold decrease
compared with 2-ME alone (P<0.0001). In addition, RU486
treatment on 2-ME (40 mg/kg BW) induced a significant 1.7-fold
decrease compared with 2-ME alone. Notably, co-treatment with RU486
in the 2-ME treatment group resulted in a 2.8-fold increase when
compared with ICI 182,780 co-treatment (Fig. 3B).
Effects of 2-ME on the regulation of Ltf
gene mRNA expression in the uterus of mice
To evaluate the potential impact of 2-ME, Ltf mRNA
levels were analyzed as an estrogen marker of rodents using RT-qPCR
(29). When compared with the VE
control, E2 at 40 µg/kg BW induced an ~125-fold increase in
Ltf mRNA expression and 2-ME at 40 mg/kg BW induced an ~78.3-fold
increase. Furthermore, treatment with 2-ME at 80 mg/kg BW resulted
in an ~60.7-fold increase in Ltf mRNA expression. In particular,
the dose of 40 mg/kg BW 2-ME demonstrated the most similarity with
E2 among the three doses of 2-ME, as shown by the increased Ltf
gene expression level (Fig.
3C).
For identification of the steroid hormone receptor
signaling pathway involved in transcriptional upregulation of Ltf
gene expression by 2-ME, mice were pre-injected with the ER
antagonist, ICI 182,780, at 10 mg/kg BW or with the PR antagonist,
RU486, at 10 mg/kg BW (28). E2 at
40 µg/kg BW and 2-ME at 40 mg/kg BW were analyzed and
co-treatment of RU486 (10 mg/kg BW) with 2-ME (40 mg/kg BW) induced
significantly increased Ltf gene expression when compared with the
VE (P<0.004). In addition, 2-ME (40 mg/kg BW) was completely
abolished by treatment with ICI 182,780 (10 mg/kg BW). Notably,
co-treatment of RU486 with 2-ME resulted in a significant 470-fold
increase when compared with co-treatment of ICI 182,780 and 2-ME
(Fig. 3D; P<0.003).
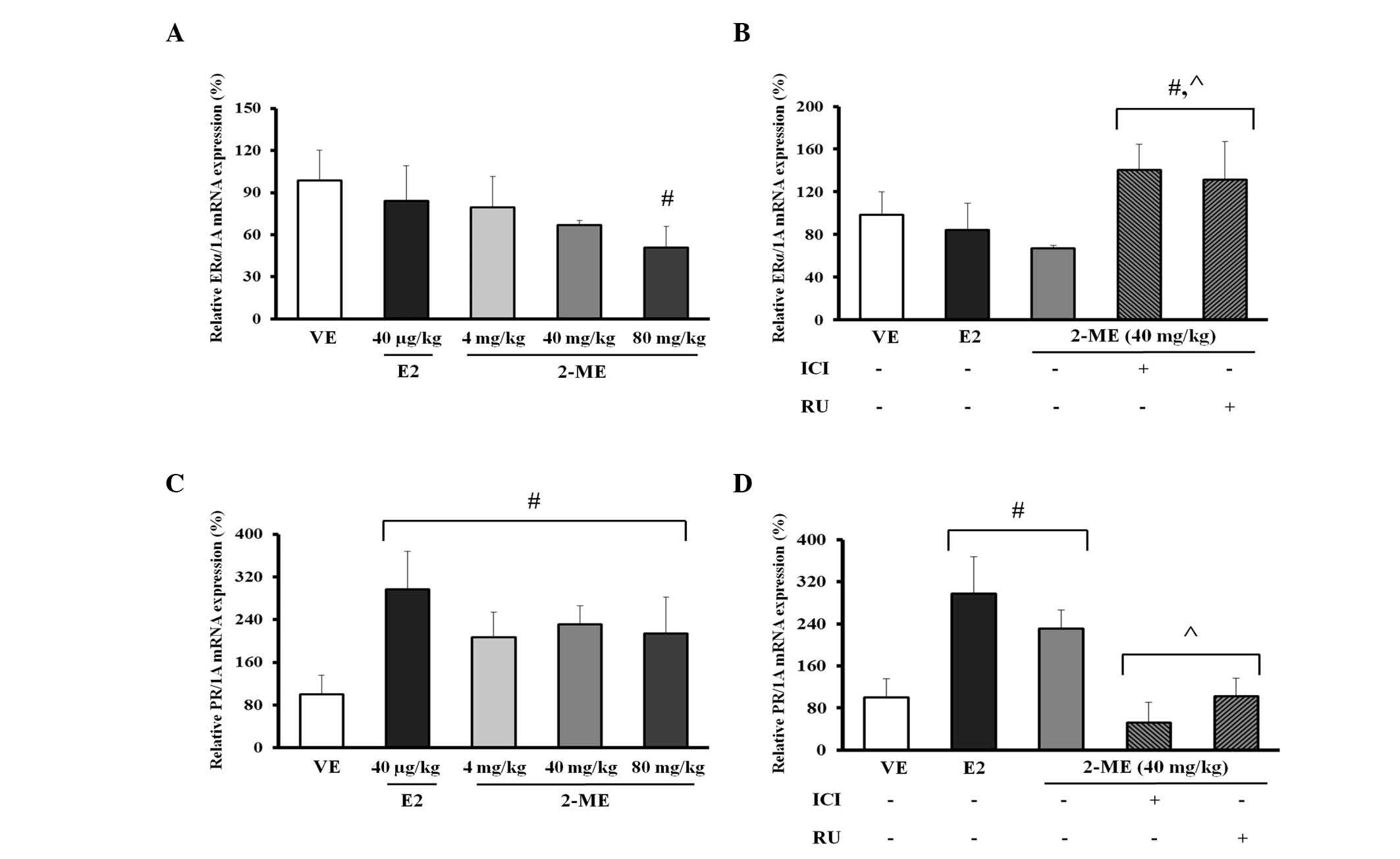
Effect of 2-ME on mRNA expression of
steroid hormone receptors in the uterus of mice
For evaluation of the potential impact of 2-ME, the
mRNA levels of ERα and PR, which respond to materials similar to
natural and synthetic estrogen were determined (27). Mice received a subcutaneous
