Introduction
MicroRNAs (miRNAs) are endogenously encoded small
noncoding RNAs, which are 21–24 nucleotides long and regulate
posttranscriptional mRNA expression (1). miRNA has been widely observed in
various organisms. One-third of human genes can be regulated by the
miRNAs (2). miRNAs are important
in translational inhibition or destabilization of the target mRNA.
It is reported that >50% of miRNA genes are located in
cancer-related genomic regions or fragile sites, suggesting that
miRNAs may be important in the progression and development of
different types of tumors (3,4).
miR-125a is a member of the miRNA family, it has a
precursor form and two mature forms (miR-125a-3p and miR-125a-5p)
(5). The mature forms have
biological activity, and the mature microRNA hsa-miR-125a-3p is
derived from the 3′ end of pre-miR-125a. Jiang et al
(6) demonstrated that
hsa-miR-125a-3p and hsa-miR-125a-5p were downregulated in non-small
cell lung cancer and have inverse effects on the invasion and
migration of lung cancer cells. Huang et al (7) reported that miRNA-125a-3p is a
negative regulator of the Ras homolog gene family (Rho)A-actomyosin
pathway in A549 cells, and loss of miR-125a-3p leads to increased
migration of tumor cells due to the upregulation of RhoA
expression.
Lung cancer is a common disease with highest
incidence and mortality (8–10).
Small-cell lung carcinoma is one of the main types of lung cancer
(11,12). The 5-year survival rate for lung
cancer is only 10% (13,14). The tumour suppressor p53 is known
to prevent cancer progression by inhibiting proliferation and
inducing apoptosis of tumor cells (15,16).
Mouse double minute 2 homolog (MDM2), also termed E3
ubiquitin-protein ligase (17,18),
is an important negative regulator of the p53 tumor suppressor.
Mdm2 protein recognizes the N-terminal trans-activation domain of
the p53 tumor suppressor and inhibits p53 transcriptional activity
(19,20). The p53-MDM2 signaling pathway is
known to control cancer invasion and metastasis (21). In the present study, the expression
of miR-125a-3p was detected in three lung cancer cell lines, and
the effects of miR-125a-3p on the proliferation and migration of
lung cancer cells were determined.
Materials and methods
Cells lines
A549, NCI-H460 and SPCA-1 human lung cancer cell
lines obtained from the American Type Culture Collection (Manassas,
VA, USA) were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) (GE Healthcare Life
Sciences, Logan, UT, USA).
MTT assay
The A549 lung cancer cell line was plated onto
48-well plates. Following adherence of the cells for 8 h, sense
hsa-miR-125a-3p and anti-sense hsa-miR-125a-3p was transfected into
A549 cells. The cells were cultured for 1, 3, 5 and 7 days,
respectively. Then, cell proliferation was evaluated by an MTT
assay (Sigma-Aldrich, St. Louis, MO, USA). Briefly, 10 µl of
5 mg/ml MTT was added into the medium. After 4 h, 150 µl
dimethylsulfoxide (Sigma-Aldrich) was added for 15 min. The plates
were then read on a microplate reader (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at a test wavelength of 490 nm and a
reference wavelength of 570 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from A549, NCI-H460 and
SPCA-1 lung cancer cells and HBE normal cells by TRIzol reagent
(Takara Bio Inc., Dalian, China), and cDNA was transcribed using
the PrimeScript® RT reagent kit (Takara Bio Inc.)
according to the manufacturer's instructions. RT-qPCR was performed
to evaluate hsa-miR-125a-3p expression on ABI 7500 system. The
following primer sequences were used: Sense, 5′-ACA GGU GAG GUU CUU
GGG AGCC-3′ and antisense, 5′-GGC UCC CAA GAA CCU CAC CUGU-3′ for
hsa-miR-125a-3p; and sense, 5′-CTC GCT TCG GCA GCACA-3′ and
antisense, 5′-AAC GCT TCA CGA ATT TGCGT-3′ for small nuclear RNA
U6RNA, which was used as a reference gene. The primers were
synthesized by Quanshijin Corporation (Beijing, China). PCR thermal
cycling conditions were as follows: 40 cycles of 12 sec at 95°C and
1 min at 60°C using SYBR Green (Takara Bio Inc.).
Western blot analysis
The A549 and NCI-H460 lung cancer cell lysates were
prepared and separated by SDS-PAGE (22–24).
