Introduction
Colorectal cancer (CRC) is the third most common
type of cancer and the third leading cause of cancer-related
mortality in the United States (1). Incidence rates decreased by ~3% per
year; however, rates increased among adults younger than 50
years-old during the past decade (2001–2010). Therapeutic outcomes
of patients with CRC are often far from satisfactory due to
recurrence and metastasis, as the tumors are biologically and
molecularly heterogeneous. In recent years, molecular targeted
therapy has progressed. However, more reliable prognostic and
curative biomarkers identifying patients with increased risk of
disease recurrence and metastasis are required.
B7-H3, a newly identified co-stimulatory molecule,
is a member of the B7 family of proteins (2). B7-H3 is highly expressed in numerous
types of cancer, and has been demonstrated to promote tumor
progression and cancer cell metastasis, as well as to correlate
with the malignancy grade and the outcome of tumor patients,
including CRC (3,4), acute leukemia (5), glioma (6), hepatocellular carcinoma (7), lung cancer (8), breast cancer (9), prostate cancer (10), osteosarcoma (11), cutaneous melanoma (12) and pancreatic cancer (13). Studies have been conducted
regarding the effects of B7-H3 in CRC. Recently, Ingebrigtsen et
al (3,14) demonstrated using tissue microarray
analysis that B7-H3 expression was associated with
clinicopathological parameters and patient outcome in CRC, and
nuclear localization of B7-H3 strongly predicts poor outcome in
colon cancer. Bin et al (4)
also demonstrated that B7-H3 was aberrantly expressed in CD133(+)
CRC cells, and the expression level was closely associated with
tumor progression. All the data suggest that B7-H3 may be a useful
prognostic and therapeutic marker for CRC. However, the exact role
of B7-H3 in CRC remains ambiguous.
Matrix metalloproteases (MMPs) are important in
tumor growth, invasion and metastasis (15). Studies have shown that matrix
metallopeptidase 9 (MMP-9), one of the most important MMPs,
functioned in the migration, invasion and metastasis of numerous
types of cancer, including CRC, through various signaling pathways
(16–23). MMP-9, activated by astrocyte
elevated gene-1 (24),
ERK-dependent induced MMP-9 (25)
and the p38-MMP-9 pathway (26),
increases the invasiveness of CRC. In a recent study, B7-H3
promoted the expression of MMP-9 in a murine inflammatory model
(11). However, to date, no
studies have been conducted to investigate the correlation between
MMP-9 and B7-H3 expression in CRC.
The present study, aimed to investigate the role of
B7-H3 in cell migration and invasion in CRC, and the possible
signaling pathway involved.
Materials and methods
Cell lines and culture
Two human CRC cell lines, SW480 and HCT8 (American
Type Culture Collection, Manassas, VA, USA), had different
expression of B7-H3. SW480 cells were constructed with high
expression of B7-H3 (SW480-B7-H3-EGFP), and HCT8 cells stably
transfected with B7-H3 siRNA (HCT8-shB7-H3) in our laboratory. At
the same time, cells transfected with pIRES2-enhanced green
fluorescent protein (EGFP) were used as negative controls (SW480-NC
and HCT8-NC). Cells were maintained in RPMI-1640 medium (HyClone GE
Healthcare Life Sciences, South Logan, UT, USA) supplemented with
10% fetal bovine serum (FBS; Gibco BRL, Grand Island, NY, USA) at
37°C in a humidified atmosphere with 5% CO2. Cells were
harvested using 0.25% trypsin/EDTA (Invitrogen Life Technologies,
Carlsbad, CA, USA).
Antibodies and reagents
Anti-human Janus kinase 2 (Jak2; 2863-1),
phosphor-Jak2 (pY1007/1008; p-Jak2) (1477-1), signal transducer and
activator of transcription 3 (Stat3; 3566-1) and phospho-Stat3
(pY705; p-Stat3) (2236-1) antibodies were purchased from Epitomics
(Burlingame, CA, USA). Antibodies against B7-H3 (sc-376769) and
MMP-9 (sc-21733) were purchased from Santa Cruz Biotechnology, Inc
(Dallas, TX, USA). The horseradish peroxidase-conjugated secondary
anti-mouse and anti-rabbit IgG antibodies and antibodies against
GAPDH were obtained from Beyotime Biotechnology Inc. (Nantong,
China). A Cytoplasmic Protein Extraction kit and a BCA Protein
Assay kit were purchased from Beyotime Biotechnology Inc.
AG490, a Jak2 protein tyrosine kinase inhibitor, was
purchased from Sigma-Aldrich (St. Louis, MO, USA; T3434) and a
stock solution of AG490 (100 mmol/l) was prepared by re-suspension
in dimethly sulfoxide (Sigma-Aldrich).
Protein preparation and western blot
analysis
SW480-B7-H3-EGFP/SW480-NC or HCT8-shB7-H3/NC/HCT8-NC
cells (5×105) were cultured for 48 h in a 6-well plate.
