Introduction
Preeclampsia, a medical condition characterized by
hypertension and proteinuria during pregnancy, can result from a
wide spectrum of pathophysiological processes involving impaired
migratory capability, endothelial dysfunction and systemic
metabolic disorders (1–3). Placenta, as the site where the
mediators of those pathophysiological processes are generated, is
essential in the development of preeclampsia (4), therefore, placenta-based
investigations may substantially improve current understanding of
the mechanism underlying the disease. Increasing evidence has
indicated that differentially expressed genes in the placenta
significantly contribute to the pathogenesis of preeclampsia
(5–8), however, the regulation of these genes
remains to be fully elucidated.
MicroRNAs (miRNAs) are a class of ~22
nucleotide-long non-protein-coding RNAs, which can regulate gene
expression by binding to the 3′ untranslated region (UTR) of target
gene messenger RNA (mRNA), resulting in translational repression
and/or mRNA degradation (9). It is
generally considered that ~1/3 human genes are regulated by
microRNA (10), and that microRNA
is key in cellular activities, including cell proliferation,
migration and apoptosis (11–13).
Since these activities have been reported to be disturbed in
preeclampsia, miRNAs may be involved in the pathogenesis of
preeclampsia (14,15). To elucidate how miRNAs are involved
in the pathogenesis of preeclampsia, the present study investigated
global placental expression levels of miRNA in preeclampsic and
normal placentas using microarray analysis, and selected the two
most significantly upregulated miRNAs, miR-355 and miR-584, for
further functional analysis due to their reported involvement in
the development of preeclampsia (14,15)
or the roles in the regulation of cell proliferation and migration
(16,17). By in-silicon analysis and miRNA
database searching, the present study aimed to identify the shared
target gene of miR-355 and miR-584.
Considering the fact that aberrant expression of
endothelial nitric oxide synthase (eNOS) may compromise the
migratory capability of trophoblast cells (18), and that a lack of eNOS may directly
exacerbate the preeclampsia-like phenotype by interfering with the
endothelin system (19), the
present study hypothesized that miR-335 and miR-584 may function as
a regulator of trophoblast cell behavior by targeting eNOS. To
assess this hypothesis, the effect of overexpressed miR-335 and
miR-584 on the proliferation and invasion of trophoblast cells, and
the potential 'rescue' effect by eNOS were investigated.
Materials and methods
Study population and data collection
The placenta micro-array investigation population in
the present study comprised 20 healthy pregnant females (age,
28.35±6.34 years) and 20 patients with severe preeclampsia (age,
28.67±7.12 years), recruited from the Department of Gynecology and
Obstetrics, Tangdu Hospital, The Fourth Military Medical University
(Xi'an, China). Normal pregnancy was defined as a previously and
currently normotensive pregnant female, who delivered a healthy
neonate following 37 weeks of gestation. Severe preeclampsia was
defined as individuals with sustained (≥2 measures, 6 h apart)
blood pressure elevation (>160/110 mmHg) following 20 weeks of
gestation, combined with significant proteinuria, which was defined
as the sustained (≥2 measures, 4 h apart) presence of elevated
protein in the urine (>30 mg/dl or >1+ on a urine dipstick).
The study was approved by the Research Ethic Committees at The
Fourth Military Medical University, and written consent was
obtained from all subjects. Individuals with a history of renal
disease, spontaneous abortion, gestational diabetes, or fetal
chromosomal or congenital abnormalities were excluded from the
investigation. Placental sample collection was performed, as
described previously (5). Briefly
~0.5 cm3 placental tissue was obtained via biopsy from
16 sites, including eight maternal and eight fetal sides, to
achieve uniformity and adequate sampling. The tissue samples
obtained were plaved into RNAlater (Qiagen Inc., Valencia, CA, USA)
and stored at −80°C.
Total RNA isolation
All tissue samples from the same placenta were
pooled together, and total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer's instructions, with little modification. The purity
of the isolated RNA was determined using a spectrophotometer (6310;
Jenway, Ltd, Essex, UK). RNA integrity was confirmed using
electrophoresis on an agarose gel (Sigma-Aldrich, St. Louis, MO,
USA).
miRNA microarray analysis
Total RNA (5 µg) from each sample was used in
the microarray expression profiling assay. An miRCURY™ Array
Labeling kit (Exiqon, Vedbaek, Denmark) was used to label the
miRNAs, and the labeled RNA samples were hybridized to the miRNA
microarray chip (Exiqon, Vedbaek, Denmark). The microarray set
consisted of 1,035 probes, including 455 probes against human
mature microRNA sequences, 344 probes against mouse miRNAs and 236
probes against rat miRNAs. A GenePix 4000B laser scanner (Axon
Instruments, Foster City, CA, USA) was used to obtain the
hybridization data, and the images were digitized and analyzed
using GenePix 4.0 software (Axon Instruments).