After transferring the protein from the gel to the membrane, the
monoclonal mouse anti-human p53 (DO-2; sc-53394), MDM-2 (SMP14;
sc-965) and β-actin (C-2; sc-8432) primary antibodies were used for
incubation. The cells were then incubated with a horseradish
peroxidase-conjugated goat anti-mouse secondary antibody. All of
the antibodies were obtained from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The western blots were visualized usin
Immobilon Western Chemiluminescent HRP Substrate (ECL) (EMD
Millipore, Billerica, MA, USA).
Flow cytometric analysis
The cell apoptosis rate was determined by flow
cytometric analysis with annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) staining (25,26).
Briefly, 1×105 lung cancer cells were plated into 6-well
plates. After 6 h, the cells were transfected with sense
hsa-miR-125a-3p, anti-sense miR-125a-3p and scrambled miRNA,
respectively, which were designed and synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China). After five days, cell
apoptosis was detected by annexin V-FITC/PI (Abcam, Cambridge, MA,
USA) dual staining analysis according to the protocols. Finally,
the cells were detected and analyzed by flow cytometry on a
FACSCalibur Cell Sorting system (BD Biosciences, Franklin Lakes,
NJ, USA).
Transwell assay
A549 cells were cultured in DMEM with 10% FBS and
Transwell compartments (Corning Incorporated, New York, NY, USA)
were prepared with 24-well format with an 8-µm pore size.
The assay was conducted using methods described previously