injection of 2-ME (4, 40 and 80 mg/kg BW), E2 (40 µg/kg BW)
or VE (24% DMSO, 38% ethanol and 38% sterile saline). Treatment
with 2-ME (4, 40 and 80 mg/kg BW) or E2 (40 µg/kg BW)
resulted in a decreasing pattern of ERα when compared with the VE,
however, the differences were not considered to be significant,
apart from 2-ME in the 80 mg/kg BW treatment group. In addition, no
dose-dependent response on ERα expression was observed at any of
the doses of 2-ME (Fig. 4A).
For identification of the steroid hormone receptor
signaling pathway involved in transcriptional upregulation of Ltf
mRNA expression by 2-ME, the mice were pretreated with the ER
antagonist, ICI 182,780 or with the PR antagonist, RU486. In the
case of ERα, co-treatment with ICI 182,780 (10 mg/kg BW) or RU486
(10 mg/kg BW) on 2-ME (10−6M) induced a marked increase
in ERα mRNA expression of 1.42-fold when compared with 2-ME alone
(Fig. 4B; P<0.02). In the case
of PR mRNA expression, PR showed an increase of ~2.94-fold in the
E2 treatment group when compared with the VE and an increase of
~2.1- to 2.3-fold was observed for all doses in the 2-ME (4, 40 and
80 mg/kg BW) treatment group; however, a dose-response effect of
2-ME was not observed (Fig.
4C).
For identification of the steroid hormone receptor
signaling pathway involved in transcriptional upregulation of Ltf
mRNA expression by 2-ME, mice were pretreated with the ER
antagonist, ICI 182,780 or with PR antagonist, RU486. When compared
with the VE control, treatment with E2 and 2-ME resulted in a
marked increase in PR mRNA expression. Co-treatment with ICI
182,780 (10 mg/kg BW) or RU486 (10 mg/kg BW) on 2-ME resulted in a
significant decrease in PR mRNA expression when compared with
administration of 2-ME (40 mg/kg BW) alone (P<0.002). In
addition, co-treatment with RU486 on 2-ME resulted in an increase
in PR mRNA expression when compared with co-treatment with ICI
182,780; however, the increase was not significant (Fig. 4D).