Cell lines and cell culture
The HTR8/Svneo cells (1×105) were
cultured in RPMI-1640 (Invitrogen Life Technologies), supplemented
with 10% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml
streptomycin (Invitrogen Life Technologies).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The target gene of the miRNA was predicted using
miRanda (http://www.miranda.org) and TargetScan
(www.targetscan.org), and the relevant
signaling pathway was analyzed using DIANA (http://diana.cslab.ece.ntua.gr/pathways/) and KEGG
(http://www.genome.jp/kegg/pathway/hsa/hsa05200.html).
The total RNA was isolated from the cultured cells or placental
tissues using TRIzol® reagent (Invitrogen Life
Technologies), according to the manufacturer's instructions.
PrimeScript RT Reagent kit (Takara Biotechnology, Co., Ltd.,
Dalian, China) was used to reverse transcribe RNA for RT-qPCR
analysis. A total of 20 µg cDNA was used as a template for
amplification of miRNA or mRNA using SYBR Premix Ex Taq™ II (Takara
Biotechnology Co., Ltd.) under the following conditions: 95°C for
30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 30
sec, then a final extension step at 72°C for 5 min. RT-qPCR was
performed using the following primer sets from Sangon Biotech Co.,
Ltd. (Shanghai, China): Forward
5′-ACACTCCAGCTGGGTCAAGAGCAATAACGAAA-3′ and reverse
5′-CTCAACTGGTGTCGTGGA -3′ for miR-335, and forward
5′-CAGGGTTGCTGTTACTGGAG-3′ and reverse 5′-AATCTAACCCCACATTTCCCC-3′
for miR-584. The following primer set: Forward
5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-3′ was
used for U6 detection, and Forward 5′-CCCTTCAGTGGCTGGTACAT-3′ and
reverse 5′-CACGATGGTGACTTTGGCTA-3′ was used for eNOS detection. A
SYBR Green real time detection system (Bio-rad Laboratories, Inc.,
Hercules, CA, USA) was used to quantitatively measure the levels of
expression. U6, an internal control, was used to normalize the
expression levels of miR-335, miR-584 and eNOS.
Western blot analysis
The cells were lysed using lysis buffer supplemented
with protease inhibitor (Sigma-Aldrich). The protein samples (20
ng) were loaded onto an 10% SDS-PAGE gel, and the separated
proteins were then transferred onto a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA) prior to being blocked
with 10 mMTris-Cl (pH 8.0), 150 mMNaCl and 0.05% Tween 20 (TBST)
containing 5% nonfat dry milk powder at room temperature for 1 h.
The membrane was subsequently incubated with rabbit anti-human eNOS
polyclonal antibody (1:1,000; cat. no. ab5589; Abcam, Cambridge,
MA, USA) at room temperature for 2 h, followed by incubation with
the goat anti-rabbit secondary antibody (1:10,000; ab6721; Abcam)
at room temperature for another 2 h. An enhanced chemiluminescence
kit (Pierce Biotechnology, Inc., Rockford, IL, USA) was used to
detect chemical fluorescence, according to the manufacturer's
instructions. The relative density of the bands were analyzed and
normalized by β-actin.
Plasmids, small interfering (si)RNA and
transfection
The siRNA, duplex against human eNOS
(anti-eNOS-siRNA), miR-335 and miR-584 mimics, miR-335 and miR-584
inhibitors (anti-miR-335 and anti-miR-584) and scramble control
were purchased from Ambion Life Technologies (Austin, TX, USA). The
coding sequence of eNOS was PCR amplified (PCR conditions: 95°C for
30 sec; 30 cycles at 95°C for 1 min, 58°C for 30 sec and 68°C for 2
min; and 72°C for 5 min) and inserted into the pcDNA4.0 vector
(Invitrogen Life Technologies) to produce a construct expressing
eNOS in trophoblast cells.
Luciferase assay
The HTR-8/SVneo cells were cultured to 70%
confluence and transfected with different constructs (pRL-SV40;
Promega Corporation, Fitchburg, WI, USA), containing the wild-type
or mutant eNOS 3′UTR, pRL-TK, and miR-584 or miR-335 mimics. A
QuickChange XL site-directed mutagenesis kit (Stratagene, La Jolla,
CA, USA) was used to introduce the variant. The cells were
collected after 24 h at room temperature and the activities of
either firefly or Renilla luciferase were determined using an LB
955 Luminometer system using the dual luciferase reporter system
(Promega Corporation), according to the manufacturer's
instructions. The activity of firefly luciferase was normalized to
that of Renilla luciferase.
