Introduction
Traditional Chinese medicine (TCM), as a
complementary and alternative medicine, has been widely used in
China for thousands of years (1).
From ancient to modern times in China, TCM has been critical in the
prevention and treatment of several diseases prior to western
medicine being introduced (2). The
benefits of TCM are gradually being recognized worldwide, which may
aid in the development of novels drugs for the treatment of various
types of disease.
Rhizoma Panacis Majoris, also named Zhu Zi Shen or
Kou Zi Qi in Chinese, has been widely used for the treatment of
several diseases due to its various pharmacological activities. In
China, Rhizoma Panacis Majoris is predominantly located in the
southwest area, including Shaanxi, Gansu, Ningxia, Henan, Hubei and
Yunnan provinces, at an altitude of 1200–4000 m in the valley of
broad-leaved forest (3). Modern
chemical investigations have demonstrated that Rhizoma Panacis
Majoris predominantly contains saponins, naphtha and organic acids.
(4,5). It has been revealed that Rhizoma
Panacis Majoris possesses several pharmacological activities. For
example, the total saponins of Rhizoma Panacis Majoris have been
demonstrated to increase the proliferation of T lymphocytes induced
by Sword bean A and Phytohemagglutinin (6). The water extract exhibits
anti-inflammatory and analgesic effects in mice (7), and protects the mice against acute
cerebral ischemia-reperfusion injury (8). Furthermore, Rhizoma Panacis Majoris
was revealed to be capable of reducing the toxicity of
chemotherapeutic drugs (9) and
exerting an anti-tumor effect (10,11).
Deglucose chikusetsusaponin IVa (DCIVa), also termed
Oleanolic acid-3-O-β-D-pyran glucuronic acid glycoside, is also
isolated from the extract of Rhizoma Panacis Majoris (12). DCIVa has been revealed to exert an
anti-arrhythmic effect (13) and
protect against hypoxia/reoxygenation-induced injury in myocardial
cells (14). DCIVa is a member of
the family of oleanane triterpenoids, which has been reported to
have numerous pharmacological activities, including cytotoxic
activity against various cancer cells, anti-inflammatory activity,
prevention of dental caries and induction of genta-micin
nephrotoxicity (15–18). However, whether DCIVa exerts an
antitumor effect remains to be elucidated. The present study aimed
to investigate the antitumor effect and the underlying mechanism of
DCIVa using HepG2 hepatocellular carcinoma cells.
Materials and methods
Reagents
Fetal bovine serum (FBS) was obtained from Minhai
Biotechnology Development (Beijing, China) and RPMI-1640 medium was
obtained from Gibco-BRL (Gaithersburg, MD, USA).
3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), dimethyl sulfoxide (DMSO), Hoechst 33258 and propidium
iodide (PI) were purchased from Sigma-Aldrich (St. Louis, MO,
USA).
Cell culture
The HepG2 hepatocellular carcinoma cells were
obtained from The Fourth Military Medical University (Xian, China).
The cells were cultured in RPMI-1640 medium supplemented with 10%
FBS, containing 100 µg/ml streptomycin, 100 U/ml penicillin
and 0.03% L-glutamine (all obtained from Sigma-Aldrich) and were
maintained at 37°C with 5% CO2 in a humidified
atmosphere.
MTT assay
The HepG2 cells were seeded in 96-well tissue
culture plates (Nunc, Roskilde, Denmark) at a density of
1×104 cells/well. Following a 12 h incubation, the cells
were treated with or without DCIVa at a concentration of 0.02,
0.04, 0.06, 0.08 or 0.1 µmol/ml, and incubated for 24, 48 or
72 h. Following incubation, MTT (5 mg/ml in phosphate-buffered
saline; PBS) was added (200 µl/well) and incubated for an
additional 4 h. DMSO (150 µl/well) was subsequently added to
dissolve the formazan product for 15 min. The absorbance at 490 nm
was measured using an ELISA reader (Bio-Tek, Winooski, VT, USA).
The experiment was performed five times. The data are
representative of three independent experiments. The cell growth
inhibition rate was calculated as follows: Cell growth inhibition
(%) = HepG2control − HepG2DCIVa /
HepG2control × 100.