Discussion
Previously, the effect of pharmacological levels of
2-ME on normal physiological systems has not been adequately
described. Although the various possible mechanisms of 2-ME were
not fully elucidated, 2-ME has been administered as an antitumor
therapy. For example, in vitro, 2-ME has been shown to
affect angiogenesis and proliferation of malignant cells, and in
vivo, 2-ME was demonstrated to inhibit the growth of tumors
arising from a subcutaneous injection in mice (30). To the best of our knowledge, the
estrogenic activity in vitro and in vivo of the
estradiol metabolite, 2-ME has not been fully determined. The
present study indicates that 2-ME exhibits estrogenicity under
in vitro and in vivo conditions.
The CaBP-9k gene is known to be controlled by sex
hormones. Expression of the CaBP-9k gene was shown to be
upregulated by estrogen and downregulated by P4 during the estrous
cycle and early pregnancy in a rat uterus (31,32).
In the current study, CaBP-9k expression in GH3 cells was
demonstrated as a useful marker for evaluating the estrogenic
potential of environmental chemicals, such as parabens (27). Thus, the present findings, showing
induction of CaBP-9k gene mRNA expression in GH3 cells demonstrate
that 2-ME may have an estrogenic affect. In addition, 2-ME shares
the effects of estradiol, such as increasing uterine weight, and
the data obtained in the current study indicated the presence of
circulating estrogens, which was indicated by the antagonism of
uterine wet weights by ICI 182,780 (33). In mice, the Ltf gene expression
caused by exposure to estrogen was shown to be associated with an
estrogen-stimulated mouse uterus (29). During the present study, it was
demonstrated that 2-ME mediates the estrogen biomarker gene.
Changes in biomarker expression could be expected to result from
this ER-dependent action that would be matched by E2-induced
effects. ERα is expressed in the uterus, mammary glands, testes,
pituitary gland, liver, kidneys and heart (34). ERβ is found in the prostate and
ovaries (35–37). According to Akbas et al, the
ERα mRNA expression of the bilateral ovariectomized uterus of adult
mice was decreased by treatment with E2 when compared with
untreated mice (38). The present
result indicated that the effect of 2-ME on ERα mRNA expression may
partly result from reduced binding to and signaling through the ERα
pathway, which is blocked by pure antiestrogen. Estrogenic
chemicals, such as parabens, induce an increase in PR gene
expression, which is known to be highly sensitive to, and enhanced
by, ER-mediated transcriptional signaling. Therefore, almost all
ER-dependent signaling may contribute significantly to the enhanced
expression of CaBP-9k and PR genes by specific paraben compounds in
in vitro models (39). In
the present study, the effect of 2-ME on Ltf and PR expression may
partly result from binding to and signaling through the PR pathway,
which is blocked by pure anti-P4.
2-ME is known as a potential novel antitumor agent
combining its anti-proliferative activity on a wide range of tumor
cell types with anti-angiogenic action (40). Combination treatment with agents
that possess anti-proliferative and anti-angiogenic activity
results in antitumor synergy and reduces the likelihood of
antitumor drug resistance (41).
For example, in vitro, 2-ME demonstrated the strongest
inhibitory effect of all of the estrogen metabolites tested, with a
half-maximal inhibitory concentration of ~0.15 µM (30). In addition, it exhibited a highly
similar growth-inhibitory effect in human breast cancer cell lines,
and this effect was not altered by the presence or absence of
exogenous E2 (42). Similarly,
in vivo, it was reported that a 100-mg/kg dose of 2-ME
inhibited tumor growth in various models with dramatic suppression
of longitudinal bone growth in ovary-intact young, growing rats
(43). However, low levels of 2-ME
may have an influence on females during pregnancy. Preeclampsia,
hypoxia and placental defects lead to deficiency in
placenta-derived hydroxyestradiols, which in turn may result in a
further decrease in the 2-ME level (44).
2-ME was found to be rapidly removed from the plasma
and was below the limit of detection (11 ng/ml) 1 h after
administration of a 10-mg/kg dose. Following, intravenous
administration of 2-ME, its half-life in plasma was ~14 min
(45). Despite numerous studies on
the effect of pharmacological levels of 2-ME on normal
physiological systems, it remains to be described fully.