Cell survival and proliferation
assay
Cell survival was evaluated using a 3-
(4,5-dimethylthiazol-2-yl) -2,5-di-phenyltetrazolium bromide (MTT)
assay. The cells were seeded in 96-well plates at a density of 800
cells/well and were incubated for 24, 48 and 72 h. MTT (5 mg/ml; 20
µl; Sigma-Aldrich) was added to each well and incubated for
4 h, and the supernatants were removed, followed by the addition of
150 ml dimethyl sulfoxide (Sigma-Aldrich). The absorbance value
(optical density; OD) of each well was measured at 490 nm using a
Jenway 6310 spectrophotometer. The experiments were performed three
times.
Transwell invasion and migration
assays
A Transwell insert invasion assay was performed in
24-well fitted inserts with membranes (8 mm pore size; Costar;
Corning Incorporated, New York City, NY, USA). The cells
transfected with the control, miRNA mimics, miRNA inhibitors or
eNOS were treated with 10 mg/ml mitomycin C (Sigma-Aldrich) for 2 h
and added to the top of the wells at the density of
2.6×105 cells/well. Cell invasion was examined using a
polycarbonate membrane cell culture insert, which was coated with
growth factor-reduced Matrigel (Corning incorporated, Corning, NY,
USA). The invaded cells on the lower surface of the membrane were
stained with trypan blue (Sigma-Aldrich) and counted under a
microscope (CX22; Olympus Corporation, Tokyo, Japan), and the
invasion index was calculated using the following equation:
Invasion index = treated / control%. Experiments were performed
three times.
Statistical analysis
Raw data were normalized using GenePix 4.0 software
(Axon Instruments, Foster City, CA), and median centered using the
Bioconductor package (www.bioconductor.org). SAM software (http://www.stat.stanford.edu/tibs.SAM)
was used to determine the differentially expressed miRNAs in the
severe preeclampsia samples, which comprised genes with a
significant (P<0.05) differential expression of ≥1.5-fold. The
Transwell insert invasion assay, proliferation assay, RT-qPCR,
western blotting and luciferase assay were repeated at least three
times, and the results are expressed as the mean ± standard
deviation. Student's t-test or one-way analysis of variance was
used to detect differences, and statistical analysis was performed
using SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
miRNA microarray analysis
The placenta microarray performed in the present
study was compared between 20 healthy pregnant females and 20
patients with severe preeclampsia. No significant difference was
present between the normal pregnant and the preeclamptic females in
the present study regarding maternal age, body mass index, glucose
tolerance, infant birth weight or gestational age (Table. I). Based on the evaluation of fold
change (absolute fold change >1.5) and statistical analysis
