Introduction
Parkinson's disease (PD) is one of the most common
neuro-degenerative disorders (1),
with prominent characteristics including the degradation of
dopaminergic cells within the substantia nigra pars compacta
(2), aberrant intracellular
protein aggregation in the dorsal motor nucleus of the vagus, a
region within the medulla oblongata (3,4).
These aggregates are known as Lewy bodies, with α-synuclein (α-Syn)
as the major component (5).
Striking evidence has confirmed that α-Syn has a key role in the
formation and progression of PD; however, the mechanism underlying
the cytotoxicity of α-Syn in PD remains to be determined (6,7).
α-Syn is a 14-KDa neuronal protein, belonging to a family of
structurally associated proteins in the brain (8,9).
Under physiological conditions, α-Syn is highly expressed at
pre-synaptic terminals and promotes the assembly of the SNARE
machinery (10), with an
importance for neurotransmitter release (11) and the protection of nerve terminals
against injury (12). α-Syn has
been widely accepted to have a natively unfolded tertiary structure
as its main physiological form in the brain (13). It is the key pathological course
for α-Syn aggregation to proceed from monomers to pathogenic
protein inclusions (14).
Aggregation of α-Syn monomers leads to the formation
of soluble oligomeric species, which, according to in vitro
experiments, further spontaneously aggregate in the absence of
other proteins, such as molecular chaperones (15). The accumulation and aggregation of
α-Syn in PD may reflect changes to its synthesis and/or degradation
(16). Besides an increased α-Syn
gene copy number (17) supporting
the role of increased α-Syn synthesis in PD, there is increasing
evidence that the impaired degradation pathways of α-Syn may also
be compromised in PD (18,19). The importance of molecular
chaperones has also been underlined by the fact that overexpression
of these molecules, such as heat shock proteins (HSPs), leads to
the re-folding of aberrant α-Syn aggregates to form non-toxic and
non-aggregated α-Syn (20,21). Therefore, functional defects of
HSPs may have a key role in PD (21,22).
Hsp70 is the most recognized molecular chaperone, and has been
linked with PD and α-Syn aggregation. Studies have confirmed the
negative regulatory role of Hsp70 in α-Syn aggregation in PD and in
α-Syn-induced toxicity in cells (22,23).
Therefore, Hsp70 is a well-defined therapeutic target, and the
upregulation of Hsp70 is an efficient strategy to block or even
reverse α-Syn-induced toxicity in PD.
The present study investigated the upregulation of
Hsp70 expression in SH-SY5Y neuroblastoma cells by glutamine (Gln)
and assessed the role of heat shock factor (HSF)-1 in this process.
Furthermore, the regulatory role of Gln in the α-Syn degradation in
α-Syn-overexpressing SH-SY5Y cells was investigated. The results of
the present study indicated that glutamine may be a potential
therapeutic agent to prevent α-Syn aggregation in PD.
Materials and methods
Reagents, cell culture and
treatments
l-Gln
was purchased from Sigma-Aldrich (St. Louis, MO, USA). The SH-SY5Y
human neuroblastoma cell line was obtained from the Type Culture
Collection of the Chinese Academy of Sciences (Beijing, China) and
was cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
10% FBS (Invitrogen Life Technologies) or maintained in DMEM
supplemented with 2% FBS. To generate α-Syn-overexpressing SH-SY5Y
cells [SH-SY5Y (Syn+)], an α-Syn coding sequence was amplified
using Phusion High-Fidelity DNA Polymerase (New England Biolabs,
Beverly, MA, USA) with the following primers: Forward,
5′-CGCGACGCGGAAGTGAGGTGC-3′ and reverse,
5′-TTCTGGGCTACTGCTGTCAC-3′, and subsequently cloned into the
pcDNA3.1(+) eukaryotic expression vector (cat. no. V790-20; Thermo
Fisher Scientific, Inc., Rockford, IL, USA). Following transfection
with the recombinant pcDNA3.1-α-Syn or pcDNA3.1-CAT plasmid (cat.