(27,28). Briefly, the lower compartment was
filled with 2.6 ml DMEM with 0.5% FBS containing 40 µg/ml
collagen I (Sigma-Aldrich). The Transwell insert was then added to
the well by merging the bottom of the insert into the medium in the
lower compartment. A549 cells (1×105) were then added to
the upper compartment. The cells were incubated in the Transwell
plate at 37°C and 5% CO2 for 8 h. Then, the insert was
carefully removed. A549 cells on the lower side of the insert
filter were quickly fixed in 5% glutaraldehyde for 10 min, and
stained with 1% crystal violet in 2% ethanol for 20 min. Finally,
excess crystal violet was removed by quickly merging the insert in
ddH2O for a few seconds and the number of cells on the
lower side of the filter was counted under a microscope (CX22;
Olympus, Tokyo, Japan).
Statistical analysis
Data were analyzed with Student's t-test using SPSS
software, version 18.0 (SPSS, Inc., Chicago, IL, USA). The data are
presented as the mean ± standard error of the mean. P<0.01 was
considered to indicate a statistically significant difference.
Results
Relative mRNA levels of hsa-miR-125a-3p
are detected in A549, NCI-H460 and SPCA-1 lung cancer cell
lines
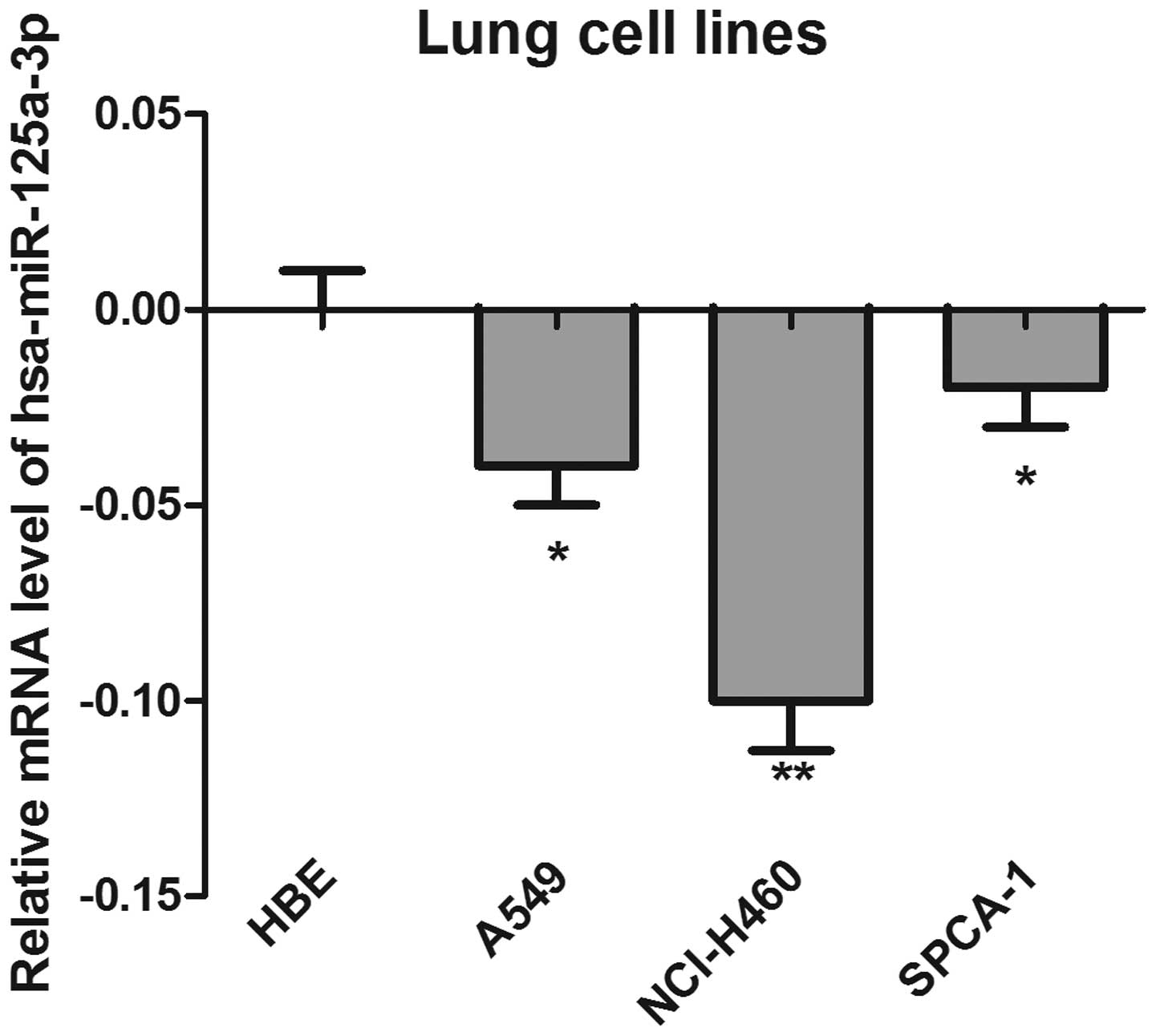
The expression of has-miR-125a-3p was detected by
RT-qPCR in a panel of three colorectal cancer cell lines. The HBE
cell line was used as a control. As shown in Fig. 1, the results demonstrated that the
mRNA levels of has-miR-125a-3p in the cancer cell lines were