(P<0.05), 18 miRNAs were significantly differentially expressed
among the preeclampsia cases, compared with the controls, which
included 12 upregulated and six downregulated miRNAs (Fig. 1). Of these, the two most
significantly upregulated miRNAs, miR-355 and miR-584, were
selected for further functional analysis due to their indicated
involvement in preeclampsia pathogenesis (14,15)
or reported regulatory roles in cell proliferation and migration
(16,17). The results from confirmatory
RT-qPCR experiments revealed the mRNA expression levels in the
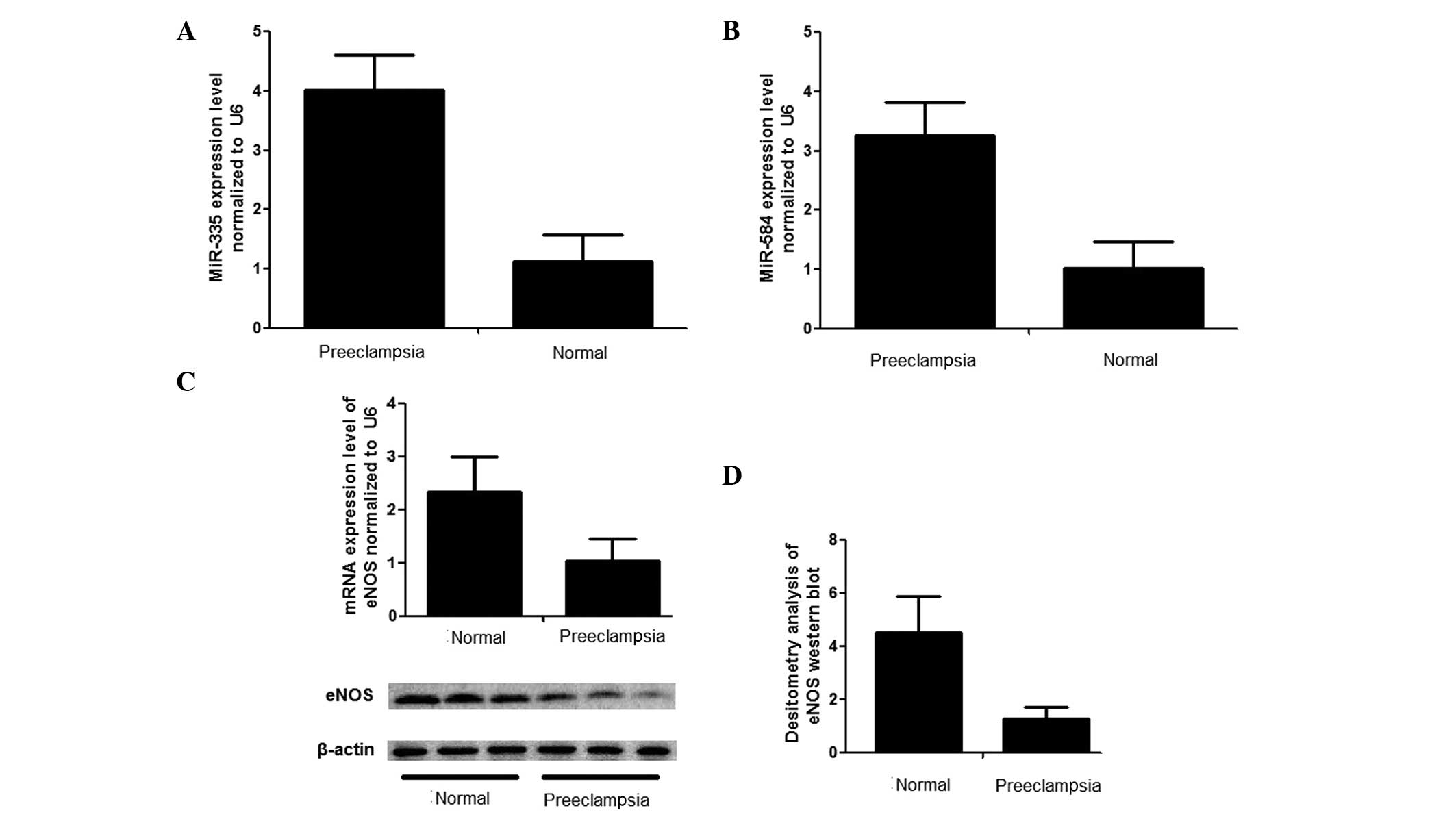
preeclampsia cases and controls for the selected miRNAs (Fig. 2A and B), and the fold changes in
expression levels identified in the RT-qPCR experiments were
comparable with the preceding microarray experiment.
 | Table IClinical characteristics of pregnant
healthy control and preeclamptic patients. |
Table I
Clinical characteristics of pregnant
healthy control and preeclamptic patients.
| Variable | Control (n=20) | Preeclampsia
(n=20) | P-value |
|---|
| Maternal age
(years) | 26.3±5.2 | 28.1±4.8 | 0.262 |
| Blood pressure
(mmHg) | | | |
| Systolic | 116±12.1 | 169±11.2 | <0.001 |
| Diastolic | 83±9.5 | 111±10.1 | <0.001 |
| 24 h urine protein
(g) | 0.01±0.03 | 4.36±2.32 | <0.001 |
| Body mass index
(kg/m2) | 25.8±4.1 | 25.9±4.5 | 0.942 |
| Infant birth weight
(g) | 3,578±243 | 3,462±215 | 0.118 |
| Placenta weight
(g) | 533±72 | 513±61 | 0.349 |
Identification of eNOS as a shared target
gene of miR-355 and miR0-584
The present study then performed searches using
miRanda and Targetscan for the potential target genes, to identify
the mediators of miR-335 and miR-584. By screening the resulting
candidate genes based on their functions, combined with information
about the microRNA using the DIANA Lab-based microRNA pathway
analysis tool (http://diana.cslab.ece.ntua.gr/pathways/) and the KEGG
database (http://www.genome.jp/kegg/pathway/hsa/hsa05200.html),
the predicted genes were categorized functionally to narrow down
the candidate genes, which were functionally associated with the
regulation of trophoblast cell behavior or preeclampsia
pathogenesis. The resulting gene identified was eNOS, a gene that
has been repeatedly reported to be compromise the migratory
capability of trophoblast cells (10), or directly exacerbate
preeclampsia-like phenotype by interfering with endothelin system
(12). The microRNAs, and their
seed sequences in the 3′-UTR of eNOS, are presented in Fig. 1A and B. The present study
demonstrated that overexpression of the miR-335 and miR-584 mimics,
but not the control mimics, markedly repressed the activity of
luciferase, when fused with the wild-type 3′-UTR of eNOS, but had
minimal effect on the luciferase activity when fused with the
mutated 3′-UTR of eNOS, as shown in Fig. 1C and D.