no. V790-20; Thermo Fisher Scientific, Inc.) using Lipofectamine
2000 (Invitrogen Life Techonologies), SH-SY5Y cells were cultured
for three passages in the presence of 800 μg/ml G418 (Life
Technologies, Grand Island, NY, USA) to select the positive
α-Syn-overexpressing clones, SH-SY5Y (Syn+), which were maintained
in the presence of 500 μg/ml G418. The HSF-1-specific small
interfering (si)RNA (with corresponding siRNA sequences as follows:
Forward, 5′-GAA CGA CAG UGG CUC AGC AUU-3′ and reverse,
5′-P-UGC-UGA GCC ACU GUC GUU CUU-3′) or control siRNA (with
scrambled siRNA sequences as follows: Forward, 5′-GUA ACU GCA ACG
AUU UCG AUG DTDT-3′ and reverse, 5′-CAU CGA AAU CGU UGC AGU UAC
DTDT-3′; Sangon, Shanghai, China) was transfected into the SH-SY5Y
(Syn+) cells with Lipofectamine 2000 at 25 or 50 nM to knock down
HSF-1 expression.
RNA isolation and reverse transcription
quantitative polymerase chain reaction (RT-qPCR)
Total cellular RNA was isolated using TRIzol
(Invitrogen Life Technologies) according to the manufacturer's
instructions, and was supplemented with RNase inhibitor (New
England Biolabs). The expression of Hsp70, HSF-1 and α-Syn mRNA was
quantified using the real-time RT-qPCR method. cDNA was synthesized
using the Quanti Tect Reverse Transcription kit (Qiagen, Valencia,
CA, USA). qPCR was performed using a SYBR PrimeScript RT-qPCR kit
(TaKaRa Bio, Inc., Tokyo, Japan) with the following primers:
Forward, 5′-AGG ACT TTC AAA GGC CAA GG-3′ and reverse, 5′-TCC TCC
AAC ATT TGT CAC TTGC-3′ for α-Syn; forward, 5′-TGT GTC TGC TTG GTA
GGA ATG GTG GTA-3′ and reverse, 5′-TTA CCC GTC CCC GAT TTG AAG
AAC-3′ for HSP70; forward, 5′-CGA CAG TGG CTC AGC ACA TTC C-3′ and
reverse, 5′-CAG CTC GGT GAT GTC GGA GAT G-3′ for HSF-1; and
forward, 5′-TGT CCA CCT TCC AGC AGA TGT-3′ and reverse, 5′-AGC TCA
GTA ACA GTC CGC CTA GA-3′ for β-actin (β-actin served as an
internal control). mRNA samples were amplified using primer sets
specific for the target gene on a Lightcycler 480 II (Roche
Diagnostics, Basel, Switzerland). Relative quantification was
performed using the ∆∆Ct method using β-actin as the reference gene
(24).
Western blot analysis
SH-SY5Y or SH-SY5Y (Syn+) cells were washed with
cold phosphate-buffered saline (PBS) and then lysed using NE-PER™
Nuclear and Cytoplasmic Extraction Reagents (cat. no. 78833; Thermo
Fisher Scientific). Protein samples were supplemented with protease
inhibitor cocktail (cat. no. 04693116001, Roche Diagnostics) and
quantified using a bicinchoninic acid protein assay reagent (cat.
no. 23234; Pierce Biotechnology, Inc., Rockford, IL, USA), and were
separated using 10% gradient SDS-PAGE. The separated proteins were
then transferred onto a polyvinylidene difluoride membrane, which
was blocked in 5% skimmed milk. The membranes were then incubated
with the primary antibody for 1 h at room temperature or overnight
at 4°C, followed by the secondary horseradish peroxidase-conjugated
anti-rabbit antibody for 1 h at room temperature. The membranes
were washed three times with PBS before each inoculation with the
primary or secondary antibodies. Target proteins were visual-ized
using an enhanced chemiluminescence detection system (RPN 2106;
Amersham Pharmacia Biotech, Amersham, UK) and analyzed with ImageJ
software (http://rsb.info.nih.gov/ij/). Rabbit
polyclonal antibodies to α-Syn (cat. no. 2642S; 1:300; Cell
Signaling Technology Inc., Danvers, MA, USA) Hsp70 (cat. no. 4872S;
1:200; Cell Signaling Technology Inc.) or β-actin (cat. no. A2066;
1:500; Sigma-Aldrich) and rabbit polyclonal antibodies to HSF-1
(cat. no. sc-9144; Santa Cruz Biotechnology, Dallas, TX, USA) were
used to quantify the expression of the target proteins.