significantly lower than those in the HBE cells, suggesting that
has-125a-3p may be a tumor suppressor gene. Notably, the expression
of has-miR-125a-3p in NCI-H460 cells was lowest, and most
significantly different compared with the control cells
(P<0.01).
Overexpression of sense hsa-miR-125a-3p
inhibits the proliferation of A549 cells
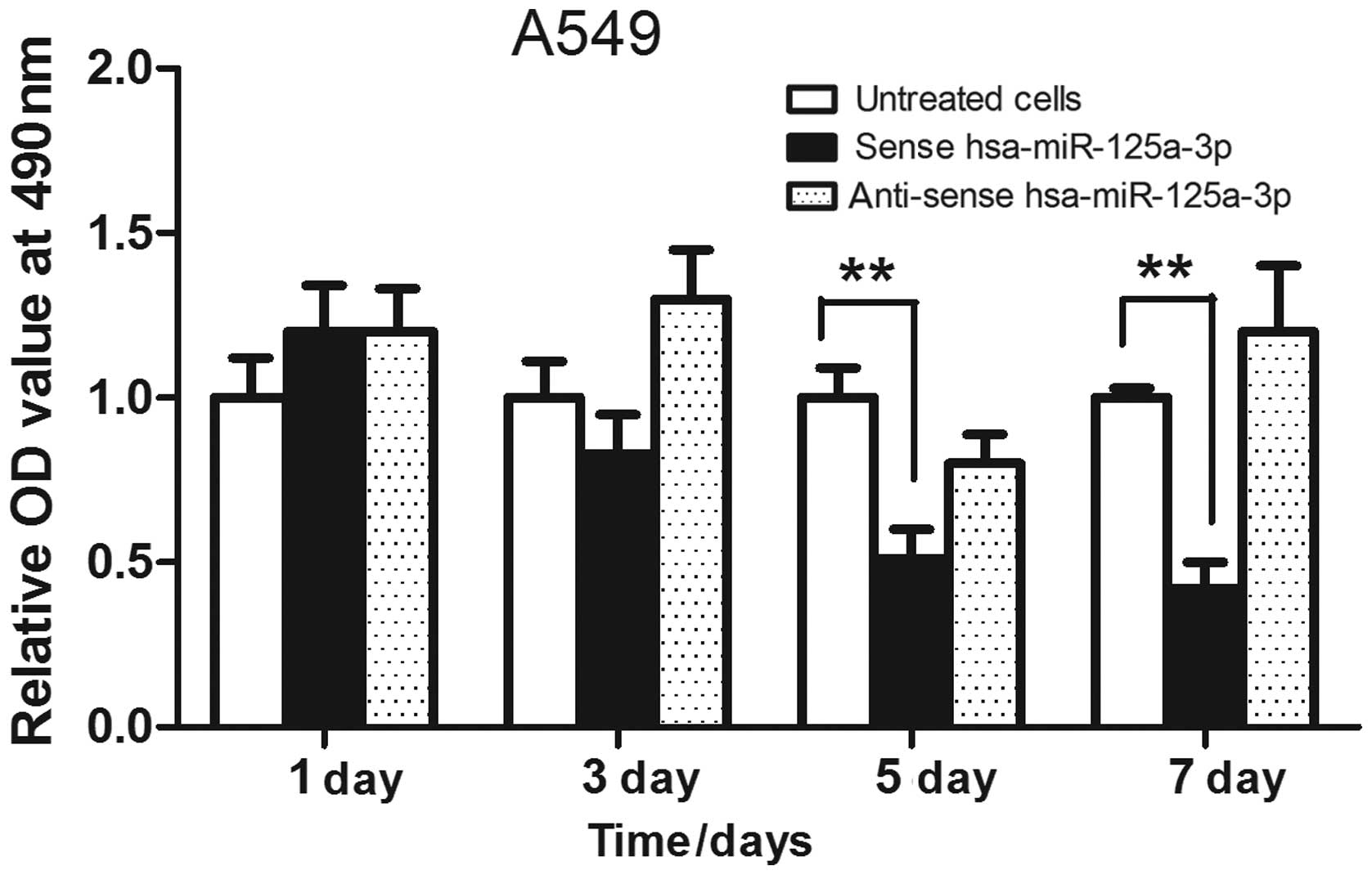
Hsa-miR-125a-3p was overexpressed in A549 lung
cancer cells, and an MTT assay was used to analyze the cell
proliferation. As shown in Fig. 2,
the lung cancer cells were transfected with sense hsa-miR-125a-3p
or anti-sense has-miR-125a-3p for 1, 3, 5 or 7 days, respectively,
and the results showed that transfection of sense hsa-miR-125a-3p
inhibited the growth of A549 cells. In addition, overexpression of
anti-sense hsa-miR-125a-3p did not suppress the proliferation of
lung cancer cells.
Apoptosis rate increases in NCI-H460 lung
cancer cells transfected with sense has-miR-125a-3p
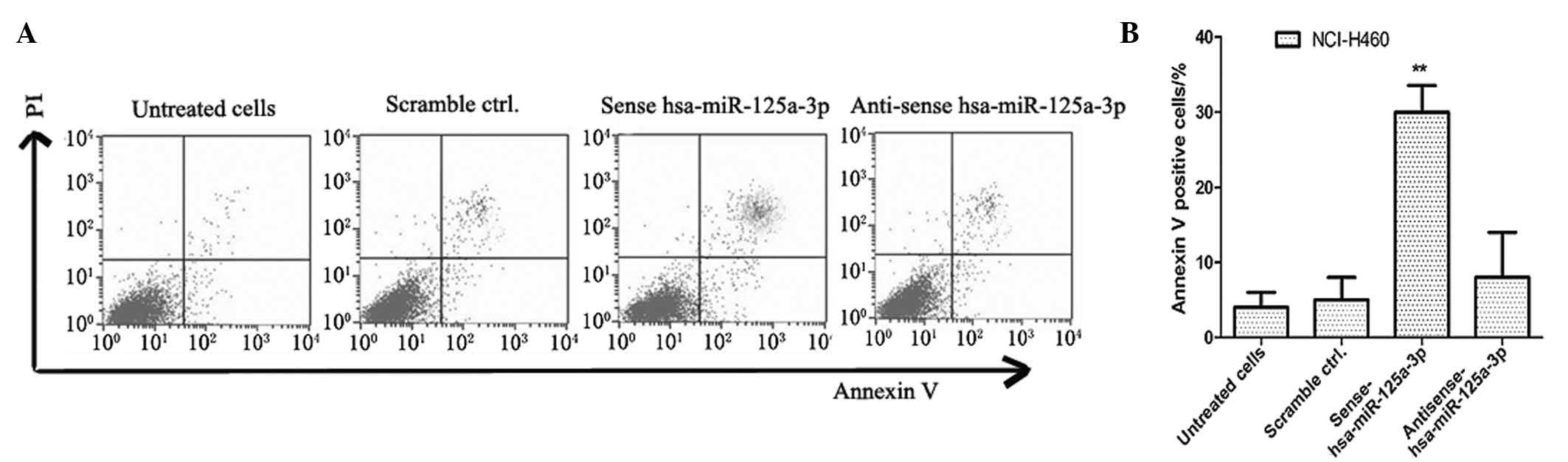
In order to detect the effects of has-miR-125a-3p on
cell apoptosis in lung cancer cells, cell apoptosis rates were
detected by annexin V-FITC and PI dual staining analysis. The
NCI-H460 lung cancer cell line was transfected with sense
has-miR-125a-3p and anti-sense has-miR-125a-3p, and cultured for 5
days. The lung cancer cells transfected with scrambled miRNA were
used as negative controls. As shown in Fig. 3, the apoptosis rate of sense
has-miR-125a-3p transfected NCI-H460 cells was significantly higher
than that of the controls (P<0.01).