Observation of morphological changes
The HepG2 cells were seeded into 6-well culture
plates at a density of 1×104 cells/well. Following a 12
h incubation, the cells were treated with different concentrations
of DCIVa for 24 h. The cellular morphology was observed using an
inverted microscope (AE21; Motic, Xiamen, China).
Transmission electron microscopy
The HepG2 cells were seeded into culture flasks at a
density of 1×106 cells/flask. Following a 12 h
incubation, the cells were treated with or without 0.06
µmol/ml DCIVa for 24 h. The cells were subsequently
collected and fixed with fixative. The cell samples were processed
into ultra-thin sections in the College of Medicine of Xian
Jiaotong University (Xian, China). The ultra-thin sections were
examined using a H600 transmission electron microscope (Hitachi,
Tokyo Japan).
Hochest 33258 staining
The cells were treated with 0, 0.06 or 0.1
µmol/ml DCIVa for 24 h and washed with ice-cold PBS twice
prior to fixation with 3 ml ethyl alcohol for 30 min at room
temperature. The fixative was discarded and the cells were washed
three times with ice-cold PBS. Hoechst 33258 (5 mg/l) was added to
the cells and incubated for 45 min in the dark. The cells were
washed twice with ice-cold PBS followed by visualization under a
Leica AF6000 fluorescence microscope (Leica, Wetzlar, Germany).
Flow cytometric analysis
For cell cycle analysis, following treatment with
different concentrations of DCIVa, the cells were harvested and
washed with ice-cold PBS. The cells were subsequently fixed in 70%
ethanol and maintained at 4°C for at least 12 h. The cell pellets
were obtained by centrifugation at 2,000 × g for 10 min and then
stained with a fluorescent probe solution, containing 50
µg/ml PI and 1 mg/ml DNase-free RNaseA in PBS on ice in the
dark for 30 min. The DNA fluorescence of the PI-stained cells was
determined using FACScan flow cytometry (Becton Dickinson, Franklin
Lakes, NJ, USA). Analysis of cell apoptosis was performed using an
Annexin V/PI apoptosis detection method (Beyotime Institute of
Biotechnology, Haimen, China). Briefly, the cells were collected
and washed with ice-cold PBS followed by resuspension in binding
buffer. The cells were incubated with 10 µl Annexin V stock
solution for 30 min at 4°C. A total of 5 µl PI was
subsequently added and the cells were incubated for 5 min. Finally,
cells were analyzed by flow cytometry.
Western blot analysis
The HepG2 cells were cultured for 24 h and the old
culture medium was replaced with fresh medium without phenol red
and FBS. Following incubation for a further 24 h, the cells were
treated with or without DCIVa at the provided concentrations and
incubated for 24 h. The adherent and floating cells were harvested
and washed with PBS. The cell pellets were obtained by
centrifugation at 2,000 × g for 10 min and then resuspended in
lysis buffer, containing 50 mM HEPES (pH 7.4), 1% Triton X-100, 2
mM sodium orthovanadate, 100 mM sodium fluoride, 1 mM edetic acid,
1 mM PMSF, 10 mg/l aprotinin and 10 mg/l leupeptin, and lysed at
4°C for 60 min. Following centrifugation at 13,000 × g for 15 min,
the protein concentration in the supernatant was determined using a
Bradford assay (Beyotime Institute of Biotechnology). A total of 20
µg protein lysate was separated by electrophoresis on 12%
SDS-polyacrylamide gels (Sangon Biotech Co., Ltd., Shanghai, China)
and transferred onto a nitrocellulose membrane (Bio-Rad, Hercules,
CA, USA). The membranes were soaked in blocking buffer (5% non-fat
milk) at 37°C for 1 h. The membranes were incubated with polyclonal
rabbit anti-human Bcl-2 antibody (1:500; cat. no. sc-492)
monoclonal mouse anti-human Bax antibody (1:1,000; sc-20067)and
polyclonal rabbit anti-human β-actin antibody (1:800;
sc-130656)obtained from Santa Cruz Biotechnology (Santa Cruz, CA,
USA) at 4°C overnight. The membrane was subsequently incubated with
anti-rabbit or anti-mouse IgG conjugated with horseradish
peroxidase (HRP) for 1 h. The proteins were detected using
3,3′-diaminobenzidine tetrahy-drochloride as the HRP substrate.