Then the conditioned medium (CM) was collected by centrifugation at
15,294 × g for 15 min at 4°C, while cells were harvested and cell
lysates were prepared using RIPA lysis buffer (Beyotime
Biotechnology Inc.) containing phosphatase inhibitor, protease
inhibitor and 1 mmol/l PMSF (Beyotime Biotechnology Inc.) for 20
min on ice and stored at –80°C for later use. The protein content
in CM and the lysates was measured by a BCA Protein Assay kit. For
western blot analysis, equal quantities of total proteins were
resolved over 10% tris-glycine polyacrylamide gels (consisting of 4
ml water, 3.3 ml of 30% Acr-Bis (29:1), 2.5ml of 1.5 mol/l Tris (pH
8.8), 100 µl of 10% SDS, 100 µl 10% ammonium
persulfate and 5 µl TEMED for a total volume of 10 ml) under
non-reduced conditions, transferred onto polyvinylidene difluoride
membranes (Merck Millipore, Billerica, MA, USA), and subsequently
incubated in blocking buffer (5% non-fat dry milk in
phosphate-buffered saline) for 1 h at room temperature. The blots
were incubated with the appropriate primary antibody, washed with
TBST (Tris-buffered saline buffer with 0.2% Tween-20), and
incubated with a horseradish peroxidase (HRP)-conjugated secondary
antibody. The blots were detected with chemiluminescence (Beyo ECL
Plus, Beyotime Biotechnology Inc.) followed by autoradiography
(Bio-Rad ChemicDoc™ XRS+imaging system and Image Lab software
version 4.0.1; Bio-Rad, Hercules, CA, USA). Relative quantities of
protein were quantified by absorbance analysis. The level was
normalized to GAPDH, a domestic loading control.
SW480-B7-H3-EGFP and SW480-NC cells
(5×105) were treated with 100 µmol/l AG490 or
left untreated. After 48 h, CM was collected by centrifugation at
15,294 × g for 15 min at 4°C, while cells were harvested and cell
lysates were prepared for western blot analysis as described
above.
Zymography experiments
To investigate the effects of overexpression of
B7-H3 on MMP-9 activation, 5×105
SW480-B7-H3-EGFP/SW480-NC or HCT8-shB7-H3/HCT8-NC cells were plated
in a 6-well plate and cultured for 48 h. The CM was collected by
centrifugation at 15,294 × g for 15 min at 4°C. The samples
containing an equal quantity of total protein were mixed with
sample buffer in the absence of reducing agent and loaded onto
zymography SDS-polyacrylamide gels containing gelatin (0.5 mg/ml)
as described previously (27). The
gels were incubated in incubation buffer (50 mmol/l Tris-HCl; pH
7.5) containing 100 mmol/l CaCl2, 1 µmol/l
ZnCl2, 1% (v/v) Triton X-100, and 0.02% (w/v)
NaN3 for 16 h. The gels were stained with Coomassie Blue
and de-stained. Negative staining showed the zones of gelatinolytic
activity of MMP-9.
To further determine the effect of AG490 on MMP-9
activation, 5×105 SW480-B7-H3-EGFP or SW480-NC cells
were plated in a 6-well plate and treated with 100 µmol/l
AG490 for 48 h or left untreated. The CM was collected, and the
gelatinolytic activity of MMP-9 was detected by zymography as
described above.
Cell migration and invasion assay
The migration and invasion assay was performed using
Transwell cell culture chambers (Corning, Corning, NY, USA) as
described previously (28).
For the migration assay, the confluent monolayers of
SW480-B7-H3-EGFP and SW480-NC cells were harvested with
trypsin-EDTA and centrifuged at 800 × g for 10 min. Cell
resuspension (200 µl, 2×105 cells/ml) in
RPMI-1640 was added to the upper chamber of the prehydrated
polycarbonate membrane filter. The lower chamber was filled with
RPMI-1640 medium with 10% FBS, which acted as a chemoattractant.
Then cells were incubated in a humidified incubator in 5%
CO2 at 37°C for 24 h. The non-migrated cells on the
upper side of the filter were scraped, and the filter was washed
with PBS and deionised water, and then air-dried. The migrated
cells on the reverse side of the filter were fixed with methanol
and stained with Giemsa. Images were captured using an inverted
microscope (Olympus IX71, Tokyo, Japan).
For the invasion assay, the prehydrated
polycarbonate membrane filter of the Transwell cell culture
chambers was pre-coated with BD Matrigel™ Basement Membrane Matrix
(356234, BD BioSciences, Franklin Lakes, NJ, USA). A 200 µl
cell re-suspension of SW480-B7-H3-EGFP and SW480-NC
(2×105 cells/ml) in RPMI-1640 was added to the upper
chamber of the Boyden chamber. RPMI-1640 medium with 10% FBS was
used in the lower chamber, which acted as a chemoattractant. After
48 h, the non-invaded cells and Matrigel from the upper side of the
filter were scraped and removed using a moist cotton swab. The
invaded cells on the lower side of the filter were fixed with
methanol and stained with Giemsa. Images were captured using an
inverted microscope (Olympus IX71).
Wound healing assay
One day prior to the wound healing assay,
SW480-B7-H3-EGFP or SW480-NC cells were plated in a 6-well plate so
that cells were 90–95% confluent at the time of the assay. Wounds
with a constant diameter were made. Cells were maintained in
RPMI-1640 with 0.5% FBS. Images of the wound area were captured
using an inverted microscope (Olympus IX71) everyday for 5–6
days.