Transfection of sense hsa-miR-125a-3p
inhibits the cell migration and invasion of A549 lung cancer
cells
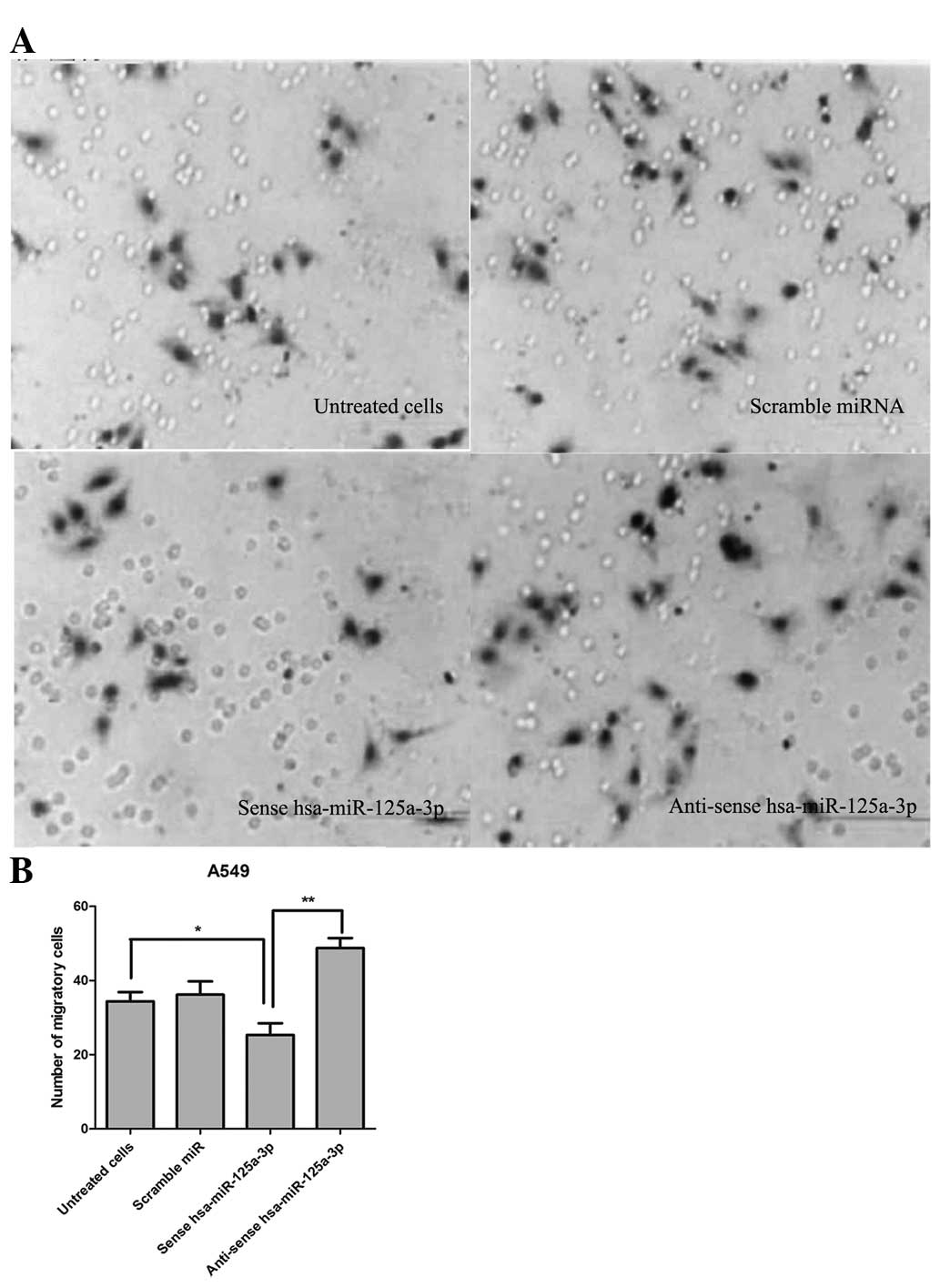
In order to detect the effect of hsa-miR-125a-3p on
cell migration and invasion of lung cancer cells, a Transwell
migration assay was performed. As shown in Fig. 4, the number of migrated A549 cells
was 33.50±2.27 in the control group without any treatment. The
number of migrated cells was 25.33±3.15, which was less than that
in the control group (P<0.01). Additionally, transfection of
anti-sense hsa-miR-125a-3p significantly promoted cell migration
compared with transfection with sense has-miR-125a-3p (P<0.01).