Statistical analysis
All data are expressed as the mean ± standard
deviation of at least three independent experiments. Statistical
comparisons between or among groups were analyzed by two-tailed
Student's t-test or one-way analysis of variance, followed by
Bonferroni post hoc. Statistical analysis was performed using SPSS
version 11.5 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
DCIVa induces cytotoxicity in a dose- and
time-dependent manner
To investigate whether DCIVa (Fig. 1A) was cytotoxic, different doses of
DCIVa were added to the HepG2 cells, and the cell growth and
viability was measured by an MTT assay. The results demonstrated
that DCIVa inhibited cell growth in a dose- and time-dependent
manner (Fig. 1B). To further
characterize the DCIVa-induced HepG2 cell growth inhibition, the
cellular morphology was observed using inverted microscopy.
Following treatment with DCIVa, the cells changed to a more round
shape and the cell number was reduced in dose-dependent manner,
compared with the control group where cells were spindle-shaped and
arranged densely (Fig. 1C).
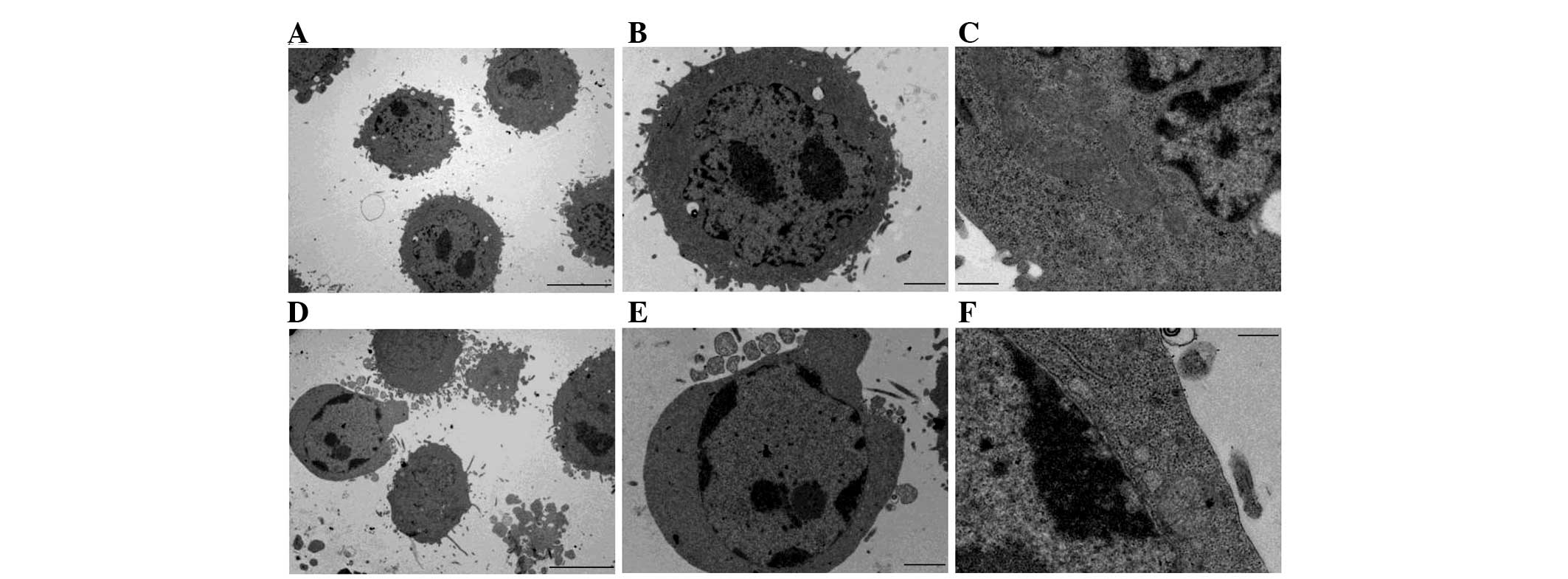
DCIVa induces an apoptotic
ultrastructure
To further investigate the effect of DCIVa on the
cells, the ultrastructure of the DCIVa-treated HepG2 cells was
determined using transmission electron microscopy. As shown in
Fig. 2A–C, the control cells
exhibited a normal cell phenotype. By contrast, the DCIVa-treated
HepG2 cells demonstrated typical apoptotic features, including
chromatin condensation, margination at the nuclear periphery and
the formation of apoptotic bodies (Fig. 2D–F).