Statistical analysis
Statistical analysis was performed with GraphPad
Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Differences
in the mRNA or protein expression of α-Syn, Hsp70 or HSF-1 between
two groups were analyzed using Student's t-test. All values are
expressed as mean ± standard error. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Gln upregulates Hsp70 expression in
SH-SY5Y neuroblastoma cells
Previous studies have shown that Gln safely enhances
HSP expression in in vitro and in vivo settings
(25–28). Given the key regulatory role of
Hsp70 in α-synuclein degradation, which is deregulated in PD, the
present study investigated the possible regulation of Hsp70
expression by Gln in SH-SY5Y neuroblastoma cells. Hsp70 mRNA
expression levels in SH-SY5Y cells post Gln treatment were
determined using RT-qPCR. Fig. 1A
shows that Gln treatment (4–16 mM) for 24 h significantly
upregulated Hsp70 mRNA expression (P<0.05 for 4 mM, P<0.01
for 8 mM and P<0.01 for 16 mM). Of note, the Gln-mediated
upregulation of Hsp70 expression was dose dependent, as there was a
significant difference in Hsp70 mRNA levels between the 4 and 8 mM
Gln groups, as well as between the 8 and 16 mM Gln groups. The
time-dependence of the Hsp70 upregulation by Gln was also
confirmed; Fig. 1B indicates that