Statistical analysis
Statistical differences were determined by Student's
t-test using GraphPad Prism 5 software (GraphPad Software Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference. All experiments were
conducted at least three times.
Results
Overexpression of B7-H3 promotes cell
migration and invasion
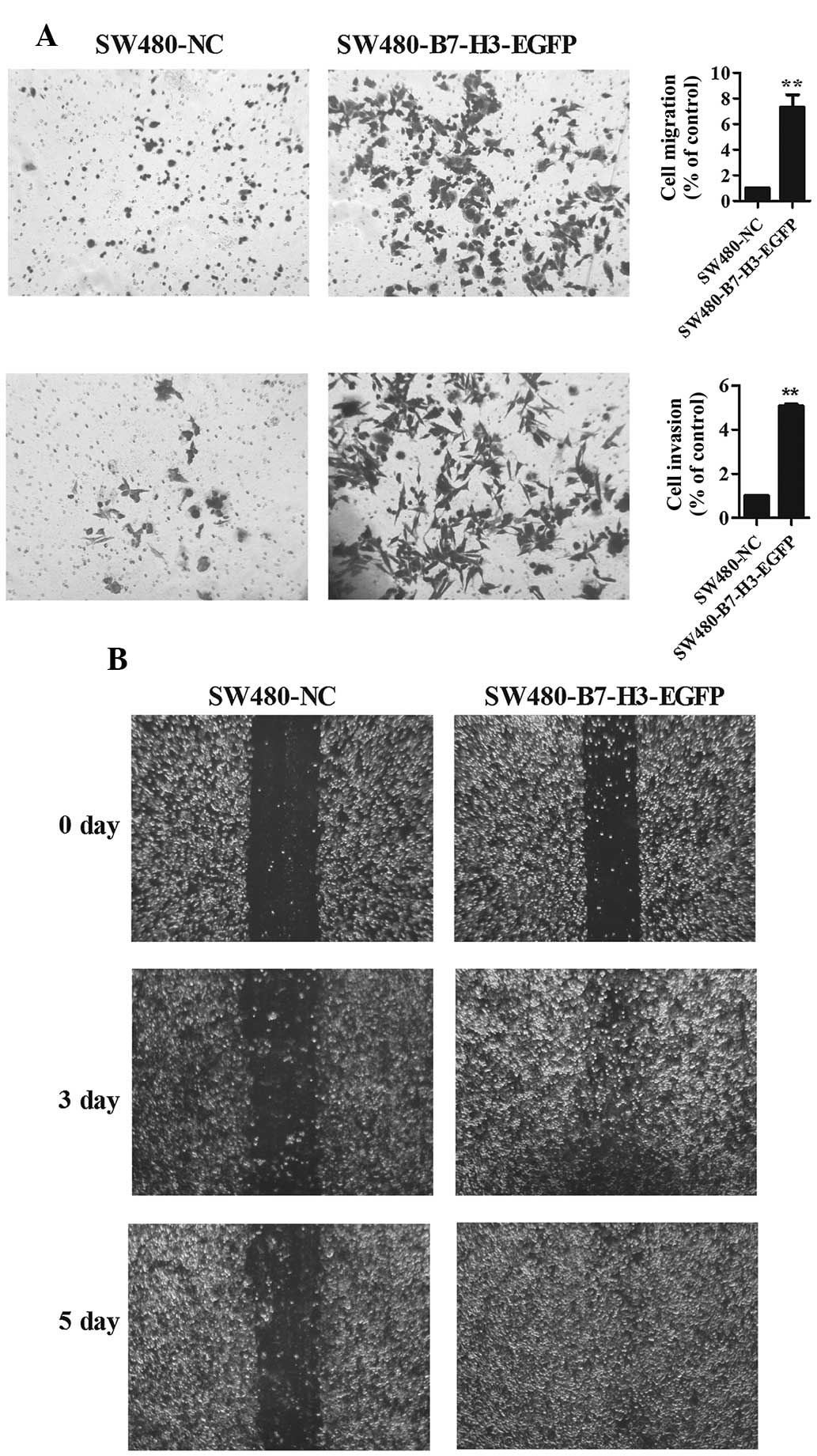
To delineate the role of B7-H3 on cell migration and
invasion in CRC cells, a wound healing assay, and a cell migration
and invasion assay were performed (Fig. 1). Transwell experiments (without
Matrigel on the filter) and wound assay results indicated that
enhanced expression of B7-H3 promoted cell migration, 7.4±1.5 fold
SW480-B7-H3-EGFP vs. SW480-NC (Fig.
1A upper panel, Fig. 1B). In
addition, enhanced expression of B7-H3 also promoted cell invasion
(with Matrigel on the filter), 5.07±0.2 fold SW480-B7-H3-EGFP vs.
SW480-NC (Fig. 1A lower
panel).