DCIVa triggers nuclear contraction and
fragmentation
The effect of DCIVa on the nucleus was assessed by
Hoechst 33258 staining. The results revealed that the HepG2 cell
nucleus was condensed and fragmented upon treatment with 0.06
(Fig. 3B) and 0.1 µmol/ml
DCIVa (Fig. 3C) treatment,
compared with the control, further confirming the pro-apoptotic
effect of DCIVa.
DCIVa induces cell apoptosis
To further determine the effect of DCIVa on cell
apoptosis, cell apoptosis was determined using an Annexin
V-fluorescein isothiocyanate/PI assay. Following treatment with
different concentrations of DCIVa, the number of cells in different
stages of the cell cycle were measured by flow cytometry. As
compared with control cells (Fig.
4A), 0.02 (Fig. 4B) and 0.04
µmol/ml (Fig. 4C) DCIVa had
no obvious effect on the number of early stage apoptotic cells.
Treatment with 0.06 (Fig. 4D) and
0.08 µmol/ml (Fig. 4E)
DCIVa significantly increased the number of early stage apoptotic
cells by 15.4 and 23.0% (Fig. 4F),
respectively, compared with the control cells. Furthermore, 0.04
(Fig. 4C), 0.06 (Fig. 4D) and 0.08 µmol/ml (Fig. 4E) DCIVa markedly increased the
number of late-stage apoptotic cells by 6.7, 17.3 and 33.5%
(Fig. 4F), respectively. However,
0.02 µmol/ml (Fig. 4B)
DCIVa caused no significant effect on the number of late stage
apoptotic cells compared with the control group.
DCIVa induces cell-cycle arrest
Next, the effect of DCIVa on cell cycle was
determined. Flow cytometric analysis demonstrated that the HepG2
cell cycles markedly changed following treatment with different
concentrations of DCIVa. The results revealed that the number of
cells in the G2/M phase were significantly increased from 17.32
(Fig. 5A) or 18.52 (Fig. 5B) to 23.08 (Fig. 5C), 31.92 (Fig. 5D) and 40.52% (Fig. 5E) upon treatment with 0, 0.02,
0.04, 0.06 and 0.08 µmol/ml DCIVa, respectively (Fig. 5F).
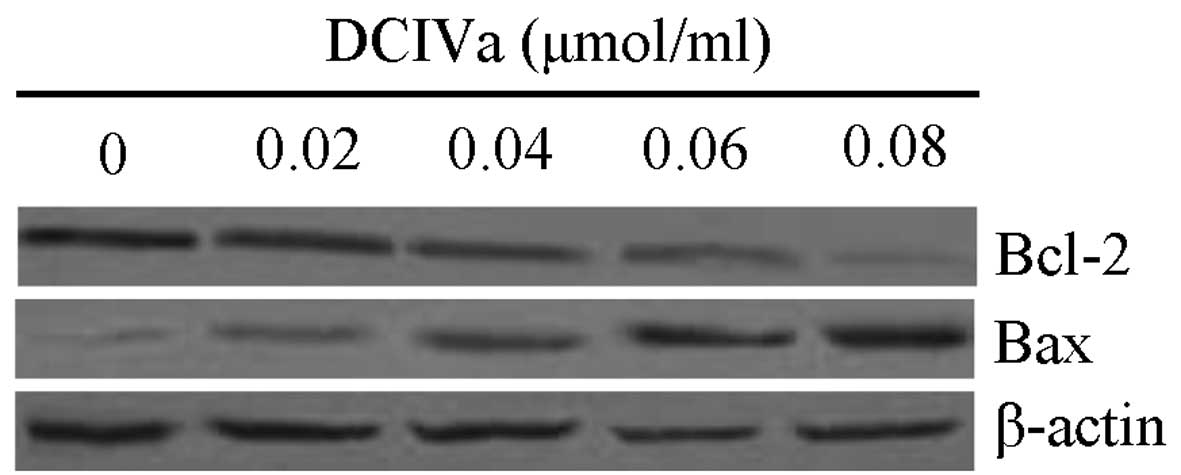
DCIVa activates pro-apoptotic
pathways
To determine the underlying mechanism of
DCIVa-induced cell apoptosis, western blot analysis was performed
to determine the expression levels of Bax and Bcl-2. The result
demonstrated that the protein expression of Bcl-2 was decreased,
whereas the expression of Bax was increased by different
concentrations of DCIVa, in a dose-dependent manner (Fig. 6).