Hsp70 mRNA was upregulated at 12 h post-Gln treatment (4 mM) and
peaked 24 h later, and there was a significant difference in Hsp70
mRNA levels between cells treated for 12 h and cells treated for 24
h (P<0.05). To reconfirm the upregulation, the Hsp70 protein
levels in SH-SY5Y cells post-Gln treatment were also examined.
Fig. 1C and D show that Gln
treatment at 4–16 mM for 24 h or at 4 mM for 24–48 h also promoted
the protein expression of Hsp70 in a dose- and time-dependent
manner (P<0.05 or P<0.01).
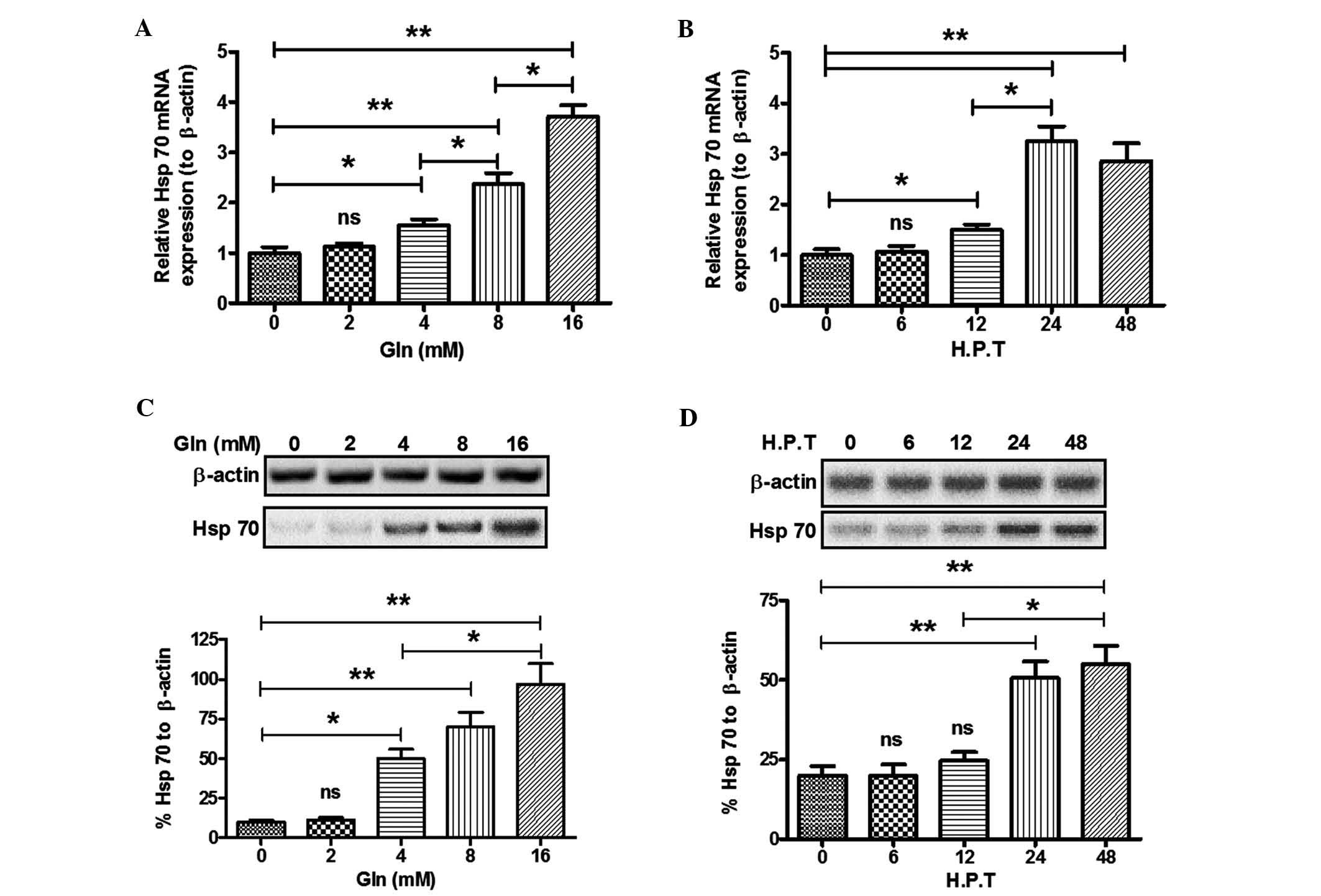 | Figure 1Glutamine treatment upregulates Hsp70
expression in SH-SY5Y neuroblastoma cells. (A) Hsp70 mRNA levels in
SH-SY5Y cells post-Gln treatment at various concentrations (0, 2,4,
8 or 16 mM) for 24 h; (B) Hsp70 mRNA levels in SH-SY5Y cells
post-Gln treatment (4 mM) for various durations [0, 6, 12, 24 or 48
h post treatment (HPT)]; (C) Western blot analysis of Hsp70 in
SH-SY5Y cells post-Gln treatment (0, 2, 4, 8 or 16 mM) for 24 h.
HSP70 levels were normalized to β-actin. (D) Hsp70 protein
expression in SH-SY5Y cells post-Gln treatment (4 mM) for various
durations (0, 6, 12, 24 or 48 HPT). Values are expressed as the
mean ± standard error of triple experiments. *P<0.05,
**P<0.01. ns, no significance; HSP, heat shock
protein; gln, glutamine. |