Overexpression of B7-H3 upregulates
MMP-9
To determine the mechanism underlying the effect of
enhanced expression of B7-H3 on the promotion of cell migration and
invasion, the present study aimed to delineate the role of MMP
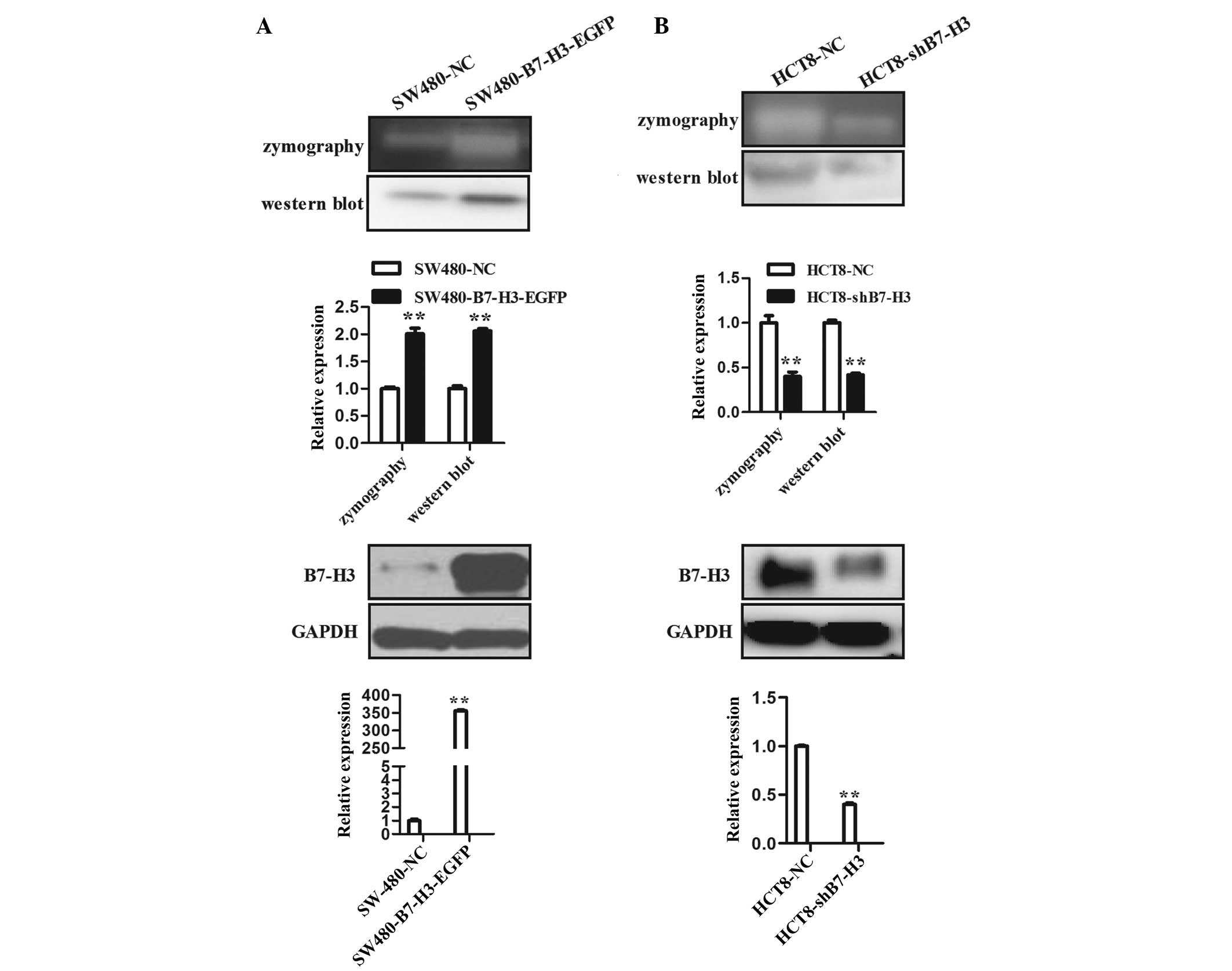
family member, MMP-9. Zymography experimental data revealed that
over-expression of B7-H3 in SW480-B7-H3-EGFP cells (Fig. 2A lower panel) significantly
elevated the proteolytic activity of MMP-9 when compared with
control cells, SW480-NC (Fig. 2A
upper panel). Moreover, western blot analysis demonstrated that
enhanced expression of B7-H3 also promoted the expression of MMP-9
protein (Fig. 2A upper panel).
B7-H3 was downregulated in HCT8-shB7-H3 cells. The proteolytic
effect of MMP-9 in CM of HCT8-shB7-H3 cells was significantly
reduced compared with that in the CM of control cells, HCT8-NC. The
expression level of MMP-9 protein in the CM of HCT8-shB7-H3 cells
was also reduced (Fig. 2B). These
results demonstrate that enhanced expression of B7-H3 promoted cell
migration and invasion, at least partially through upregulation of
MMP-9.
