Introduction
Cameron and Pauling first observed that ascorbic
acid (AsA) exhibits antitumor properties and applied this finding
to a clinical setting, however, their investigations date back ~30
years (1). At that time, the
effectiveness of AsA for different purposes in addition to the
treatment of cancer. However, the anticancer activity of AsA was
not confirmed by clinical investigations at the Mayo Clinic
(Rochester, MN, USA), and AsA had not been considered for primary
clinical therapies until recently (2). Previous studies have demonstrated
that it is possible to administer a high intravenous dose of AsA,
and that this can be applied for the treatment of cancer (3). The direct intravenous use of AsA in
cancer treatment was demonstrated by Padayatty et al, who
reported that three malignant tumor cases were controlled primarily
by treatment with AsA (4).
Furthermore, Padayatty et al reported side effects in only
101 of the 9,328 patients treated intravenously with AsA, and these
were mostly minor effects (5).
Therefore, the present study investigated whether
AsA in combination with X-ray irradiation exhibited an increased
antitumor effect against HL60 cells, with the aim to use AsA in
combination with radiation therapy in the future (6). Regarding the mechanism of apoptosis,
it has been suggested that the involvement of B-cell-associated X
protein and caspase 8 differ following X-ray irradiation or
treatment with AsA alone compared with the combined treatment with
X-ray irradiation and AsA (7). The
present study was performed to investigate the effect of the
combined use of AsA and X-ray irradiation on epithelial cancer
cells and its potential use in future clinical treatment.
Materials and methods
Cell culture
Human HT1080 fibrosarcoma cells were purchased from
American Tissue Culture Collection (Rockville, IL, USA) and human
A549 lung cancer cells and human SAS oral squamous cell carcinoma
cells were purchased from the RIKEN Bio-Resource Center (Tsukuba,
Japan). The HT1080 and SAS cells were maintained in RPMI-1640
(Gibco Life Technologies, Carlsbad, CA, USA), supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Japan Bioserum Co. Ltd.,
Fukuyama, Japan) in a 5% CO2 incubator at 37°C. The A549
cells were maintained in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich, St. Louis, MO, USA), supplemented with 10%
heat-inactivated FBS in 5% CO2 at 37°C. All three cell
lines were passaged at 75–90% confluence.
X-ray irradiation and AsA treatment in
vitro
Culture dishes or microtiter plates containing the
cells were exposed to X-rays from an X-ray machine (MBR-1520R-3;
Hitachi, Tokyo, Japan) at 150 kV and 20 mA through a 0.5-mm
aluminum and 0.3-mm copper filter, at a dose rate of 1.0 Gy/min.
Prior to X-ray irradiation and/or treatment with AsA (Wako Pure
Chemical Industries Ltd., Osaka, Japan), 10×105 cells in
10 ml of medium were prepared in 10 cm dishes for 24 h. The dishes
were divided into four groups: Control, AsA alone, X-ray
irradiation alone and X-ray irradiation combined with AsA. The AsA
was dissolved to a final concentration of 5 mM in medium following
titration of the solution with NaOH (Wako Pure Chemical Industries
Ltd.) to pH 7.4. The dishes treated with X-ray irradiation alone
and X-ray irradiation combined with AsA, were irradiated 1 h after
treatment with AsA.
Cell survival
HT1080 cells do not form colonies, and the cell
survival rates of these cells as well as A549 and SAS cells were
estimated without using colony assay in the present study. To
remove all cells from the dishes following incubation, the cell
monolayer was washed with phosphate buffered saline (PBS) and 0.1%
trypsin/EDTA (1 ml per 25 cm2 of surface area; Gibco
Life Technologies, Carlsbad, CA, USA) was added to each well and
incubated at 37°C for 2–10 min. When all the cells had detached,
the number of viable cells were quantified using a trypan blue
exclusion test (Wako Pure Chemical Industries Ltd.). The cells were
stained with 0.2% trypan blue for 1 min and counted to estimate the
number of viable cells in 1 ml medium.