Upregulation of Hsp70 by Gln in SH-SY5Y
cells is HSF-1-dependent
In previous studies, HSF has been confirmed to bind
to a target sequence, the heat shock element (HSE), located in the
promoters of heat-induced genes and promote the expression of HSPs,
including Hsp70 (29–31). In order to identify the signaling
pathways of Gln-induced upregulation of Hsp70 expression in SH-SY5Y
cells, the present study investigated the effect of HSF-1 knockdown
on Gln-induced Hsp70 expression. RNA interference technology was
adopted to knockdown HSF-1 expression, and the results shown in
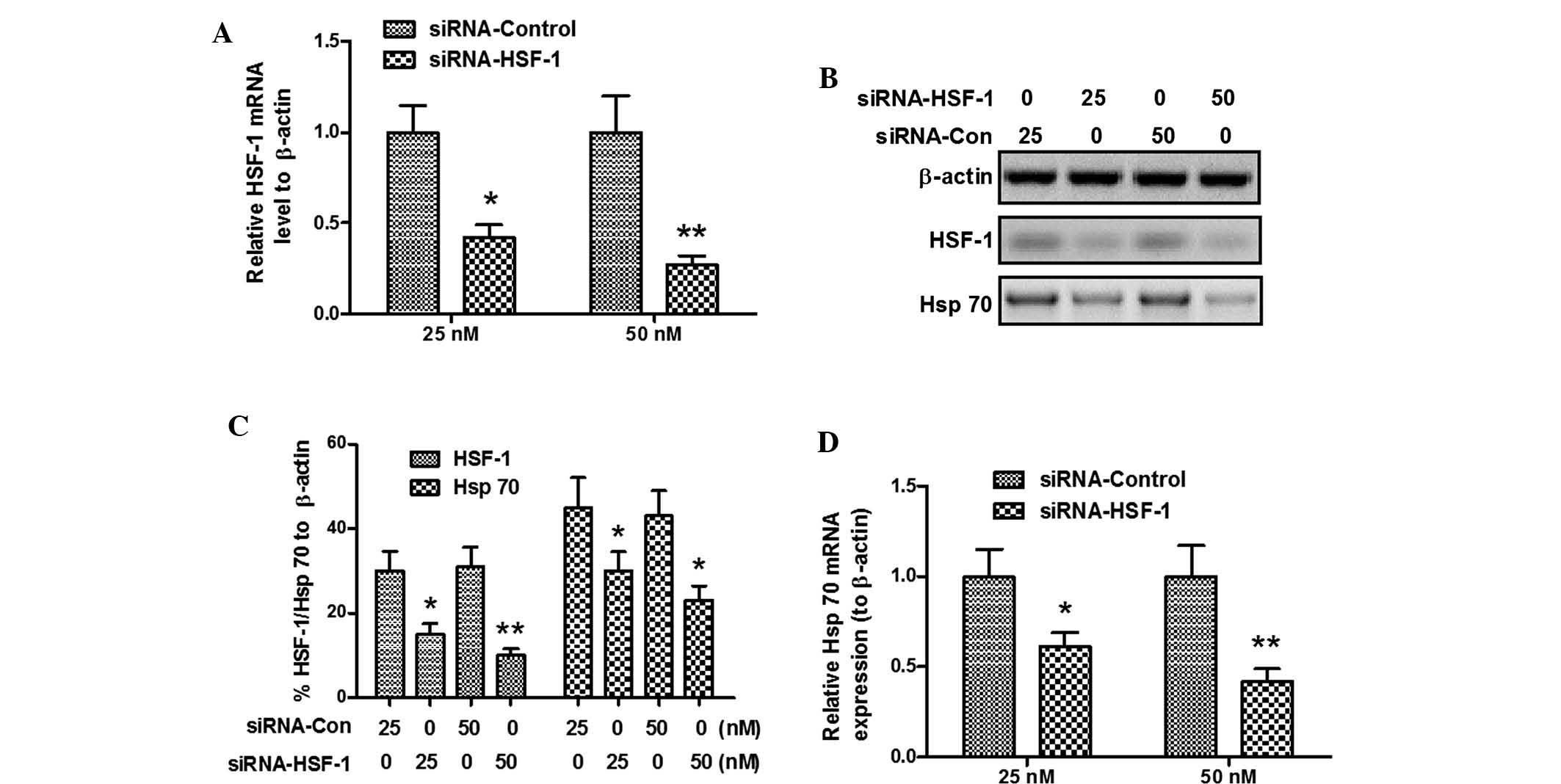
Fig. 2A demonstrated that
HSF-1-specific siRNA, siRNA-HIF-1, significantly downregulated the
HSF-1 mRNA expression (P<0.05 for 25 nM and P<0.01 for 50 nM)
in SH-SY5Y cells following Gln treatment (8 mM for 24 h). HSF-1
protein levels were also downregulated following siRNA-HSF-1
transfection (P<0.05 or P<0.01, respectively; Fig. 2B and C). Furthermore, the Hsp70 was
also significantly downregulated at the protein (P<0.05;
Fig. 2B and C) and mRNA (P<0.05
for 25 nM and P<0.01 for 50 nM; Fig. 2B and C) level, compared to that in
the siRNA control-transfected cells. These results confirmed that
the Gln-induced Hsp70 expression was HSF-1-dependent.