mRNA and protein expression levels of
miR-335 and miR-584 are increased in preeclampsic placenta
As shown in Fig. 2A and
B, the relative expression levels of miR-335 and miR-584 in the
preeclampsic placentas were significantly upregulated to ~400% and
320%, respectively, compared with normal placentas. In addition,
the mRNA expression levels were measured uisng RT-qPCR, which
revealed that the mRNA expression level of eNOS in the placentas
derived from severe preeclampsic women was significantly lower than
that observed in the normal placentas (Fig. 2C). The protein levels of eNOS were
also examined using western blot analysis, and the relative density
of eNOS in the tissue samples from severe preeclampsic placentas
was ~4.5-fold higher than in the control, as shown in Fig. 2D and E.
miR-335 and miR-584 inhibit the migration
of trophoblast cells
In addition, the HTR8/SVneo cells were transfected
with either miR-335 and miR584 mimics or their inhibitors to
further investigate the effects of miR-335 and miR-584 on
trophpoblast cell behavior. Inhibitory effects of miR-335 and
miR-584 on the expression levels of eNOS in the HTR8/SVneo cells
were observed, and the inhibitory effect of specific anti-eNOS
siRNA were similar to that of miR-335 or miR-584. The
overexpression of miR-335 and miR584 markedly suppressed the
migratory capability of the HTR8/SVneo cells individually and
synergically (Fig 3D), however,
the effect on the proliferation of the cells was minimal (data not
shown).
 | Figure 3(A) Suppression of miR-335 by its
inhibitor, anti-miR-335, in HTR8/SVneo cells; (B) Suppression of
miR-584 by its inhibitor, anti-miR-584, in HTR8/SVneo cells. (C)
Transwell insert assay to examine cell invasiveness following
transfection with the two inhibitors and control. (D) Effect of the
upregulation of miR-335/miR-584 on cell invasion in the HTR8/SVneo
cells; upper panel, suppression of eNOS by miR-335/miR-584 mimics
and anti-eNOS siRNA in the HTR8/SVneo cells. The histogram
represents results of a Transwell insert assay to examine cell
invasiveness following transfection of HTR8/SVneo cells with
control, miR-335 mimic, miR-584 mimic, siRNA for eNOS and anti-eNOS
siRNA. A 'rescue' effect was observed by the overexpression of eNOS
in the HTR8/SVneo cells transfected with the miR-335/miR-584 mimics
and anti-eNOS siRNA. Data are expressed as the mean ± standard
deviation. miR, microRNA; eNOS, endothelial nitric oxide synthase;
siRNA, small interfering RNA. |
eNOS rescues the inhibition of migration
by miR-335 and miR-584 in trophoblast cells
To assess whether eNOS was directly involved in the
inhibitory effect of miR-335 and miR-584, the present study
transfected eNOS (pcDNA4-eNOS) into the HTR8/SVneo cells
overexpressing miR-335, miR-584 or anti-eNOS-siRNA, respectively.
The results indicated overexpression of eNOS not only completely
inhibited the effect of anti-eNOS-siRNA, miR-335/miR-584 in the
HTR8/SVneo cells, but it also 'overcorrected' the migratory
capability in those cells (Fig.
3).
Inhibition of miR-335 and miR-584
increases the migration of trophoblast cells
To further confirm the function of miR-335 and
miR-584, a 'loss-of-function' assessment was performed by
transfecting the HTR8/SVneo cells with miR-335 inhibitors and
miR-584 inhibitors. The expression levels of miR-335 and miR-584
were significantly reduced 48 h after transfection with the
inhibitors (Fig 3A and B). As
expected, the migratory ability of the HTR8/SVneo cells transfected
with the miR-335 inhibitors and miR-584 inhibitors were markedly
higher than in the control, and was even higher in the cells
transfected with both inhibitors (Fig
3C).
Discussion
miRNAs have emerged as potential novel diagnostic
and therapeutic biomarkers for various medical disorders, including
cancer (20), pregnancy (21,22)
and tissue injury (23).
Preeclampsia is a life threatening medical condition complicated by
hypertension and proteinuria during pregnancy, and is the most
common cause of maternal mortality, morbidity, perinatal mortality
and intrauterine growth retardation (24). Despite progression in understanding
of this medical disorder, the mechanisms underlying preeclampsia
remain to be fully elucidated (25). Investigation of the roles of
differentially expressed miRNAs in the placenta of patients with
preeclampsia are required to advancu current understanding of the
pathogenesis of preeclampsia, and improve its diagnosis and
management.