Animal experiments
The animals used in the present study were
maintained, and the experiments were performed, according to the
Principles of Laboratory Animal Care established by the NIH. All
animal experiments described were previously approved by the ethics
committee of Hirosaki University (Hirosaki, Japan) and were
performed in accordance with the Guidelines for the Care and Use of
Laboratory Animals in Hirosaki University. Female, 5-week-old
BALB/cAJcl-nu/nu mice (CLEA Japan, Tokyo, Japan) were used in the
present study. The mice were injected in the lateral neck with 200
µl PBS, containing HT1080 cells (5×106 cells/ml)
1 week after arrival at Hirosaki University. The mice were divided
into four groups: Control, AsA alone, X-ray irradiation alone and
X-ray irradiation combined with AsA. The treatment consisted of
X-ray irradiation and/or AsA injection 7 days and 9 days after
implantation of the HT1080 cells (Fig.
2A). AsA was dissolved in PBS at a concentration of 5 mM and
titrated with NaOH at pH 7.4. Each mouse in the AsA alone group and
in the X-ray irradiation combined with AsA group were injected with
200 µl AsA solution. In the X-ray irradiation group and
X-ray irradiation combined with AsA group, a field (40×40 mm) was
made using a lead plate, which was placed centrally over the tumor.
The mice were anesthetized by intraperitoneal injection of sodium
pentobarbital (50 mg/kg; Nembutor, Abbott Laboratories, Abbott
Park, Il, USA) and were fixed in the prone position using synthetic
rubber bands tied to each leg of the mouse. The mice were
irradiated twice with 4 Gy with a total dose of 8 Gy. The
irradiation was the same as that used for the cells treated in
vitro, as described above. The mice in the X-ray irradiation
and AsA combination groups were irradiated 15 min following
injection with the AsA. The long diameter and short diameter of the
tumors on the skin were measured daily. All mice were sacrificed by
cervical dislocation following 5% halothane (Wako Pure Chemical
Industries Ltd.) inhalation, 13 days after implantation of the
HT1080 cells. The tumor mass was subsequently removed from the
lateral neck of the mice and was weighed.
Detection of apoptosis
The extent of apoptosis was determined by annexin
V-Fluorescein isothiocyanate (FITC; BioLegend, San Diego, CA, USA)
and propidium iodide (PI; Sigma-Aldrich) staining, according to the
manufacturer's instructions. Following X-ray irradiation and/or
treatment with AsA, the cells were cultured for 48 h at 37°C. The
cells were washed with PBS and suspended in 100 µl annexin V
binding buffer (BioLegend). The annexin V-FITC (2.5 µg/ml)
and PI solution (50 µg/ml) were added to the cell suspension
and incubated for 15 min at room temperature in the dark. The
number of apoptotic cells were determined using flow cytometry
(Cytomics FC500; Beckman-Coulter, Fullerton, CA, USA). In the
annexin V/PI quadrant gating, annexin V(−)/PI(−), annexin
V(+)/PI(−) and annexin V(+)/PI(+) were used to identify the
fraction of viable cells, early apoptotic cells and late
apoptotic/necrotic cells, respectively (8).
Detection of activated caspase 3
An FITC-conjugated monoclonal active caspase 3
antibody kit for apoptosis (BD Biosciences, San Diego, CA, USA) was
used to detect active caspase 3, according to the manufacturer's
instructions. The cells were cultured for 48 h following X-ray
irradiation and/or treatment with AsA. The cells were washed with
PBS, suspended in 500 µl Cytofix/Cytoperm™ (BD Biosciences)
and incubated for 20 min on ice. Following incubation, the cells
were washed with Perm/Wash™ buffer (BD Biosciences) and resuspended
in Perm/Wash™ buffer, containing 5% FITC-conjugated rabbit
anti-active caspase 3 antibody (cat. no. 559341; BD Pharmingen, San
Diego, CA, USA). Following 30 min incubation at room temperature in
the dark, the cells were washed and then analyzed by flow cytometry
(Cytomics FC500; Beckman-Coulter).
Cell cycle analysis by flow
cytometry
The HT1080 cells were seeded in 10 ml RPMI-1640
(1×104 cells/ml) in 100 mm culture dishes (Iwaki, Tokyo,
Japan) and incubated at 37°C for 24 h, in order to adhere to the
dish. Following X-ray irradiation and/or treatment with AsA, the
cells were cultured for a specific duration. The cells were fixed
with 70% ethanol (Wako Pure Chemical Industries Ltd.) overnight at
4°C, washed with PBS and subsequently treated with RNase (200
µg/ml; Sigma-Aldrich) at 37°C for 30 min to hydrolyze the
RNA. Following treatment, the cells were washed with PBS and
stained with PI (25 µg/ml) for 30 min in the dark. A flow
cytometer was used to analyze the cell cycle distribution.