Overexpression of B7-H3 enhances cell
migration and invasion in CRC cells via activation of the
Jak2-Stat3 pathway
The phenomenon that B7-H3 enhanced cell migration
and invasion through upregulation of MMP-9 was observed, and the
present study aimed to identify the signaling pathway involved. The
Jak2/Stat3 pathway has been reported to be key in cell migration,
invasion and metastasis, and inhibition of Jak2/Stat3 signaling
induced CRC cell apoptosis, cell arrest and reduced tumor cell
invasion (29–31). Thus, it was analyzed whether this
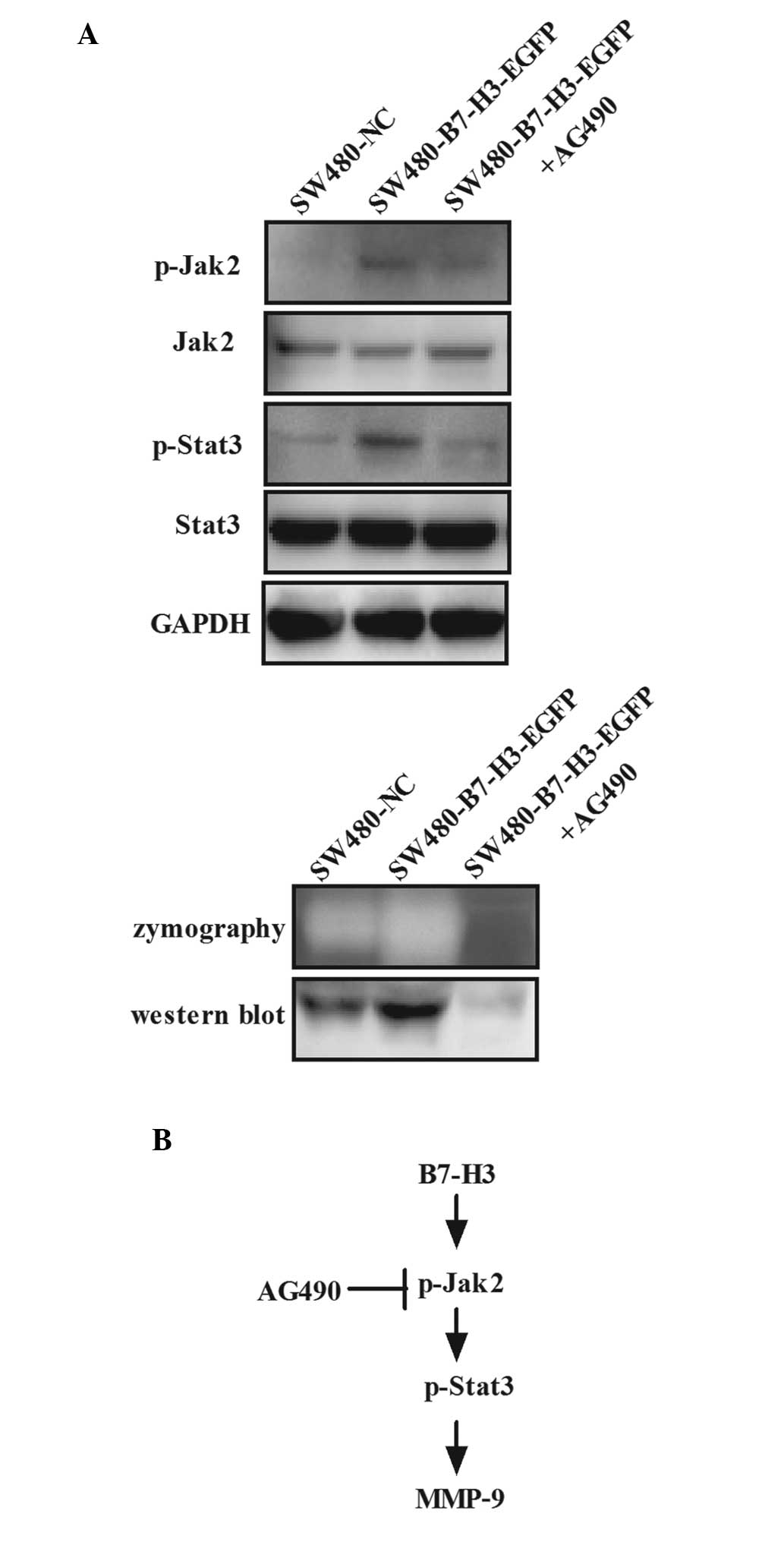
pathway could be activated by B7-H3 in CRC. SW480-B7-H3-EGFP cells
were treated with AG490, a Jak2-selective inhibitor, at a final
concentration of 100 µmol/l for 48 h. CM was collected and
used for detection of MMP-9 by western blot analysis and
zymography, and whole-cell lysates were used for detection of Jak2,
Stat3 and their phosphorylated forms by western blot analysis
(Fig. 3A). Data showed that the
phosphorylation levels of Jak2 and Stat3 increased following
upregulation of B7-H3 expression; however, both were significantly
reduced after AG490 treatment due to the inhibition of tyrosine
phosphorylation of Jak2 (Fig. 3A
upper panel). In addition, the proteolytic activity and protein
expression of MMP-9 increased following upregulated expression of
B7-H3, but significantly reduced after AG490 treatment, as
determined by zymography and western blot analysis, paralleled with
the regulation of the phosphorylation level of Jak2 and Stat3
(Fig. 3A lower panel). These
results indicate that MMP-9 is a downstream target of B7-H3 and the
upregulation of MMP-9 induced by B7-H3 can be blocked by AG490 (a
Jak2/Stat3 signaling pathway specific inhibitor). Thus, it was
hypothesized that overexpression of B7-H3 increased the
phosphorylation of Jak2, which led to increased phosphorylation of
Stat3, resulting in increased expression of MMP-9 (Fig. 3B). Thus, it was confirmed that the
Jak2/Stat3/MMP-9 signaling pathway was important in regulating the
cell migration and invasion induced by B7-H3 in CRC.