In conclusion, the results of the present study
(based on uterine wet weight gain, and increasing levels of
estrogen biomarkers in in vitro and in vivo models)
demonstrate that all experimental doses of 2-ME, which were lower
than anticancer therapeutic concentrations, exerted an estrogenic
effect. In addition, the estrogenic activity of 2-ME was observed
to be blocked by the ER and PR antagonists, ICI 182,780 and RU486,
respectively, in the in vitro and in vivo models.
Since 2-ME shows proliferative effects, it is necessary that
potential risk of 2-ME should be further investigated as cancer
therapeutic reagents., which may affect endocrine homeostasis
and/or function. Overall, the present results reveal that 2-ME may
impact the ER and PR, and maintain the body in a state of
disturbance of the endocrine system.
Acknowledgments
The present study was supported by a National
Research Foundation of Korea grant from the Korean government,
Ministry of Education, Science and Technology (grant no.
NRF-2013R1A2A2A05004582).
References
|
1
|
Wang SH, Myc A, Koenig RJ, Bretz JD,
Arscott PL and Baker JR: 2-Methoxyestradiol, an endogenous estrogen
metabolite, induces thyroid cell apoptosis. Mol Cell Endocrinol.
165:163–172. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Männistö PT and Kaakkola S:
Catechol-O-methyltransferase (COMT): Biochemistry, molecular
biology, pharmacology, and clinical efficacy of the new selective
COMT inhibitors. Pharmacol Rev. 51:593–628. 1999.PubMed/NCBI
|
|
3
|
Zhang Y, Gaikwad NW, Olson K, Zahid M,
Cavalieri EL and Rogan EG: Cytochrome P450 isoforms catalyze
formation of catechol estrogen quinones that react with DNA.
Metabolism. 56:887–894. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spady TJ, McComb RD and Shull JD: Estrogen
action in the regulation of cell proliferation, cell survival, and
tumorigenesis in the rat anterior pituitary gland. Endocrine.
11:217–233. 1999. View Article : Google Scholar
|
|
5
|
Pribluda VS, Gubish ER Jr, Lavallee TM,
Treston A, Swartz GM and Green SJ: 2-Methoxyestradiol: An
endogenous antiangiogenic and antiproliferative drug candidate.
Cancer Metastasis Rev. 19:173–179. 2000. View Article : Google Scholar
|
|
6
|
Hamel E, Lin CM, Flynn E and D'Amato RJ:
Interactions of 2-methoxyestradiol, an endogenous mammalian
metabolite, with unpolymerized tubulin and with tubulin polymers.
Biochemistry. 35:1304–1310. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banerjeei SK, Zoubine MN, Sarkar DK,
Weston AP, Shah JH and Campbell DR: 2-Methoxyestradiol blocks
estrogen-induced rat pituitary tumor growth and tumor angiogenesis:
Possible role of vascular endothelial growth factor. Anticancer
Res. 20:2641–2645. 2000.PubMed/NCBI
|
|
8
|
D'Amato RJ, Lin CM, Flynn E, Folkman J and
Hamel E: 2-Methoxyestradiol, an endogenous mammalian metabolite,
inhibits tubulin polymerization by interacting at the colchicine
site. Proc Natl Acad Sci USA. 91:3964–3968. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Attalla H, Westberg JA, Andersson LC,
Adlercreutz H and Mäkelä TP: 2-Methoxyestradiol-induced
phosphorylation of Bcl-2: Uncoupling from JNK/SAPK activation.
Biochem Biophys Res Commun. 247:616–619. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang I, Majid S, Saini S, Zaman MS,
Yamamura S, Chiyomaru T, Shahryari V, Fukuhara S, Deng G, Dahiya R,
et al: Hrk mediates 2-methoxyestradiol-induced mitochondrial
apoptotic signaling in prostate cancer cells. Mol Cancer Ther.
12:1049–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farhat GN, Parimi N, Chlebowski RT, Manson
JE, Anderson G, Huang AJ, Vittinghoff E, Lee JS, Lacroix AZ, Cauley
JA, et al: Sex hormone levels and risk of breast cancer with
estrogen plus progestin. J Natl Cancer Inst. 105:1496–1503. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yue W, Yager JD, Wang JP, Jupe ER and
Santen RJ: Estrogen receptor-dependent and independent mechanisms
of breast cancer carcinogenesis. Steroids. 78:161–170. 2013.