α-Syn overexpression in SH-SY5Y cells has
no influence on Hsp70 and HSF-1 expression
Functional defects of HSPs are thought to have a key
role in PD (21,22). Hsp70 is the most investigated
molecular chaperone and is known to negatively regulate α-Syn
aggregation in PD and to mediate α-Syn-induced toxicity in cells
(22,23). To explore the influence of Hsp70 on
α-Syn degradation following Gln-induced Hsp70 upregulation, the
present study constructed an SH-SY5Y cell clone which
overex-pressed wild-type α-Syn, termed SH-SY5Y (Syn+). The coding
sequence of wild-type α-Synn was cloned into the eukaryotic
expression vector, pcDNA3.1(+). The α-Syn-overexpressing SH-SY5Y
cell clone was selected using 800 μg/ml G418 and maintained
in complete medium containing 500 μg/ml G418. Significantly
higher and stably expressed levels of α-Syn mRNA were detected in
the SH-SY5Y (Syn+) cells following various passages (P<0.01;
Fig. 3A). α-Syn expression at the
protein level was also significantly upregulated in the SH-SY5Y
(Syn+) cells (P<0.01) (Fig. 3B and
C) according to western blot analysis. Furthermore, α-Syn
overexpression did not vary among various passages (Fig. 3A–D). To further investigate the
influence of α-Syn overexpression on Hsp70 and HSF-1, the mRNA
expression of Hsp70 and HSF-1 was assessed by RT-qPCR and their
protein levels by western blot analysis. Fig. 3A, B and D demonstrates that there
was no difference in the mRNA and protein levels of Hsp70 and HSF-1
between SH-SY5Y cells transfected with the control CAT-pcDNA3.1(+)
and SH-SY5Y (Syn+) cells, or among various passages of SH-SY5Y
(Syn+) cells. Thus, the stably α-Syn-overexpressing SH-SY5Y cells
are suitable to be used for investigating the influence of Hsp70
and HSF-1 on α-Syn degradation.
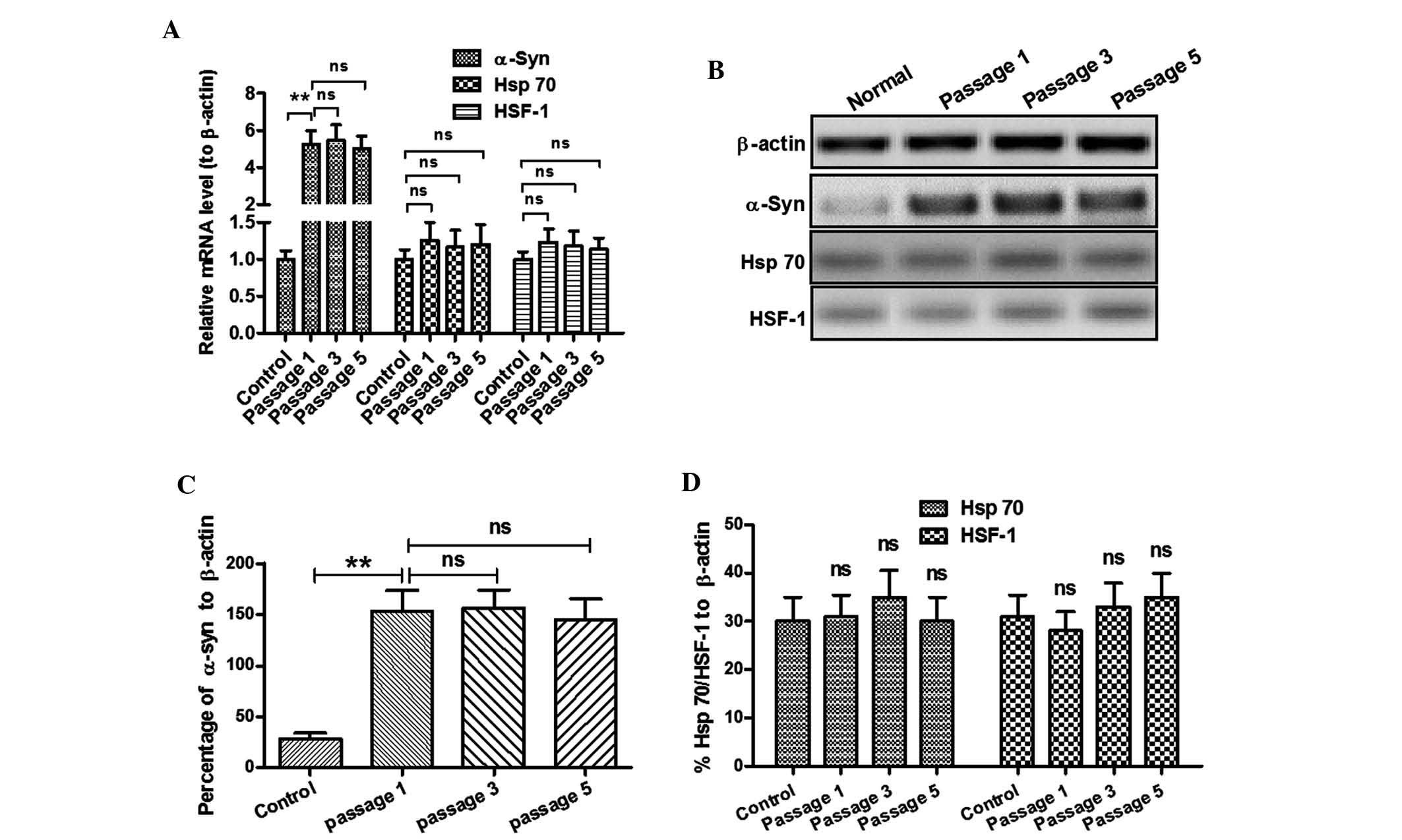 | Figure 3Expression of HSF-1 and Hsp70 in
SH-SY5Y (Syn+) cells. (A) mRNA levels of α-Syn, HSF-1 and Hsp70 in