The data demonstrated that transfection of sense hsa-miR-125a-3p
effectively inhibited the cell migration and invasion of A549 lung
cancer cells.
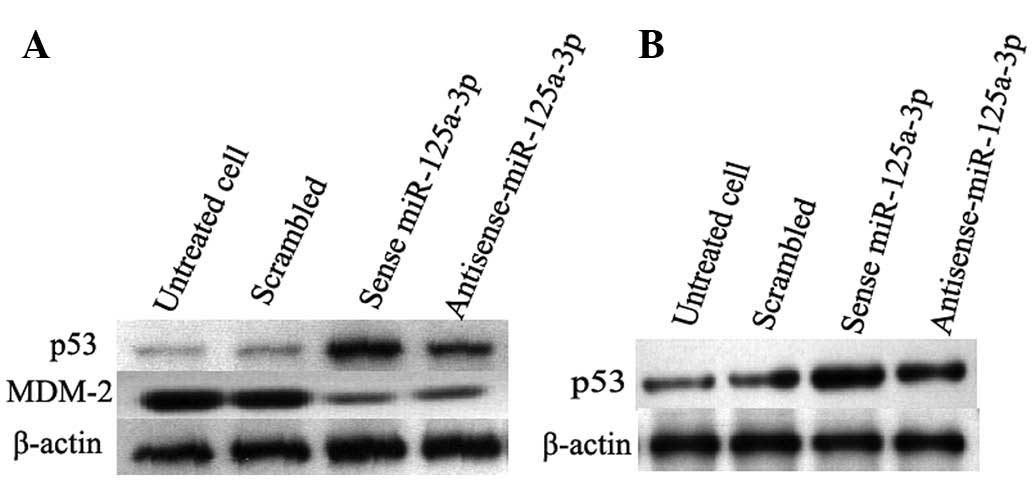
Transfection of sense miR-125a-3p
promotes the expression of tumor suppressor p53 and inhibits the
expression of MDM-2
To further investigate the mechanism of
hsa-miR-125a-3p the regulation of apoptosis of lung cancer cells,
western blot analysis was used to detect the expression of p53 and
MDM-2. As shown in Fig. 5,
transfection of sense miR-125a-3p increased the expression of p53
and inhibited the expression of MDM-2 in A549 cells. This is
consistent with that observed in the NCI-H460 lung cancer cell
line. Untreated cells and the cells transfected with scrambled
miRNA were used as negative controls. These results suggested that
hsa-miR-125a-3p could promote cell apoptosis by inhibiting the
expression of MDM-2 to upregulate the expression of tumor
suppressor p53.
Discussion
miRNA is an endogenous small RNA, which are not
encoded into proteins (29). It is
well conserved in eukaryotic organisms and is hypothesized to be a
vital and evolutionarily component of genetic regulation (30). Studies have demonstrated that
miR-125a is important in carcinogenesis (31). Bi et al (32) demonstrated that decreased miR-125a
was observed in HCC tissues and cell lines, and it was associated
with aggressive pathological features. It was also reported that
miR-125a-3p was downregulated in human gastric cancer, and the low
expression levels of miR-125a-3p were associated with indicators of
enhanced malignant potential, such as tumor size, tumor invasion,
lymph node metastasis, liver metastasis, peritoneal dissemination,
advanced clinical stage and poor prognosis (33).
In the present study, the role of hsa-miR-125a-3p
was observed in the proliferation and metastasis of lung cancer
cells. Firstly, the expression of hsa-miR-125a-3p in different lung
cancer cell lines was detected. The results demonstrated that
relative mRNA levels of hsa-miR-125a-3p are decreased in A549,
NCI-H460 and SPCA-1 lung cancer cell lines compared with the HBE
cell line, which suggested hsa-miR-125a-3p may act as a tumor
suppressor gene to inhibit the tumor progression. Subsequently, the
miR-125a-3p was overexpressed in lung cancer cells to detect the
role of miR-125a-3p on the proliferation of A549 cells. The results
showed that overexpression of sense hsa-miR-125a-3p inhibited the
growth of A549 cells; however, transfection of anti-sense
hsa-miR-125a-3p could not suppress the proliferation of lung cancer
cells. In addition, transfection of sense miR-125a-3p could enhance
the apoptosis rate of NCI-H460 cells, which was detected by annexin
V-FITC/PI dual staining analysis.