Cell proliferation assay
Cell proliferation was measured using a cell
proliferation ELISA, bromodexyuridine (BrdU) colorimetric kit
(Roche, Basel, Switzerland). The cells (20,000/ml) in 200 µl
of medium were placed into each well of a 96 well microtiter plate
24 h prior to treatment. Following X-ray irradiation and/or
treatment with AsA, the cells were added to 20 µl BrdU
(Roche) and cultured for a specific duration. The cells were
incubated with 20 µl/well FixDenat solution (Roche) for 30
min and Anti-BrdU peroxidase (Roche) for 90 min at room
temperature. The substrate reaction was measured using a
spectrophotometer (Benchmark; Bio-Rad, Foster City, CA, USA) at 370
nm.
Statistical analysis
All the data are expressed as the mean ± standard
deviation of at least three independent experiments. Statistical
comparisons between the groups were performed using analysis of
variance and Student's t-test. All statistical analyses were
conducted using SPSS version 16.0 for Windows (SPSS Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
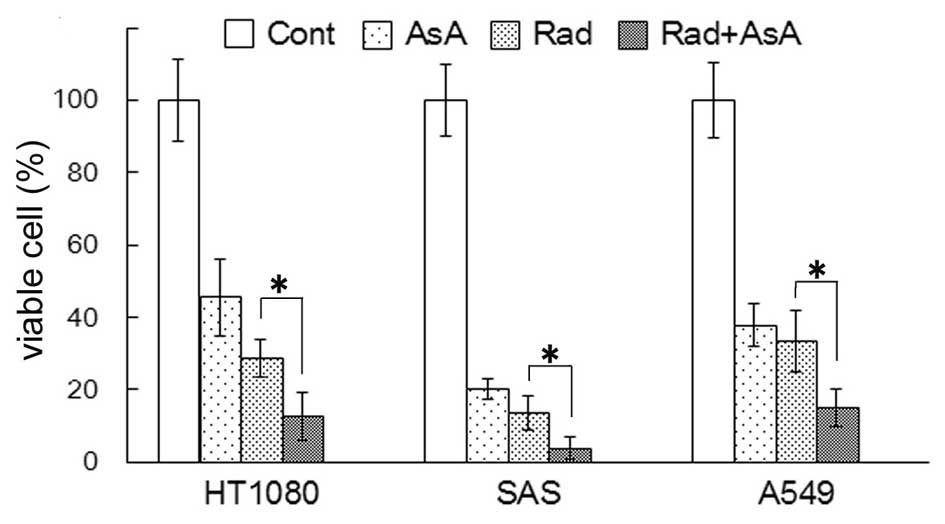
Growth suppression of HT1080, SAS and
A549 cells by X-ray irradiation and/or AsA in vitro
The number of viable cells were quantified 72 h
after 4 Gy X-ray irradiation and/or treatment with 5 mM AsA. The
percentage of viable cells compared with the control are shown in
Fig. 1. The percentage of viable
cells following treatment with AsA alone and X-ray irradiation
alone significantly decreased in all three cell lines (P<0.05).
In all three cell lines, the percentage of viable cells following
X-ray irradiation combined with AsA treatment were significantly
lower compared with X-ray irradiation alone (P<0.05).