Discussion
B7 family members are regarded as
co-stimulatory/co-inhibitory immune molecules that integrate T cell
receptor signaling to regulate T cell function. In this study, it
was demonstrated that B7-H3 exhibits a non-immune role in CRC,
promoting MMP-9 expression in CRC cells. The elevated MMP-9
expression level in CRC cell supernatants can partly explain the
phenomena that the expression level of B7-H3 in CRC tissue is
positively correlated with T stage of patients (3,4) and
negatively correlated with overall survival of CRC (14). Furthermore, it has previously been
shown that B7-H3 regulates the expression of Bcl-2, Bcl-xl and Bax
via the Jak2/Stat3 signaling pathway in order to increase the
anti-apoptotic ability of cancer cells (32). In the present study it was
demonstrated that B7-H3 promotes cell migration and invasion
through the Jak2/Stat3/MMP9 signaling pathway.
Ingebrigtsen et al (3,14)
and Bin et al (4) showed that high
B7-H3 expression predicted poor outcome in patients with colon
cancer, and the high expression level in CD133(+) CRC cells was
associated with tumor progression as determined by tissue
microarray analysis. However, the molecular regulatory mechanisms
have not yet been investigated. To the best of our knowledge, the
present study demonstrated that B7-H3 promoted cancer cell
migration and invasion via Jak2/Stat3/MMP-9 signaling pathway for
the first time.
MMPs degrade all types of extracellular matrix
proteins, and are regarded as a marker of malignant tumor invasion
and metastasis (33–35). MMP-2 and MMP-9 are the most
important molecules in the MMP family. B7-H3 and MMP-2 were shown
to correlate with infiltration depth in pancreatic cancer (4), and knock-down of B7-H3 led to reduced
expression of MMP-2 (36). In
addition, B7-H3 was shown to increase the expression of MMP-9 in
murine models of inflammation (11). The correlation between B7-H3 and
MMP-9 requires further investigation in malignant tumors. In the
present study, it was shown that overexpression of B7-H3 elevated
the MMP-9 expression level in CRC. However, whether B7-H3 can
affect the levels of other MMPs, such as MMP-2, remains to be
investigated.
Abnormalities in the Jak2/Stat3 pathway are involved
in the pathogenesis of CRC. Inhibition of Jak2/Stat3 signaling
induces CRC cell apoptosis, cell arrest and reduces tumor cell
invasion (29–31). MMPs, including MMP-2, -9 and -10,
have been reported to be downstream of this pathway (37–39).
Another study showed that blocking B7-H3 resulted in inhibition of
activated Jak2/Stat3 in breast cancer cells (40). It was also previously demonstrated
that B7-H3 possesses an anti-apoptotic function through the
Jak2-Stat3 signaling pathway (32). In the present study, B7-H3
activated the Jak2/Stat3 signaling pathway, and the high
phosphorylation level of Jak2 and Stat3, led to the upregulation of
MMP-9. Furthermore, AG490, a specific inhibitor of Jak2, was used
to inhibit Jak2/Stat3 signaling. It was demonstrated that AG490
could significantly reduce the phosphorylation level of Jak2 and
Stat3, and downregulate the expression of MMP-9. These results
indicate that MMP-9 is a downstream target of the B7-H3/Jak2/Stat3
signaling pathway, and B7-H3 promotes CRC invasion through the
Jak2/Stat3/MMP-9 pathway. Due to the complex signaling pathways
present in cancer cells, successful targeted therapies are becoming
more difficult to identify. Each cellular process is controlled by
various signaling networks. Thus, B7-H3 may also promote CRC cell
migration and invasion through other signal networks, which
required to be determined.
Based on above results, we plan to analyze other MMP
family members and tissue inhibitor of metalloproteinase family
members in cells with B7-H3 overexpression and knock down in the
subsequence studies, and to identify possible proteins that mediate
B7-H3 signaling resulting in Jak2 phosphorylation.
In conclusion, it was demonstrated that high B7-H3
expression could promote cancer cell migration and invasion in CRC.
This is the first report to show the molecular mechanisms
underlying this effect. Overexpressed B7-H3 elevated MMP-9
resulting in pro-migratory and pro-invasive abilities through the
Jak2-Stat3 pathway. These findings indicate a novel role for B7-H3
in the regulation of the invasive capacity of CRC cells and it may
be a potential therapeutic target to prevent metastasis.
Acknowledgments
This study has been supported in part by the grants
from the Jiangsu Provincial Health Bureau of Medical (grant no.
RC2011030), the Wuxi Hospital Management Center (grant no. YCZ1108)
and the Natural Science Foundation of Jiangsu Province (grant no.
BK2012542).