Abnormal development of the placenta is one of the
major causes of preeclampsia (26), and delivery of the placenta remains
the only definitive treatment for preeclampsia (27). Several studies (28–30)
have examined the differential expression of miRNAs in preeclampsic
placentas. In the present study, the differential expression of
miRNAs in tissue samples derived from preeclampsic placentas were
compared with those from normal controls. In total, 12 miRNAs were
found to be significantly upregulated and six miRNAs were
significantly downregulated in patients with severe preeclampsia.
Of the identified miRNAs, miR-355 and miR-584 were selected for
further functional analysis due to their indicated involvement in
preeclampsia pathogenesis (14,15)
or reported regulatory roles in cell proliferation and migration
(16,17), and eNOS was identified as a shared
target gene of miR-355 and miR-584.
Previously, the preeclampsia susceptibility locus
has been mapped to chromosome 7q35–36 and eNOS has been identified
as one of the susceptible genes of preeclampsia by fine mapping
(31). A common variant in eNOS
predisposes carriers to preeclampsia by promoting degradation of
the enzyme (32). eNOS knockout
leads to elevation of blood pressure and reduces the production of
NO (33). Consistently, decreased
maternal eNOS/NO exacerbates preeclampsia-like phenotype (19), and a significant reduction in the
expression of eNOS is observed in the umbilical vessel of pregnant
female with preeclampsia (34).
Since eNOS was confirmed as a shared target of miR-335 and miR-584,
the present study examined the effect of the two molecules on
trophoblast cells. The results of this investigation revealed that
miR-335 and miR-584 suppressed the expression of eNOS in the
trophoblast cells, individually and synergically, and the
expression pattern of miR-335, miR-584 and eNOS in the preeclampsic
and normal placentas was examined. Consistent with the microarray
results, significantly higher levels of miR-335 and miR-584, and a
lower levels of eNOS were detected in the 20 preeclampsic
placentas, compared with the 20 normal controls.
It is generally accepted that trophoblast cells
contain various cytokines and biochemical effectors that are
essential for metastatic invasion, a crucial process for the
successful implantation and penetration of the endometrial stroma
and blood vessels, which is necessary for a mature placenta and a
viable fetus (35). Impaired
trophoblast invasiveness may result in compromised placental
perfusion during pregnancy, which is considered to be associated
with various pathological processes, including fetal growth
retardation, preeclampsia and spontaneous abortion (36). Accumulating evidence has indicated
that inhibition of NO may cause preeclampsia-like conditions.
Buhimschi et al demonstrated that administration of L-NAME,
which competes with L-arginine and inhibits NO synthesis, to
pregnant rats results in a preeclampsia-like condition.
Additionally, administration of L-arginine to rats infused with
L-NAME reversed the preeclampsia-like symptoms, which were observed
in the pregnant rats treated with L-NAME alone (37).
Consistent with the data of the present study,
miR-335 and miR-584 have repeatedly been observed to be
differentially expressed in previous preeclampsia microarray
studies (14,15,38),
and the two miRNAs have been demonstrated to be significantly
associated with the invasive and migratory capabilities of human
cells (39,40). In the present study, miR-335 and
miR-584 suppressed the migration and invasion of trophoblast cells
via the regulation of eNOS. To investigate the function of miR-335
and miR-584 in vitro, miR-335 and miR-584 mimics or their
inhibitors were transfected into HTR8/Svneo cells. miR-335 and
miR-584 was observed to have an inhibitory effect on trophoblast
cell migration and invasion individually, and the combined effect
of co-transfection of the two small molecules was even more marked.
Similarly, downregulation of miR-335 and miR-584 promoted
trophoblast cell migration and invasion, individually and
synergically, suggesting that endogenous miR-335 and miR-584 are
involved in regulating cell behavior. In addition, the inhibitory
effect of miR-335, miR-584 and anti-eNOS-siRNA on the migration of
trophoblast cells was 'rescued' by overexpression of eNOS, however,
simultaneous 'overcorrection' was observed in the microRNA and
siRNA groups (Fig. 3D). As cGMP,
the major downstream messanger of NO, also mediates migration in
human cells (41), the present
study hypothesized that the accumulation of cGMP, generated by
intracellular overexpression of NO, may be responsible for this
overcorrection in the migratory ability of the cells.
Taken together, the data of the present study
revealed for the first time, to the best of our knowledge, that
miR-335 and miR-584 exert an inhibitory effect in regulating the
migration and invasion of trophoblast cells through targeting eNOS,
which may contribute to the pathogenesis of preeclampsia. These
results advance current understanding of trophoblast disease, and
miR335 and miR-584 may offer potential as novel therapeutic targets
in the treatment of preeclampsia.