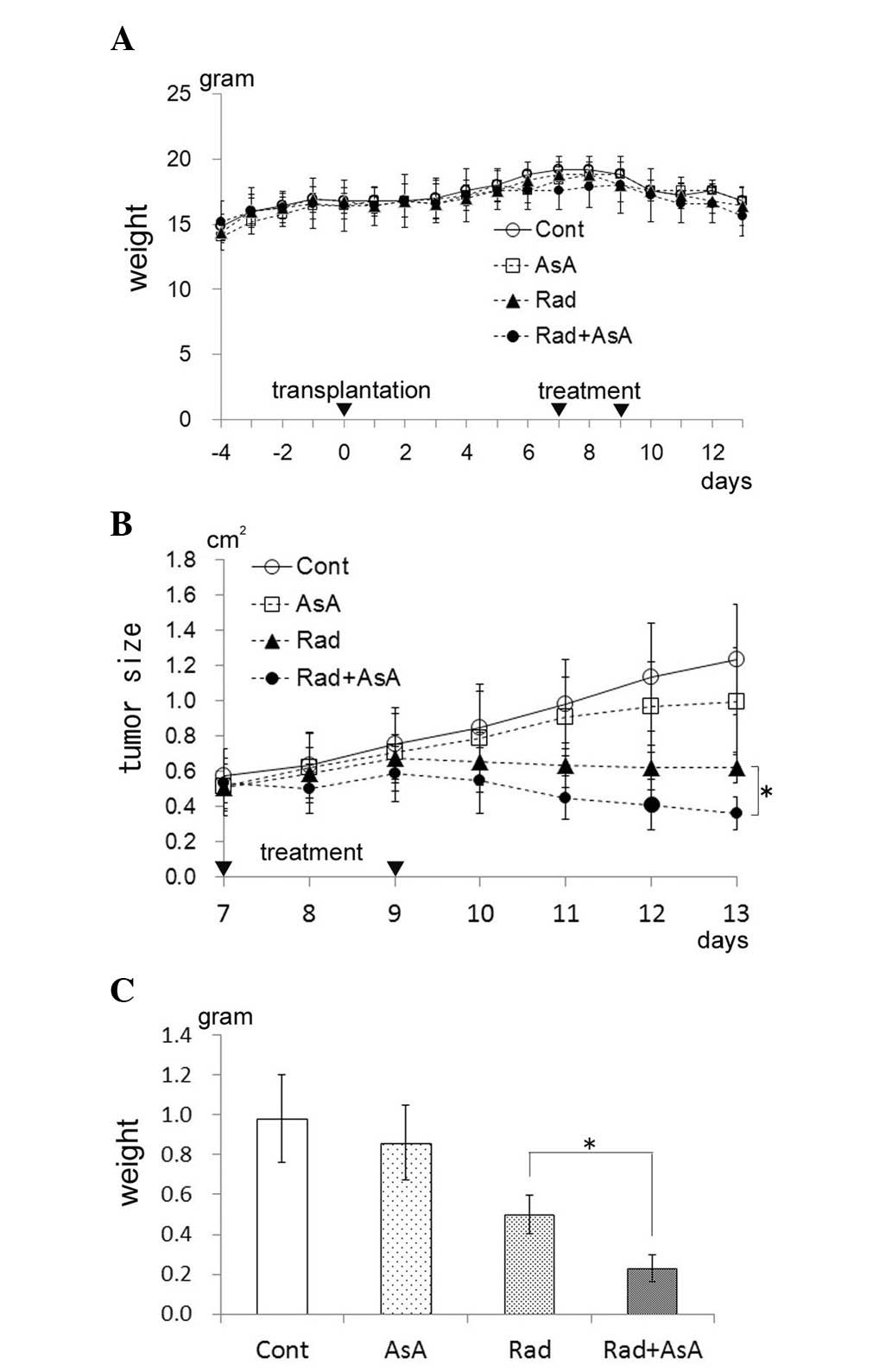
Tumor growth suppression of transplanted
HT-1080 cells by X-ray irradiation and/or AsA treatment in
vivo
The initial weight of the mice increased relative to
natural growth, however, on the eighth day following
transplantation of the HT-1080 cells, the weight of the mice
decreased (Fig. 2A). No
significant difference in weight was observed between the four
groups. The results of the tumor sizes (long diameter x short
diameter) are presented in Fig. 2B
and demonstrate that, in the control mice, the tumor size increased
daily. No significant difference in tumor size was observed between
the control group and those subjected to treatment with AsA alone,
although the tumor size did not increase following the second AsA
treatment. The tumor size in the groups treated with X-ray
irradiation alone and X-ray irradiation combined with AsA decreased
following the second treatment. Furthermore, the tumor size in the
X-ray irradiation combined with AsA group was lower compared with
that in the X-ray irradiation alone group, with a significant
difference between the two groups on the 13th day following
transplantation of the HT-1080 cells (P<0.05). The weights of
the tumor mass for each group on the 13th day following
the transplantation of HT-1080 cells is shown in Fig. 2C. The average tumor weight in the
AsA alone group appeared lower compared with the control group,
however, no significant difference was determined between the two
groups. Statistical analysis revealed significant differences
between the control group and the X-ray irradiation alone group,
and between control group and X-ray irradiation combined AsA
treatment group (P<0.05). The average weight of the tumor in the
X-ray irradiation combined with AsA group was significantly lower
than that in the X-ray irradiation alone group (P<0.05).