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun M, Richards S, Prasad DV, Mai XM,
Rudensky A and Dong C: Characterization of mouse and human B7-H3
genes. J Immunol. 168:6294–6297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ingebrigtsen VA, Boye K, Nesland JM,
Nesbakken A, Flatmark K and Fodstad Ø: B7-H3 expression in
colorectal cancer: Associations with clinicopathological parameters
and patient outcome. BMC Cancer. 14:6022014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bin Z, Guangbo Z, Yan G, Huan Z, Desheng L
and Xueguang Z: Overexpression of B7-H3 in CD133+
colorectal cancer cells is associated with cancer progression and
survival in human patients. J Surg Res. 188:396–403. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu Y, Lv X, Wu Y, Xu J, Wang L, Chen W,
Zhang W, Li J, Zhang S and Qiu H: Expression of costimulatory
molecule B7-H3 and its prognostic implications in human acute
leukemia. Hematology. 20:187–195. 2015. View Article : Google Scholar
|
|
6
|
Baral A, Ye HX, Jiang PC, Yao Y and Mao Y:
B7-H3 and B7-H1 expression in cerebral spinal fluid and tumor
tissue correlates with the malignancy grade of glioma patients.
Oncol Lett. 8:1195–1201. 2014.PubMed/NCBI
|
|
7
|
Wang F, Wang G, Liu T, Yu G, Zhang G and
Luan X: B7-H3 was highly expressed in human primary hepatocellular
carcinoma and promoted tumor progression. Cancer Invest.
32:262–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun J, Mao Y, Zhang YQ, Guo YD, Mu CY, Fu
FQ and Zhang XG: Clinical significance of the induction of
macrophage differentiation by the costimulatory molecule B7-H3 in
human non-small cell lung cancer. Oncol Lett. 6:1253–1260.
2013.PubMed/NCBI
|
|
9
|
Maeda N, Yoshimura K, Yamamoto S, Kuramasu
A, Inoue M, Suzuki N, Watanabe Y, Maeda Y, Kamei R, Tsunedomi R, et
al: Expression of B7-H3, a potential factor of tumor immune evasion
in combination with the number of regulatory T cells, affects
against recurrence-free survival in breast cancer patients. Ann
Surg Oncol. 21(Suppl 4): S546–S554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roth TJ, Sheinin Y, Lohse CM, Kuntz SM,
Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML,
et al: B7-H3 ligand expression by prostate cancer: A novel marker
of prognosis and potential target for therapy. Cancer Res.
67:7893–7900. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Bai Y, Cui W, Wang Z, Zhang G, Xu
Y, Zhu X, Li Y and Wang JH: Effects of B7-H3 on the inflammatory
response and expression of MMP-9 in mice with pneumococcal
meningitis. J Mol Neurosci. 50:146–153. 2013. View Article : Google Scholar
|
|
12
|
Wang J, Chong KK, Nakamura Y, Nguyen L,
Huang SK, Kuo C, Zhang W, Yu H, Morton DL and Hoon DS: Hoon = B7-H3
associated with tumor progression and epigenetic regulatory
activity in cutaneous melanoma. J Invest Dermatol. 133:2050–2058.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao X, Li DC, Zhu XG, Gan WJ, Li Z, Xiong
F, Zhang ZX, Zhang GB, Zhang XG and Zhao H: B7-H3 overexpression in
pancreatic cancer promotes tumor progression. Int J Mol Med.
31:283–291. 2013.
|
|
14
|
Ingebrigtsen VA, Boye K, Tekle C, Nesland
JM, Flatmark K and Fodstad O: B7-H3 expression in colorectal
cancer: Nuclear localization strongly predicts poor outcome in
colon cancer. Int J Cancer. 131:2528–2536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Artacho-Cordón F, Ríos-Arrabal S, Lara PC,
Artacho-Cordón A, Calvente I and Nńñez MI: Matrix
metalloproteinases: Potential therapy to prevent the development of
second malignancies after breast radiotherapy. Surg Oncol.
21:e143–e151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu S, Li L, Yeh S, Cui Y, Li X, Chang HC,
Jin J and Chang C: Infiltrating T cells promote prostate cancer
metastasis via modulation of FGF11 >miRNA-541> androgen
receptor (AR) >MMP9 signaling. Mol Oncol. 9:44–57. 2015.
View Article : Google Scholar
|
|
17
|
Jian H, Zhao Y, Liu B and Lu S: SEMA4b
inhibits MMP9 to prevent metastasis of non-small cell lung cancer.
Tumour Biol. 35:11051–11056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jang SY, Kim A, Kim JK, Kim C, Cho YH, Kim
JH, Kim CH and Lee JY: Metformin inhibits tumor cell migration via
down-regulation of MMP9 in tamoxifen-resistant breast cancer cells.
Anticancer Res. 34:4127–4134. 2014.PubMed/NCBI
|
|
19
|
Zhou DN, Deng YF, Li RH, Yin P and Ye CS:
Concurrent alterations of RAGE, RECK and MMP9 protein expression
are relevant to Epstein-Barr virus infection, metastasis and
survival in nasopharyngeal carcinoma. Int J Clin Exp Pathol.
7:3245–3254. 2014.
|
|
20
|
Huang Q, Lan F, Wang X, Yu Y, Ouyang X,
Zheng F, Han J, Lin Y, Xie Y, Xie F, et al: IL-1β-induced
activation of p38 promotes metastasis in gastric adenocarcinoma via
upregulation of AP-1/c-fos, MMP2 and MMP9. Mol Cancer. 13:182014.