View Article : Google Scholar
|
|
13
|
Gökmen-Polar Y, Escuin D, Walls CD, Soule
SE, Wang Y, Sanders KL, Lavallee TM, Wang M, Guenther BD,
Giannakakou P, et al: Beta-Tubulin mutations are associated with
resistance to 2-methoxyestradiol in MDA-MB-435 cancer cells. Cancer
Res. 65:9406–9414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu BT and Conney AH: Is
2-methoxyestradiol an endogenous estrogen metabolite that inhibits
mammary carcinogenesis? Cancer Res. 58:2269–2277. 1998.PubMed/NCBI
|
|
15
|
Brueggemeier RW and Singh U: Inhibition of
rat liver microsomal estrogen 2-hydroxylase by 2-methoxyestrogens.
J Steroid Biochem. 33:589–593. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tinnanooru P, Dang VH, Nguyen TH, Lee GS,
Choi KC and Jeung EB: Estrogen regulates the localization and
expression of calbindin-D9k in the pituitary gland of immature male
rats via the ERalpha-pathway. Mol Cell Endocrinol. 285:26–33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee GS, Choi KC, Park SM, An BS, Cho MC
and Jeung EB: Expression of human Calbindin-D (9k) correlated with
age, vitamin D receptor and blood calcium level in the
gastrointestinal tissues. Clin Biochem. 36:255–261. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dang VH, Nguyen TH, Choi KC and Jeung EB:
A calcium-binding protein, calbindin-D9k, is regulated through an
estrogen-receptor mediated mechanism following xenoestrogen
exposure in the GH3 cell line. Toxicol Sci. 98:408–415. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong EJ, Park SH, Choi KC, Leung PC and
Jeung EB: Identification of estrogen-regulated genes by microarray
analysis of the uterus of immature rats exposed to endocrine
disrupting chemicals. Reprod Biol Endocrinol. 4:492006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walmer DK, Wrona MA, Hughes CL and Nelson
KG: Lactoferrin expression in the mouse reproductive tract during
the natural estrous cycle: Correlation with circulating estradiol
and progesterone. Endocrinology. 131:1458–1466. 1992.PubMed/NCBI
|
|
21
|
Newbold RR, Teng CT, Beckman WC Jr,
Jefferson WN, Hanson RB, Miller JV and McLachlan JA: Fluctuations
of lactoferrin protein and messenger ribonucleic acid in the
reproductive tract of the mouse during the estrous cycle. Biol
Reprod. 47:903–915. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujimoto N, Jinno N and Kitamura S:
Activation of estrogen response element dependent transcription by
thyroid hormone with increase in estrogen receptor levels in a rat
pituitary cell line, GH3. J Endocrinol. 181:77–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Banerjee SN, Sengupta K, Banerjee S,
Saxena NK and Banerjee SK: 2-Methoxyestradiol exhibits a biphasic
effect on VEGF-A in tumor cells and upregulation is mediated
through ER-alpha: A possible signaling pathway associated with the
impact of 2-ME2 on proliferative cells. Neoplasia. 5:417–426. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang H, Nguyen TT, An BS, Choi KC and
Jeung EB: Synergistic effects of parabens on the induction of
calbindin-D (9k) gene expression act via a progesterone
receptor-mediated pathway in GH3 cells. Hum Exp Toxicol.
31:134–144. 2012. View Article : Google Scholar
|
|
25
|
Vo TT, An BS, Yang H, Jung EM, Hwang I and
Jeung EB: Calbindin-D9k as a sensitive molecular biomarker for
evaluating the synergistic impact of estrogenic chemicals on GH3
rat pituitary cells. Int J Mol Med. 30:1233–1240. 2012.PubMed/NCBI
|
|
26
|
Jung EM, An BS, Choi KC and Jeung EB:
Potential estrogenic activity of triclosan in the uterus of
immature rats and rat pituitary GH3 cells. Toxicol Lett.