Detection of apoptosis following X-ray
irradiation and/or treatment with AsA
Annexin V/PI staining methods were used to analyze
the induction of apoptosis. The percentage of early apoptotic
annexin V(+)/PI(−) cells and annexin V(+)/PI(+) late apoptotic
cells were significantly higher in the groups treated with X-ray
irradiation and X-ray irradiation in combination with AsA, compared
with the control and the group treated with AsA alone (P<0.05;
Fig. 3A and C). However, no
statistically significant difference was observed between the
control cells and those treated with AsA alone. In addition, no
statistically significant difference was observed between the cells
treated with X-ray irradiation alone and X-ray irradiation combined
with AsA. To further investigate the induction of apoptosis, the
expression levels of activated caspase 3, an executioner of
apoptosis, were analyzed. The activation of caspase 3 was higher in
cells treated with X-ray irradiation and X-ray irradiation combined
with AsA compared with the control and the group treated with AsA
alone (Fig. 3B and D). However, no
statistically significant difference was observed in the activation
of caspase 3 between the control cells and those treated with AsA.
In addition, no statistically significant difference was observed
between the cells from the X-ray irradiation and X-ray combined AsA
groups.
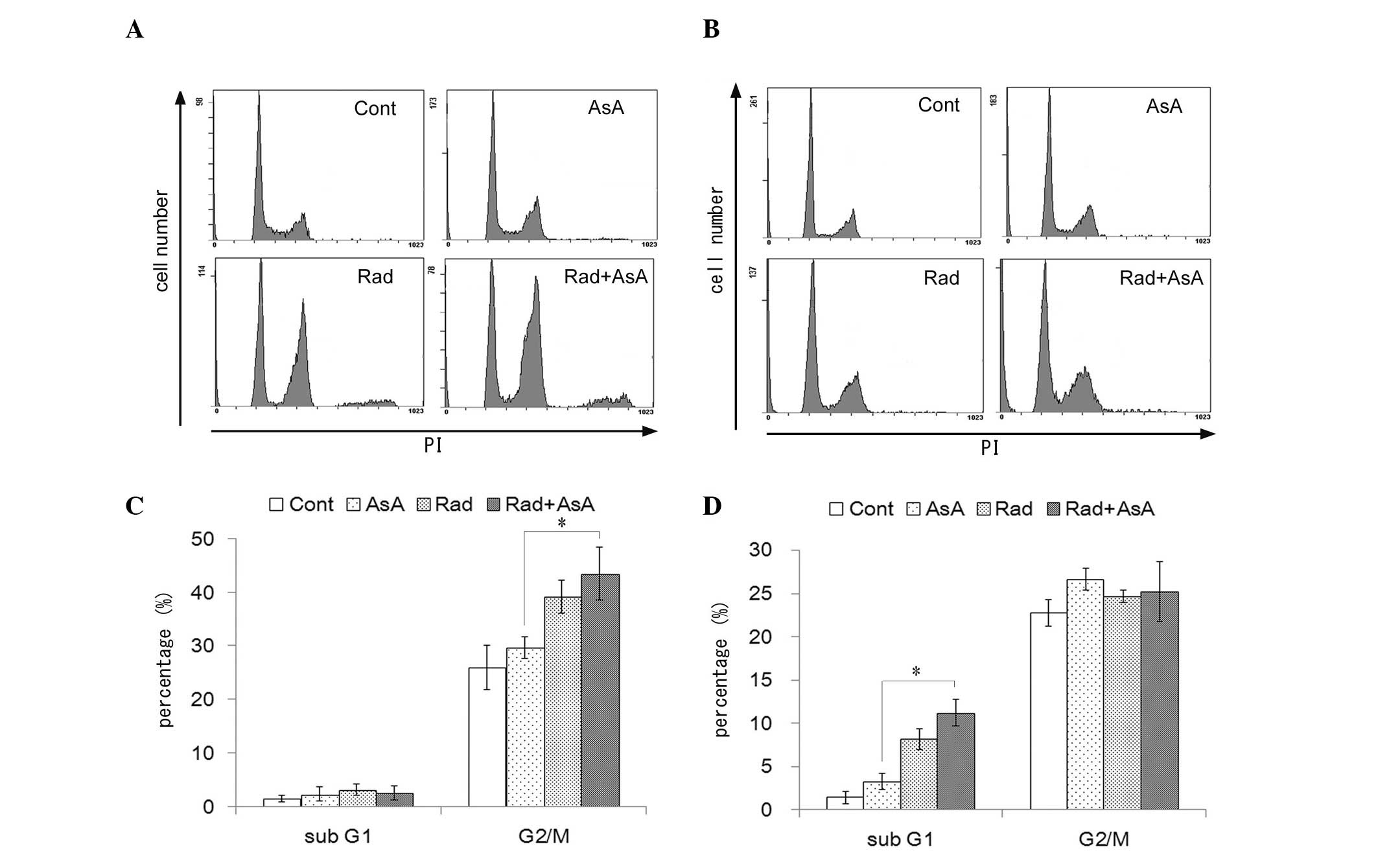
Changes in cell cycle profile and cell
proliferation following X-ray irradiation and/or treatment with
AsA
The effect of X-ray irradiation and/or treatment
with AsA on the cell cycle profile of HT1080 cells was analyzed
(Fig. 4). X-ray irradiation and
X-ray irradiation combined with AsA increased the number of cells
in the G2/M fraction following 12 h treatment, compared with the
control and AsA alone groups (P<0.05; Fig. 4A). Treatment with X-ray irradiation
alone and combined with AsA significantly increased the sub-G1
fraction following treatment for 72 h compared with the control and
treatment with AsA alone (P<0.05; Fig. 4B). By contrast, no statistically
significant differences were observed in the cell cycle profiles
between the control cells and those treated with AsA alone, nor
between the cells treated with X-ray irradiation alone and in
combination with AsA. However, the proliferation of cells following
treatment with AsA, with or without X-ray irradiation were lower