Acknowledgments
The present study was fully sponsored by the Natural
Science Foundation of Shanxi Province (China; grant. no.
2014JM4133).
References
|
1
|
Sibai B, Dekker G and Kupferminc M:
Pre-eclampsia. Lancet. 365:785–799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Redman CW and Sargent IL: Latest advances
in understanding preeclampsia. Science. 308:1592–1594. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grill S, Rusterholz C, Zanetti-Dallenbach
R, Tercanli S, Holzgreve W, Hahn S and Lapaire O: Potential markers
of preeclampsia-a review. Reprod Biol Endocrinol. 7:702009.
View Article : Google Scholar
|
|
4
|
Huppertz B: Placental origins of
preeclampsia: Challenging the current hypothesis. Hypertension.
51:970–975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oudejans CB and van Dijk M: Placental gene
expression and pre-eclampsia. Placenta. 29(Suppl A): S78–S82. 2008.
View Article : Google Scholar
|
|
6
|
Sitras V, Paulssen RH, Grønaas H, Leirvik
J, Hanssen TA, Vårtun A and Acharya G: Differential placental gene
expression in severe preeclampsia. Placenta. 30:424–433. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Founds SA, Conley YP, Lyons-Weiler JF,
Jeyabalan A, Hogge WA and Conrad KP: Altered global gene expression
in first trimester placentas of women destined to develop
preeclampsia. Placenta. 30:15–24. 2009. View Article : Google Scholar :
|
|
8
|
Enquobahrie DA, Meller M, Rice K, Psaty
BM, Siscovick DS and Williams MA: Differential placental gene
expression in preeclampsia. Am J Obstet Gynecol. 199:566e561–e511.
2008.PubMed/NCBI
|
|
9
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu J, Wang F, Yang GH, Wang FL, Ma YN, Du
ZW and Zhang JW: Human microRNA clusters: Genomic organization and
expression profile in leukemia cell lines. Biochem Bioph Res
Commun. 349:59–68. 2006. View Article : Google Scholar
|
|
11
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calame K: MicroRNA-155 function in B
Cells. Immunity. 27:825–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jopling CL, Yi M, Lancaster AM, Lemon SM
and Sarnow P: Modulation of hepatitis C virus RNA abundance by a
liver-specific MicroRNA. Science. 309:1577–1581. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu Y, Li P, Hao S, Liu L, Zhao J and Hou
Y: Differential expression of microRNAs in the placentae of Chinese
patients with severe pre-eclampsia. Clin Chem Lab Med. 47:923–929.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mouillet JF, Chu T, Nelson DM, Mishima T
and Sadovsky Y: MiR-205 silences MED1 in hypoxic primary human
trophoblasts. FASEB J. 24:2030–2039. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skrzypek K, Tertil M, Golda S, Ciesla M,
Weglarczyk K, Collet G, Guichard A, Kozakowska M, Boczkowski J, Was
H, et al: Interplay between heme oxygenase-1 and miR-378 affects
non-small cell lung carcinoma growth, vascularization and
metastasis. Antioxid Redox Signal. 19:644–660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen LT, Xu SD, Xu H, Zhang JF, Ning JF
and Wang SF: MicroRNA-378 is associated with non-small cell lung
cancer brain metastasis by promoting cell migration, invasion and
tumor angiogenesis. Med Oncol. 29:1673–1680. 2012. View Article : Google Scholar
|
|
18
|
Corthorn J, Germain AA, Chacón C, Rey S,
Soto GX, Figueroa CD, Müller-Esterl W, Duarte I and Valdés G:
Expression of kallikrein, bradykinin b2 receptor and endothelial
nitric oxide synthase in placenta in normal gestation, preeclampsia
and placenta accreta. Endocrine. 29:491–499. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li F, Hagaman JR, Kim HS, Maeda N,
Jennette JC, Faber JE, Karumanchi SA, Smithies O and Takahashi N:
eNOS deficiency acts through endothelin to aggravate sFlt-1-induced
pre-eclampsia-like phenotype. J Am Soc Nephrol. 23:652–660. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chim SS, Shing TK, Hung EC, Leung TY, Lau
TK, Chiu RW and Lo YM: Detection and characterization of placental
microRNAs in maternal plasma. Clin Chem. 54:482–490. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gilad S, Meiri E, Yogev Y, Benjamin S,
Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed
N, et al: Serum microRNAs are promising novel biomarkers. PLoS One.