208:142–148. 2012. View Article : Google Scholar
|
|
27
|
Vo TT, Jung EM, Choi KC, Yu FH and Jeung
EB: Estrogen receptor α is involved in the induction of Calbindin-D
(9k) and progesterone receptor by parabens in GH3 cells: A
biomarker gene for screening xenoestrogens. Steroids. 76:675–681.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahn HJ, Yang H, An BS, Choi KC and Jeung
EB: Expression and regulation of Enpp2 in rat uterus during the
estrous cycle. J Vet Sci. 12:379–385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Tu Y, Han F, Xu Z and Wang J:
Developmental gene expression of lactoferrin and effect of dietary
iron on gene regulation of lactoferrin in mouse mammary gland. J
Dairy Sci. 88:2065–2071. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fotsis T, Zhang Y, Pepper MS, Adlercreutz
H, Montesano R, Nawroth PP and Schweigerer L: The endogenous
oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and
suppresses tumour growth. Nature. 368:237–239. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krisinger J, Setoyama T and Leung PC:
Expression of calbindin-D9k in the early pregnant rat uterus:
Effects of RU 486 and correlation to estrogen receptor mRNA. Mol
Cell Endocrinol. 102:15–22. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krisinger J, Dann JL, Currie WD, Jeung EB
and Leung PC: Calbindin-D9k mRNA is tightly regulated during the
estrous cycle in the rat uterus. Mol Cell Endocrinol. 86:119–123.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sibonga JD, Lotinun S, Evans GL, Pribluda
VS, Green SJ and Turner RT: Dose-response effects of
2-methoxyestradiol on estrogen target tissues in the ovariectomized
rat. Endocrinology. 144:785–792. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Williams K, McKinnell C, Saunders PT,
Walker M, Fisher JS, Turner KJ, Atanassova N and Sharpe M: Neonatal
exposure to potent and environmental oestrogens and abnormalities
of the male reproductive system in the rat: Evidence for importance
of the androgenoestrogen balance and assessment of the relevance to
man. Hum Reprod Update. 7:236–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Swedenborg E, Pongratz I and Gustafsson
JA: Endocrine disruptors targeting ERbeta function. Int J Androl.
33:288–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Keightley MC: Steroid receptor isoforms:
Exception or rule? Mol Cell Endocrinol. 137:1–5. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mangelsdorf DJ, Thummel C, Beato M,
Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M,
Chambon P, et al: The nuclear receptor superfamily: The second
decade. Cell. 83:835–839. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Akbas GE, Fei X and Taylor HS: Regulation
of HOXA10 expression by phytoestrogens. Am J Physiol Endocrinol
Metab. 292:E435–E442. 2007. View Article : Google Scholar
|
|
39
|
Kim YR, Jung EM, Choi KC and Jeung EB:
Synergistic effects of octylphenol and isobutyl paraben on the
expression of calbindin-D (9)k in GH3 rat pituitary cells. Int J
Mol Med. 29:294–302. 2012.
|
|
40
|
Mooberry SL: New insights into
2-methoxyestradiol, a promising antiangiogenic and antitumor agent.
Curr Opin Oncol. 15:425–430. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Browder T, Butterfield CE, Kräling BM, Shi
B, Marshall B, O'Reilly MS and Folkman J: Antiangiogenic scheduling
of chemotherapy improves efficacy against experimental
drug-resistant cancer. Cancer Res. 60:1878–1886. 2000.PubMed/NCBI
|
|
42
|
Liu ZJ and Zhu BT: Concentration-dependent
mitogenic and antiproliferative actions of 2-methoxyestradiol in
estrogen receptor-positive human breast cancer cells. J Steroid
Biochem Mol Biol. 88:265–275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Turner RT and Evans GL: 2-Methoxyestradiol
inhibits longitudinal bone growth in normal female rats. Calcif
Tissue Int. 66:465–469. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takanashi K, Honma T, Kashiwagi T, Honjo H
and Yoshizawa I: Detection and measurement of urinary
2-hydroxyestradiol 17-sulfate, a potential placental antioxidant
during pregnancy. Clin Chem. 46:373–378. 2000.PubMed/NCBI
|
|
45
|
Ireson CR, Chander SK, Purohit A, Perera
S, Newman SP, Parish D, Leese MP, Smith AC, Potter BV and Reed MJ:
Pharmacokinetics and efficacy of 2-methoxyoestradiol and
2-methoxyoestradiol-bis-sulphamate in vivo in rodents. Br J Cancer.
90:932–937. 2004. View Article : Google Scholar : PubMed/NCBI
|