compared with the control, and a significant difference was
observed between the groups treated with X-ray irradiation alone
and X-ray in combination with AsA treatment (P<0.05; Fig. 5).
Discussion
AsA exhibits cytotoxic effects on tumor cells, which
have a low concentration of intracellular catalase to degrade
hydrogen peroxide (H2O2) (9). Previous studies have demonstrated
that tumor cells are easily damaged by H2O2,
and the production of adenosine triphosphate is decreased as a
result of mitochondrial damage, leading to tumor cell death
(10,11). When AsA is added to culture in the
presence of catalase, the cytotoxic effects of AsA disappear
(6). The majority of the cell
death induced by X-ray irradiation depends on the production of
intracellular reactive oxygen species (ROS), which are produced
during irradiation. Within several hours of irradiation, secondary
ROS production occurs intracellularly, which induces apoptosis
(12). When cell cultures are
exposed to X-ray irradiation in the presence of catalase, the
cytotoxic effects of the X-rays do not disappear (13). This suggests that the production of
ROS following X-ray irradiation differs from that produced
following treatment with AsA (6).
Padayatty et al demonstrated that an AsA
concentration ≥1 mM in the blood, administered by intravenous
injection, is required for the effective treatment of cancer, and
that a concentration of ~5 mM is optimal (4). In clinical investigations performed
by Hoffer et al, it was observed that when AsA is
administered at a blood concentration of 5 mM, no side effects are
observed in humans (14). In the
present study, the effects of a combined treatment of 5 mM AsA and
X-ray irradiation on epithelial cancer cells and sarcoma cells were
examined. Several previous studies have also used 5 mM AsA in their
investigations to determine its direct effect in vitro and
by diffusion into the human body (15–17).
In the present study, X-ray irradiation combined
with AsA suppressed the growth of the epithelial cancer and sarcoma
cells in vitro (Fig. 1) and
suppressed the growth of implanted HT-1080 tumor cells in
vivo (Fig. 2). The suppression
on cell growth observed following X-ray irradiation combined with
AsA was significantly higher compared with that observed following
X-ray irradiation alone. These data indicated that combining X-ray
irradiation with AsA treatment is beneficial for cancer therapy.