3:e31482008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang K, Zhang S, Marzolf B, Troisch P,
Brightman A, Hu Z, Hood LE and Galas DJ: Circulating microRNAs,
potential biomarkers for drug-induced liver injury. Proc Natl Acad
Sci USA. 106:4402–4407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sibai B, Dekker G and Kupferminc M:
Pre-eclampsia. Lancet. 365:785–799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levine RJ, Lam C, Qian C, Yu KF, Maynard
SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, et al:
CPEP Study Group: Soluble endoglin and other circulating
antiangiogenic factors in preeclampsia. N Engl J Med. 355:992–1005.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Myatt L: Role of placenta in preeclampsia.
Endocrine. 19:103–111. 2002. View Article : Google Scholar
|
|
27
|
Maynard S, Epstein FH and Karumanchi SA:
Preeclampsia and angiogenic imbalance. Annu Rev Med. 59:61–78.
2008. View Article : Google Scholar
|
|
28
|
Pineles BL, Romero R, Montenegro D, Tarca
AL, Han YM, Kim YM, Draghici S, Espinoza J, Kusanovic JP, Mittal P,
et al: Distinct subsets of microRNAs are expressed differentially
in the human placentas of patients with preeclampsia. Am J Obstet
Gynecol. 196:261e261–e266. 2007.PubMed/NCBI
|
|
29
|
Roman H, Marpeau L and Hulsey TC:
Surgeons' experience and interaction effect in randomized
controlled trials regarding new surgical procedures. Am J Obstet
Gynecol. 199:108e101–e106. 2008.PubMed/NCBI
|
|
30
|
Hu Y, Li P, Hao S, Liu L, Zhao J and Hou
Y: Differential expression of microRNAs in the placentae of Chinese
patients with severe pre-eclampsia. Clin Chem Lab Med. 47:923–929.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo G, Lade JA, Wilton AN, Moses EK,
Grehan M, Fu Y, Qiu H, Cooper DW and Brennecke SP: Genetic
susceptibility to pre-eclampsia and chromosome 7q36. Hum Genet.
105:641–647. 1999. View Article : Google Scholar
|
|
32
|
Demircubuk AG, Coskun MY, Demiryurek S,
Dokuyucu R, Öztuzcu S, Taviloğlu ZŞ, Arslan A and Sivaslı E:
Endothelial NOS gene Glu298Asp polymorphism in preterm neonates
with respiratory distress syndrome. Pediatr Pulmonol. 48:976–980.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duplain H, Burcelin R, Sartori C, Cook S,
Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B,
et al: Insulin resistance, hyperlipidemia and hypertension in mice
lacking endothelial nitric oxide synthase. Circulation.
104:342–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiang W, Chen H, Hu L and Xu X:
Endothelial nitric oxide synthase traffic inducer in the umbilical
vessels of the patients with pre-eclampsia. J Huazhong U Sci
Technolog Med Sci. 29:243–245. 2009. View Article : Google Scholar
|
|
35
|
Soundararajan R and Rao AJ: Trophoblast
'pseudo-tumorigenesis': Significance and contributory factors.
Reprod Biol Endocrinol. 2:152004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lala PK and Chakraborty C: Factors
regulating trophoblast migration and invasiveness: Possible
derangements contributing to pre-eclampsia and fetal injury.
Placenta. 24:575–587. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Buhimschi I, Yallampalli C, Chwalisz K and
Garfield RE: Pre-eclampsia-like conditions produced by nitric oxide
inhibition: Effects of L-arginine, D-arginine and steroid hormones.
Hum Reprod. 10:2723–2730. 1995.PubMed/NCBI
|
|
38
|
Enquobahrie DA, Abetew DF, Sorensen TK,
Willoughby D, Chidambaram K and Williams MA: Placental microRNA
expression in pregnancies complicated by preeclampsia. Am J Obstet
Gynecol. 204:178e112–e121. 2011.
|
|
39
|
Wang H, Li M, Zhang R, Wang Y, Zang W, Ma
Y, Zhao G and Zhang G: Effect of miR-335 upregulation on the
apoptosis and invasion of lung cancer cell A549 and H1299. Tumour
Biol. 34:3101–3109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ueno K, Hirata H, Shahryari V, Chen Y,
Zaman MS, Singh K, Tabatabai ZL, Hinoda Y and Dahiya R: Tumour
suppressor microRNA-584 directly targets oncogene Rock-1 and
decreases invasion ability in human clear cell renal cell
carcinoma. Br J Cancer. 104:308–315. 2011. View Article : Google Scholar :
|
|
41
|
Caterina MJ and Devreotes PN: Molecular
insights into eukaryotic chemotaxis. FASEB J. 5:3078–3085.
1991.PubMed/NCBI
|