Discussion
DCIVa, an active constituent extracted from Rhizoma
Panacis Majoris, has been demonstrated to have exert
pharmacological activities, including an anti-arrhythmic effect
(13) and a protective effect
against hypoxia/reoxygenation-induced injury on myocardial cells
(14). However, the antitumor
effect of DCIVa remains to be elucidated. In the present study,
evidence suggested for the first time, to the best of our
knowledge, that DCIVa exerted an antitumor effect by inducing cell
apoptosis and cell cycle arrest in the HepG2 cells. Therefore,
DCIVa may be a promising compound for the development of anticancer
drugs.
The cytotoxic activity of oleanane triterpenoids has
been reported in numerous previous studies. Zhang et al
(16) demonstrated that
oleanane-type triterpene saponins isolated from Albizia
inundata exhibited cytotoxicity against melanoma cells and
human head and neck squamous cells. The oleanane synthetic
triterpenoid, CDDO-Me, revealed anti-tumorigenic activity against
prostate cancer by inactivation of Akt and nuclear factor-κB
(15,17). Group B oleanane triterpenoid
extract, containing Soy saponins I and III from Soy Flou induced
apoptosis in the HepG2 cells (18). The present study identified and
characterized DCIVa, a type of oleanane triterpenoids, and revealed
that this exhibited an antitumor effect in HepG2 cells. Using an
MTT assay, it was revealed that DCIVa inhibited HepG2 cell growth
in a dose-dependent manner. The alteration of cell morphology was
essential for the medicinal mechanism, which triggers drug-mediated
apoptosis in the cells. In the present study, it was demonstrated
that DCIVa caused cell atrophy and induced typical apoptotic
features, including chromatin condensation, margination at the
nuclear periphery, apoptotic bodies formation and nuclear
condensation and fragmentation. These findings suggested that DCIVa
induced apoptosis in the HepG2 cells. The results of flow
cytometric analysis demonstrated that DCIVa induced an increase in
early stage apoptotic cells and late stage apoptotic cells, further
confirming that DCIVa induced cell apoptosis.
Alteration in the Bax/Bcl-2 ratio has been suggested
to be crucial for the activation of the mitochondrial apop-totic
pathway (19,20). Various anticancer drugs have been
demonstrated to induce cell apoptosis through regulating Bax/Bcl-2.
Boldine was revealed to exert cytotoxic and chemotherapeutic
properties by downregulating the expression of Bcl-2 and
simultaneously promoting the expression of Bax, therefore leading
to cell apoptosis in breast cancer cells (21). Rice bran phytic acid was reported
to induce apop-tosis via regulation of Bcl-2/Bax in HepG2 cells
(22) and Persea declinata (Bl.)
Kosterm bark methanolic crude extract induced cell apoptosis
through upregulation of Bax and downregulation of Bcl-1 in human
breast cancer cells (23). In the
present study, it was further demonstrated that treatment with
DCIVa activated the mitochondrial apoptotic pathway. The data
revealed that the pro-apoptotic protein, Bax, was induced by DCIVa,
whereas the anti-apoptotic protein, Bcl-2, was inhibited by DCIVa.
A novel oleanane triterpenoid, meth
yl-25-hydroxy-3-oxoolean-12-en-28-oate, has been demonstrated to
induce apoptosis by regulating the expression of Bax and Bcl-2
(24). This data suggested that
DCIVa, as a type of oleanane triterpenoid, exerted cytotoxicity
through the induction of cell apoptosis, by mediating the
expression levels of Bax and Bcl-2.