Metastasis of tumor cells is one of the predominant
reasons for the high mortality rate in lung cancer patients
(13,34). Thus, the present study analyzed
whether overexpression of sense miR-125a-3p could inhibit the
invasion and migration of lung cancer cells. The Transwell
migration assay was performed and the data demonstrated that the
number of migrated cells in the sense miR-125a-3p transfected group
was less than that of the control group, suggesting transfection of
sense hsa-miR-125a-3p could inhibit the cell migration and invasion
of lung cancer cells. Mdm2 acts as a p53 tumor suppressor and
inhibits the transcriptional activity of p53 by regulating its
stability. The results showed that transfection of sense
miR-125a-3p could inhibit the expression of MDM-2 and promote the
expression of tumor suppressor p53. Thus, it may exhibit an
antitumor role in lung cancer cells by regulating the MDM-2/p53
signaling pathway. In conclusion, the results demonstrated that
upregulation of hsa-miR-125a-3p may be a promising approach for
lung cancer therapy.
Acknowledgments
This study was supported by grants from the Youth
Innovation Fund of the Afflilated Hospital of Zhengzhou
University.
References
|
1
|
Xu X, Yang X, Xing C, Zhang S and Cao J:
miRNA: The nemesis of gastric cancer (Review). Oncol Lett.
6:631–641. 2013.PubMed/NCBI
|
|
2
|
Cai Y, Yu X, Hu S and Yu J: A brief review
on the mechanisms of miRNA regulation. Genomics Proteomics
Bioinformatics. 7:147–154. 2009. View Article : Google Scholar
|
|
3
|
Lodewijk L, Prins AM, Kist JW, et al: The
value of miRNA in diagnosing thyroid cancer: a systematic review.
Cancer Biomark. 11:229–238. 2012.PubMed/NCBI
|
|
4
|
Srivastava K and Srivastava A:
Comprehensive review of genetic association studies and
meta-analyses on miRNA polymorphisms and cancer risk. PLoS ONE.
7:e509662012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scott GK, Goga A, Bhaumik D, Berger CE,
Sullivan CS and Benz CC: Coordinate suppression of ERBB2 and ERBB3
by enforced expression of micro-RNA miR-125a or miR-125b. J Biol
Chem. 282:1479–1486. 2007. View Article : Google Scholar
|
|
6
|
Jiang L, Zhang Q, Chang H, Qiu X and Wang
E: hsa-miR-125a-5p enhances invasion in non-small cell lung
carcinoma cell lines by upregulating rock-1. Zhongguo Fei Ai Za
Zhi. 12:1069–1073. 2009.In Chinese.
|
|
7
|
Huang B, Luo W, Sun L, et al:
MiRNA-125a-3p is a negative regulator of the RhoA-actomyosin
pathway in A549 cells. Int J Oncol. 42:1734–1742. 2013.PubMed/NCBI
|
|
8
|
Li X, Sundquist J, Zöller B and Sundquist
K: Neighborhood deprivation and lung cancer incidence and
mortality: a multilevel analysis from sweden. J Thorac Oncol.
10:256–263. 2015. View Article : Google Scholar
|
|
9
|
Janković M, Samarzija M, Jakopović M,
Kulis T and Znaor A: Trends in lung cancer incidence and mortality
in Croatia, 1988–2008. Croat Med J. 53:93–99. 2012. View Article : Google Scholar
|
|
10
|
Ondrusova M, Muzik J, Hunakova L, et al:
Trends in the lung cancer incidence and mortality in the Slovak and
Czech Republics in the contexts of an international comparison.
Clin Transl Oncol. 659–666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song Y and Yao Y: Lung cancer
screening:review and prospect. Zhonghua Jie He He Hu Xi Za Zhi.
37:164–166. 2014.In Chinese. PubMed/NCBI
|
|
12
|
Pendharkar D, Ausekar BV and Gupta S:
Molecular biology of lung cancer-a review. Indian J Surg Oncol.