Few studies have investigated the use of AsA with radiotherapy in
tumor control. This may be due to the fact that AsA is a well-known
free radical scavenger and that the anticancer effects of X-ray
irradiation, which attack tumor cells by generating OH radicals may
disappear (18,19). However, treatment with AsA has
failed to inhibit the anticancer effects of X-ray irradiation when
used in combination with X-ray irradiation (6,7).
The results of the present study demonstrated that
X-ray irradiation increased the apoptotic rate of HT1080 cells. An
increase in the G2/M fraction at 12 h and sub-G1 fraction at 72 h
was observed in the HT1080 cells exposed to X-ray irradiation. It
is likely that X-ray irradiation induced DNA damage and resulted in
G2/M arrest in preparation for programmed cell death (20). However, the apoptotic rate was not
increased in the HT1080 cells treated with 5 mM AsA, and no changes
were observed in the G2/M fraction at 12 h or sub-G1 fraction at 72
h. By contrast, treatment with AsA caused higher levels of
suppression on cell proliferation compared with X-ray irradiation
alone. Considering these results, 5 mM AsA may slow the cell cycle
without changing the cell cycle fraction rate, leading to reduced
tumor growth.
Several studies have investigated apoptosis as part
of tumor inhibition by AsA (21,22).
The present study confirmed that apoptosis in the HT1080 cells was
induced and G2/M cell cycle arrest was observed following treatment
with >10 mM AsA (data not shown). It is well-known that cell
cycle arrest can be readily induced in blood cells (23). The present study demonstrated that,
even with 5 mM AsA, cell growth was suppressed in the epithelial
cancer and sarcoma cells, which were relatively radioresistant
without apoptosis. The marked inhibition of cell growth observed in
the cells treated with X-ray irradiation combined with AsA
treatment appeared to be attributed to the combined effect of X-ray
irradiation and the added suppressive effect of AsA on cancer
cells. Therefore, X-ray irradiation combined with AsA treatment may
be effective against solid tumors (24).
In conclusion, X-ray irradiation combined with AsA
treatment suppressed the growth of HT1080, SAS and A549 cells in
vitro, and reduced tumor mass following implantation of HT-1080
in vivo. Treatment with 5 mM AsA caused a higher suppression
of cell proliferation compared with X-ray irradiation alone,
although apoptosis was not increased. This may explain why X-ray
irradiation combined with AsA led to more marked inhibition of
cancer cell growth compared with X-ray irradiation alone. Since AsA
administration was observed to be non-toxic in humans, AsA may be
important in potential future radiotherapy (23).
Acknowledgments
The present study was supported by the Japan Society
for the Promotion of Science (Grants-in-Aid for Scientific
Research, project nos. 21591603 and 24591831).
References
|
1
|
Cameron E and Pauling L: Ascorbic acid and
cancer: a review. Proc Natl Acad Sci USA. 75:4538–3542. 1987.
View Article : Google Scholar
|
|
2
|
Creagan ET, Moertel CG, O'Fallon JR,
Schutt AJ, O'Connell MJ, Rubin J and Frytak S: Failure of high-dose
vitamin C (ascorbic acid) therapy to benefit patients with advanced
cancer. A controlled trial. N Engl J Med. 301:687–690. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duconge J, Miranda-Massari JR, Gonzalez
MJ, Jackson JA, Warnock W and Riordan NH: Pharmacokinetics of
vitamin C: insights into the oral and intravenous administration of
ascorbate. P R Health Sci J. 27:7–19. 2008.PubMed/NCBI
|
|
4
|
Padayatty SJ, Riordan HD, Hewitt SM, Katz
A, Hoffer LJ and Levine M: Intravenously administered vitamin C as
cancer therapy: three cases. CMAJ. 174:937–942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Padayatty SJ, Sun AY, Chen Q, Espey MG,
Drisko J and Levine M: Vitamin C: intravenous use by complementary
and alternative medicine practitioners and adverse effects. PLoS
One. 5:e114142010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Terashima S, Hosokawa Y, Yoshino H,
Yamaguchi M and Nakamura T: Effect of ascorbic acid and
X-irradiation on HL-60 human leukemia cells: the kinetics of
reactive oxygen species. Oncol Rep. 30:2653–2658. 2013.PubMed/NCBI
|
|
7
|
Shinozaki K, Hosokawa Y, Hazawa M,
Kashiwakura I, Okumura K, Kaku T and Nakayama E: Ascorbic acid
enhances radiation-induced apoptosis in an HL60 human leukemia cell
line. J Radiat Res (Tokyo). 52:229–237. 2011. View Article : Google Scholar
|
|
8
|
Wakasaya T, Yoshino H, Fukushi Y,
Yoshizawa A and Kashiwakura I: A liquid crystal-related compound
induces cell cycle arrest at the G2/M phase and apoptosis in the
A549 human non-small cell lung cancer cell line. Int J Oncol.