In addition, the present study demonstrated that
DCIVa induced a cell cycle arrest in the G2/M phase. Cell cycle
checkpoints are critical events for cell proliferation, which
guarantee the integrity of the DNA and proper timing of cell
division. If the DNA is damaged, the cell cycle is inhibited at the
G2/M phase to initiate the repair mechanism for DNA (25). However, excessive DNA damage
results in cell apoptosis. Using flow cytometric analysis, it was
demonstrated that DCIVa induced G2/M cell cycle arrest in a
dose-dependent manner. However, various genes are involved in the
regulation of the G2/M checkpoint and the effect of DCIVa on the
G2/M checkpoint regulatory proteins and the underlying mechanism
remain to be elucidated.
In conclusion, the present study provided evidence
that DCIVa exhibits a potent cytotoxic effect on HepG2 cells
through the induction of cell apoptosis and cell cycle arrest.
These findings contributed to the development of DCIVa as a
potential anticancer agent. However, further investigation into the
antitumor effects of DCIVa in vitro and in vivo are
required.
Acknowledgments
The present study was supported by the National
Natural Sciences Foundation of China (grant nos. 81102805 and
81373978) and the Innovation Program on Science and Technology
Project of Shaanxi Province (grant nos. 2011KTCQ03-02 and
2013KTCQ03-14).
Abbreviations:
|
DCIVa
|
deglucose chikusetsusaponin IVa
|
|
TCM
|
traditional Chinese medicine
|
|
FBS
|
fetal bovine serum
|
|
MTT
|
3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
DMSO
|
dimethyl sulfoxide
|
|
PI
|
propidium iodide
|
|
HRP
|
horseradish peroxidase
|
References
|
1
|
Xu Q, Bauer R, Hendry BM, Fan TP, Zhao Z,
Duez P, Simmonds MS, Witt CM, Lu A, Robinson N, et al: The quest
for modernisation of traditional Chinese medicine. BMC Complement
Altern Med. 13:1322013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang L, Li Y, Li J, Zhang M, Xu L, Yuan W,
Wang G and Hopewell S: Quality of reporting of trial abstracts
needs to be improved: Using the CONSORT for abstracts to assess the
four leading Chinese medical journals of traditional Chinese
medicine. Trials. 11:752010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao R, Zhao Y, Li D, Wang J and Zeng Y:
Research progress of panax japonicus. Modern Chinese Medicine.
10:3–6. 2008.
|
|
4
|
Zou K, Liu Z, Zhu S, Cai S and Komatsu K:
Research of ginsenosides in kou zi qi using HPLC-MS-MS. Yao Xue Xue
Bao. 39:385–388. 2004.In Chinese. PubMed/NCBI
|
|
5
|
Wang DQ, Feng BS, Wang XB, Yang CR and
Zhou J: Further study on dammarane saponins of leaves of Panax
japonicus var. Major collected in Quinling Mountains China. Yao Xue
Xue Bao. 24:633–636. 1989.In Chinese.
|
|
6
|
Zhu XH, Hou WJ and Li CD: Study of effect
of Panax Japonicus Saponin on spleen lymphocyte proliferous
response. Academic Journal of Kunming Medical College. 15:65–67.
1994.In Chinese.
|
|
7
|
He H, Shi M, Chen T, Lu X, Qin N and Chen
S: Anti-inflammatory and analgesic effect of water extractive of
Panacis Majoris Rhizoma in mice. Acta Academiae Medicinae Militaris
Tertiae. 20:202010.
|
|
8
|
Mengqiong S, Haibo H, Ningling Q and
Shuwei C: Effect of pretreatment with water extract from Rhizoma
Panacis majoris on cerebral ischemia-reperfusion injury in mice.
Acta Academiae Medicinae Militaris Tertiae. 3:0232011.
|
|
9
|
Chen T, Gong Z and Fu Y: Rhizoma Panacis
majori reduces toxicity of chemotherapy in S180-bearing mice. Zhong
Xi Yi Jie He Xue Bao. 6:1255–1258. 2008.In Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen C, Wu W, Xu X, Zhang L, Liu Y and
Wang K: Chain conformation and anti-tumor activity of derivatives
of polysac-charide from Rhizoma Panacis Japonici. Carbohydr Polym.