View Article : Google Scholar
|
|
21
|
Li L, Tan J, Zhang Y, Han N, Di X, Xiao T,
Cheng S, Gao Y and Liu Y: DLK1 promotes lung cancer cell invasion
through upregulation of MMP9 expression depending on Notch
signaling. PLoS One. 9:e915092014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han S, Han L, Yao Y, Sun H, Zan X and Liu
Q: Activated hepatic stellate cells promote hepatocellular
carcinoma cell migration and invasion via the activation of
FAK-MMP9 signaling. Oncol Rep. 31:641–648. 2014.
|
|
23
|
Feng X, Miao G, Han Y and Xu Y: CARMA3 is
overexpressed in human glioma and promotes cell invasion through
MMP9 regulation in A172 cell line. Tumour Biol. 35:149–154. 2014.
View Article : Google Scholar
|
|
24
|
Song H, Tian Z, Qin Y, Yao G, Fu S and
Geng J: Astrocyte elevated gene-1 activates MMP9 to increase
invasiveness of colorectal cancer. Tumour Biol. 35:6679–6685. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim HC, Kim YS, Oh HW, Kim K, Oh SS, Kim
JT, Kim BY, Lee SJ, Choe YK, Kim DH, et al: Collagen triple helix
repeat containing 1 (CTHRC1) acts via ERK-dependent induction of
MMP9 to promote invasion of colorectal cancer cells. Oncotarget.
5:519–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei SC, Tsao PN, Weng MT, Cao Z and Wong
JM: Flt-1 in colorectal cancer cells is required for the tumor
invasive effect of placental growth factor through a p38-MMP9
pathway. J Biomed Sci. 20:392013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kale S, Raja R, Thorat D, Soundararajan G,
Patil TV and Kundu GC: Osteopontin signaling upregulates
cyclooxy-genase-2 expression in tumor-associated macrophages
leading to enhanced angiogenesis and melanoma growth via α9β1
integrin. Oncogene. 33:2295–2306. 2014. View Article : Google Scholar
|
|
28
|
Rangaswami H, Bulbule A and Kundu GC:
Nuclear factor-inducing kinase plays a crucial role in
osteopontin-induced MAPK/IkappaBalpha kinase-dependent nuclear
factor kappaB-mediated promatrix metalloproteinase-9 activation. J
Biol Chem. 279:38921–38935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du W, Hong J, Wang YC, Zhang YJ, Wang P,
Su WY, Lin YW, Lu R, Zou WP, Xiong H and Fang JY: Inhibition of
JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via
mitochondrial pathway. J Cell Mol Med. 16:1878–1888. 2012.
View Article : Google Scholar
|
|
30
|
Xiong H, Chen ZF, Liang QC, Du W, Chen HM,
Su WY, Chen GQ, Han ZG and Fang JY: Inhibition of DNA
methyl-transferase induces G2 cell cycle arrest and apoptosis in
human colorectal cancer cells via inhibition of JAK2/STAT3/STAT5
signalling. J Cell Mol Med. 13:3668–3679. 2009. View Article : Google Scholar
|
|
31
|
Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang
QC, Zhang YJ, Lu R, Chen YX and Fang JY: Inhibition of JAK1,
2/STAT3 signaling induces apoptosis, cell cycle arrest and reduces
tumor cell invasion in colorectal cancer cells. Neoplasia.
10:287–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang T, Jiang B, Zou ST, Liu F and Hua D:
Overexpression of B7-H3 augments anti-apoptosis of colorectal
cancer cells by Jak2-STAT3. World J Gastroenterol. 21:1804–1813.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Curran S and Murray GI: Matrix
metalloproteinases: Molecular aspects of their roles in tumour
invasion and metastasis. Eur J Cancer. 36:1621–1630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tekle C, Nygren MK, Chen YW, Dybsjord I,
Nesland JM, Maelandsmo GM and Fodstad O: B7-H3 contributes to the
metastatic capacity of melanoma cells by modulation of known
metastasis-associated genes. Int J Cancer. 130:2282–2290. 2012.
View Article : Google Scholar
|
|
37
|
Zhang X, Yin P, Di D, Luo G, Zheng L, Wei
J, Zhang J, Shi Y, Zhang J and Xu N: IL-6 regulates MMP-10
expression via JAK2/STAT3 signaling pathway in a human lung
adenocar-cinoma cell line. Anticancer Res. 29:4497–4501.
2009.PubMed/NCBI
|
|
38
|
Senft C, Priester M, Polacin M, Schröder
K, Seifert V, Kögel D and Weissenberger J: Inhibition of the
JAK-2/STAT3 signaling pathway impedes the migratory and invasive
potential of human glioblastoma cells. J Neurooncol. 101:393–403.
2011. View Article : Google Scholar
|
|
39
|
Reis ST, Pontes-Junior J, Antunes AA,
Dall'Oglio MF, Dip N, Passerotti CC, Rossini GA, Morais DR,
Nesrallah AJ, Piantino C, et al: miR-21 may acts as an oncomir by
targeting RECK, a matrix metalloproteinase regulator, in prostate
cancer. BMC Urol. 12:142012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu H, Tekle C, Chen YW, Kristian A, Zhao
Y, Zhou M, Liu Z, Ding Y, Wang B, Mælandsmo GM, et al: B7-H3
silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3
phosphorylation. Mol Cancer Ther. 10:960–971. 2011. View Article : Google Scholar : PubMed/NCBI
|