4:120–124. 2013. View Article : Google Scholar :
|
|
13
|
Di JZ, Peng JY and Wang ZG: Prevalence,
clinicopathological characteristics, treatment, and prognosis of
intestinal metastasis of primary lung cancer: A comprehensive
review. Surg Oncol. 23:72–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thunnissen E, van der Oord K and den
Bakker M: Prognostic and predictive biomarkers in lung cancer. A
review Virchows Arch. 464:347–358. 2014. View Article : Google Scholar
|
|
15
|
Kumar S, Mohan A and Guleria R: Prognostic
implications of circulating anti-p53 antibodies in lung cancer - a
review. Eur J Cancer Care (Engl). 18:248–254. 2009. View Article : Google Scholar
|
|
16
|
Bergqvist M: Radiosensitivity in lung
cancer with focus on p53: a review based on a doctoral thesis. Ups
J Med Sci. 108:87–127. 2003.PubMed/NCBI
|
|
17
|
Wang AL, Liu ZX, Li G and Zhang LW:
Expression and significance of P53 protein and MDM-2 protein in
human gliomas. Chin Med J (Engl). 124:2530–2533. 2011.
|
|
18
|
Asher G, Lotem J, Sachs L, Kahana C and
Shaul Y: Mdm-2 and ubiquitin-independent p53 proteasomal
degradation regulated by NQO1. Proc Natl Acad Sci USA.
99:13125–13130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jabbur JR, Tabor AD, Cheng X, et al: Mdm-2
binding and TAF(II)31 recruitment is regulated by hydrogen bond
disruption between the p53 residues Thr18 and Asp21. Oncogene.
21:7100–7113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Almog N, Milyavsky M, Stambolsky P,
Falcovitz A, Goldfinger N and Rotter V: The role of the C' terminus
of murine p53 in the p53/mdm-2 regulatory loop. Carcinogenesis.
22:779–785. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kondo I, Iida S, Takagi Y and Sugihara K:
MDM2 mRNA expression in the p53 pathway may predict the potential
of invasion and liver metastasis in colorectal cancer. Dis Colon
Rectum. 51:1395–1402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishitani H, Sugimoto N, Roukos V, et al:
Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1
for proteolysis. EMBO J. 25:1126–1136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peng L, Xu Z, Zhou Y, Yang T, Liang ZQ and
Zhang M: Effect of rosiglitazone on cells cycle, apoptosis and
expression of Skp2 and p27Kip1 in hepatocellular carcinoma cell
line. Zhonghua Gan Zang Bing Za Zhi. 18:148–149. 2010.In Chinese.
PubMed/NCBI
|
|
24
|
Schulman BA, Carrano AC, Jeffrey PD, et
al: Insights into SCF ubiquitin ligases from the structure of the
Skp1-Skp2 complex. Nature. 408:381–386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krishan A: Rapid flow cytofluorometric
analysis of mammalian cell cycle by propidium iodide staining. J
Cell Biol. 66:188–193. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buchegger F, Dupertuis YM and
Perillo-Adamer F: A pitfall of propidium iodide staining in
fluorescence-activated cell sorting cell cycle analysis? Cancer
Res. 67:5576–5577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rüster B, Grace B, Seitz O, Seifried E and
Henschler R: Induction and detection of human mesenchymal stem cell
migration in the 48-well reusable transwell assay. Stem Cells Dev.
14:231–235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Primiceri E, Chiriacò MS, Dioguardi F, et
al: Automatic transwell assay by an EIS cell chip to monitor cell
migration. Lab Chip. 11:4081–4086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tilghman SL, Rhodes LV, Bratton MR, et al:
Phytoalexins, miRNAs and breast cancer: a review of
phytochemical-mediated miRNA regulation in breast cancer. J Health
Care Poor Underserved. 24(Suppl): 36–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim JK, Noh JH, Jung KH, et al: Sirtuin7
oncogenic potential in human hepatocellular carcinoma and its
regulation by the tumor suppressors MiR-125a-5p and MiR-125b.
Hepatology. 57:1055–1067. 2013. View Article : Google Scholar
|
|
31
|
Jiang L, Huang Q, Chang J, Wang E and Qiu
X: MicroRNA HSA-miR-125a-5p induces apoptosis by activating p53 in
lung cancer cells. Exp Lung Res. 37:387–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bi Q, Tang S, Xia L, et al: Ectopic
expression of MiR-125a inhibits the proliferation and metastasis of
hepatocellular carcinoma by targeting MMP11 and VEGF. PLoS ONE.
7:e401692012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashiguchi Y, Nishida N, Mimori K, et al:
Down-regulation of miR-125a-3p in human gastric cancer and its
clinicopathological significance. Int J Oncol. 40:1477–1482.
2012.PubMed/NCBI
|
|
34
|
Collins J, Noble S, Chester J, Coles B and
Byrne A: The assessment and impact of sarcopenia in lung cancer: a
systematic literature review. BMJ Open. 4:e0036972014. View Article : Google Scholar : PubMed/NCBI
|