42:1205–121. 2013.PubMed/NCBI
|
|
9
|
Chen Q, Espey MG, Sun AY, et al: Ascorbate
in pharmacologic concentrations selectively generates ascorbate
radical and hydrogen peroxide in extracellular fluid in vivo. Proc
Natl Acad Sci USA. 104:8749–8754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takemura Y, Satoh M, Satoh K, Hamada H,
Sekido Y and Kubota S: High dose of ascorbic acid induces cell
death in mesothelioma cells. Biochem Biophys Res Commun.
394:249–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bordignon B, Mones S, Rahman F, Chiron J,
Peiretti F, Vidal N and Fontes M: A derivative of ascorbic acid
modulates cAMP production. Biochem Biophys Res Commun. 439:137–141.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kam WW and Banati RB: Effects of ionizing
radiation on mitochondria. Free Radic Biol Med. 65:607–619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klingelhoeffer C, Kämmerer U, Koospal M,
et al: Natural resistance to ascorbic acid induced oxidative stress
is mainly mediated by catalase activity in human cancer cells and
catalase-silencing sensitizes to oxidative stress. BMC Complement
Altern Med. 12:612012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoffer LJ, Levine M, Assouline S, et al:
Phase I clinical trial of iv ascorbic acid in advanced malignancy.
Ann Oncol. 19:1969–1974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Putchala MC, Ramani P, Sherlin HJ,
Premkumar P and Natesan A: Ascorbic acid and its pro-oxidant
activity as a therapy for tumours of oral cavity - a systematic
review. Arch Oral Biol. 58:563–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du J, Cullen JJ and Buettner GR: Ascorbic
acid: chemistry, biology and the treatment of cancer. Biochim
Biophys Acta. 1826:443–457. 2012.PubMed/NCBI
|
|
17
|
Ha YM, Park MK, Kim HJ, Seo HG, Lee JH and
Chang KC: High concentrations of ascorbic acid induces apoptosis of
human gastric cancer cell by p38-MAP kinase-dependent up-regulation
of transferrin receptor. Cancer Lett. 277:48–54. 2009. View Article : Google Scholar
|
|
18
|
Fukumura H, Sato M, Kezuka K, et al:
Effect of ascorbic acid on reactive oxygen species production in
chemotherapy and hyperthermia in prostate cancer cells. J Physiol
Sci. 62:251–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Witenberg B, Kletter Y, Kalir HH, et al:
Ascorbic acid inhibits apoptosis induced by X irradiation in HL60
myeloid leukemia cells. Radiat Res. 152:468–478. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta A, Hunt CR, Chakraborty S, et al:
Role of 53BP1 in the regulation of DNA double-strand break repair
pathway choice. Radiat Res. 181:1–8. 2014. View Article : Google Scholar :
|
|
21
|
Vuyyuri SB, Rinkinen J, Worden E, Shim H,
Lee S and Davis KR: Ascorbic acid and a cytostatic inhibitor of
glycolysis synergistically induce apoptosis in non-small cell lung
cancer cells. PLoS One. 8:e670812013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Traber MG and Stevens JF: Vitamins C and
E: beneficial effects from a mechanistic perspective. Free Radic
Biol Med. 51:1000–1013. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harakeh S, Diab-Assaf M, Khalife JC, et
al: Ascorbic acid induces apoptosis in adult T-cell leukemia.
Anticancer Res. 27(1A): 289–298. 2007.PubMed/NCBI
|
|
24
|
Stephenson CM, Levin RD, Spector T and Lis
CG: Phase I clinical trial to evaluate the safety, tolerability and
pharmacokinetics of high-dose intravenous ascorbic acid in patients
with advanced cancer. Cancer Chemother Pharmacol. 72:139–146. 2013.
View Article : Google Scholar : PubMed/NCBI
|