105:308–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Z, Ren H, Duan X and Zhang L: Chain
conformation and bioactivity of water-soluble polysaccharide
extracted from Rhizoma Panacis Japonici. Biopolymers. 93:383–390.
2010.PubMed/NCBI
|
|
12
|
Song X, Li L, Yang G and Cai B: HPLC
determination of chiku-setsusaponin IVa in Rhizoma Panacis Majoris
from different producing areas. Zhongguo Zhong Yao Za Zhi.
35:885–887. 2010.In Chinese. PubMed/NCBI
|
|
13
|
Sun GB, Xu HB, Wen FC, Ding T and Sun XB:
Study of anti-experimental arrhythmia effect of deglucose
Chikusetsu Saponin IVa. Chin J Pharmacol Toxicol. 20:377–380.
2006.In Chinese.
|
|
14
|
Sun GB, Xu HB, Wen FC, Zhang W, Ding T and
Sun XB: Protective Effects of Deglucose Chikusetsu Saponin IVa on
Cultured Myocardial Cells Subjected to Anoxia Reoxygenation Injury.
Chin J Pharmacol Toxicol. 19:424–427. 2005.In Chinese.
|
|
15
|
Liu Y, Gao X, Deeb D and Gautam SC:
Oleanane triterpenoid CDDO-Me inhibits Akt activity without
affecting PDK1 kinase or PP2A phosphatase activity in cancer cells.
Biochem Biophys Res Commun. 417:570–575. 2012. View Article : Google Scholar :
|
|
16
|
Zhang H, Samadi AK, Rao KV, Cohen MS and
Timmermann BN: Cytotoxic oleanane-type saponins from Albizia
inundata. J Nat Prod. 74:477–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao X, Deeb D, Liu Y, Arbab AS, Divine GW,
Dulchavsky SA and Gautam SC: Prevention of prostate cancer with
oleanane synthetic triterpenoid CDDO-Me in the TRAMP mouse model of
prostate cancer. Cancers (Basel). 3:3353–3369. 2011. View Article : Google Scholar
|
|
18
|
Zhang W and Popovich DG: Group B oleanane
triterpenoid extract containing soyasaponins I and III from soy
flour induces apoptosis in Hep-G2 cells. J Agric Food Chem.
58:5315–5319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Antonsson B and Martinou JC: The Bcl-2
protein family. Exp Cell Res. 256:50–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reed JC: Double identity for proteins of
the Bcl-2 family. Nature. 387:773–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paydar M, Kamalidehghan B, Wong YL, Wong
WF, Looi CY and Mustafa MR: Evaluation of cytotoxic and
chemotherapeutic properties of boldine in breast cancer using in
vitro and in vivo models. Drug Des Devel Ther. 8:719–733.
2014.PubMed/NCBI
|
|
22
|
Al-Fatlawi AA, Irshad M, Zafaryab M, Rizvi
MM and Ahmad A: Rice bran phytic acid induced apoptosis through
regulation of Bcl-2/Bax and p53 genes in HepG2 human hepatocellular
carcinoma cells. Asian Pac J Cancer Prev. 15:3731–3736. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Narrima P, Paydar M, Looi CY, Wong YL,
Taha H, Wong WF, Ali Mohd M and Hadi AH: Persea declinata (Bl.)
Kosterm bark crude extract induces apoptosis in MCF-7 cells via
G0/G1 cell cycle arrest, Bcl-2/Bax/Bcl-xl signaling pathways and
ROS generation. Evid Based Complement Alternat Med.
2014:2481032014. View Article : Google Scholar
|
|
24
|
Bishayee A, Mandal A, Thoppil RJ, Darvesh
AS and Bhatia D: Chemopreventive effect of a novel oleanane
triterpenoid in a chemically induced rodent model of breast cancer.
Int J Cancer. 133:1054–1063. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hartwell LH and Weinert TA: Checkpoints:
controls that ensure the order of cell cycle events. Science.
246:629–634. 1989. View Article : Google Scholar : PubMed/NCBI
|