SH-SY5Y (Syn+) cells at various passages. (B) Western blot analysis
of α-Syn, HSF-1 and Hsp70 protein levels in SH-SY5Y (Syn+) cells at
various passages. (C) Stable overexpression of α-Syn protein in
SH-SY5Y (Syn+) cells at various passages; (D) Protein expression of
HSF-1 or Hsp70 was not dependent on α-Syn overexpression.
Normal/Control: CAT-pcDNA3.1(+)-transfected SH-SY5Y cells; passage
1, 3 or 5: SH-SY5Y (Syn+) cells which were propagated for 1, 3 or 5
passages. Values are expressed as the mean ± standard deviation
(n=3). **P<0.01. ns, no significance; SH-SY5Y (Syn+),
α-Syn-overexpressing SH-SY5Y cells; HSP, heat shock protein; HSF,
heat shock factor; Gln, glutamine; α-Syn, α-synuclein. |
Upregulation of Hsp70 by Gln increases
α-Syn degradation in SH-SY5Y (Syn+) cells
First, the possible regulation of α-Syn expression
in mRNA expression by Gln was investigated by RT-qPCR. Fig. 4A shows that 8 mM Gln had no effect
on α-Syn mRNA levels in the SH-SY5Y (Syn+) cells at 6–12 h
post-treatment. Furthermore, the protein levels of α-Syn in SH-SY5Y
(Syn+) cells post-Gln treatment were investigated. Fig. 4B demonstrates that Gln treatment
for 24 h reduced the protein levels of α-Syn (P<0.05 for 4 mM
and P<0.01 for 8 and 16 mM). A dose-dependence of the α-Syn
reduction was observed, as there was a significant difference
between the 4- and 8 mM Gln groups (P<0.05) as well as between
the 8- and 16 mM Gln groups (P<0.05) (Fig. 4B). In addition, the present study
investigated the effect of HSF-1 knockdown on the α-Syn reduction
by Gln treatment. The results showed that 25 or 50 nM siRNA-HIF-1
inhibited the α-Syn reduction by Gln compared to that in cells
transfected with the control siRNA (P<0.01 for either
concentration) (Fig. 4C and D).
These results indicated that Gln treatment promoted α-Syn
degradation in SH-SY5Y (Syn+) cells, which was HSF-1-dependent.
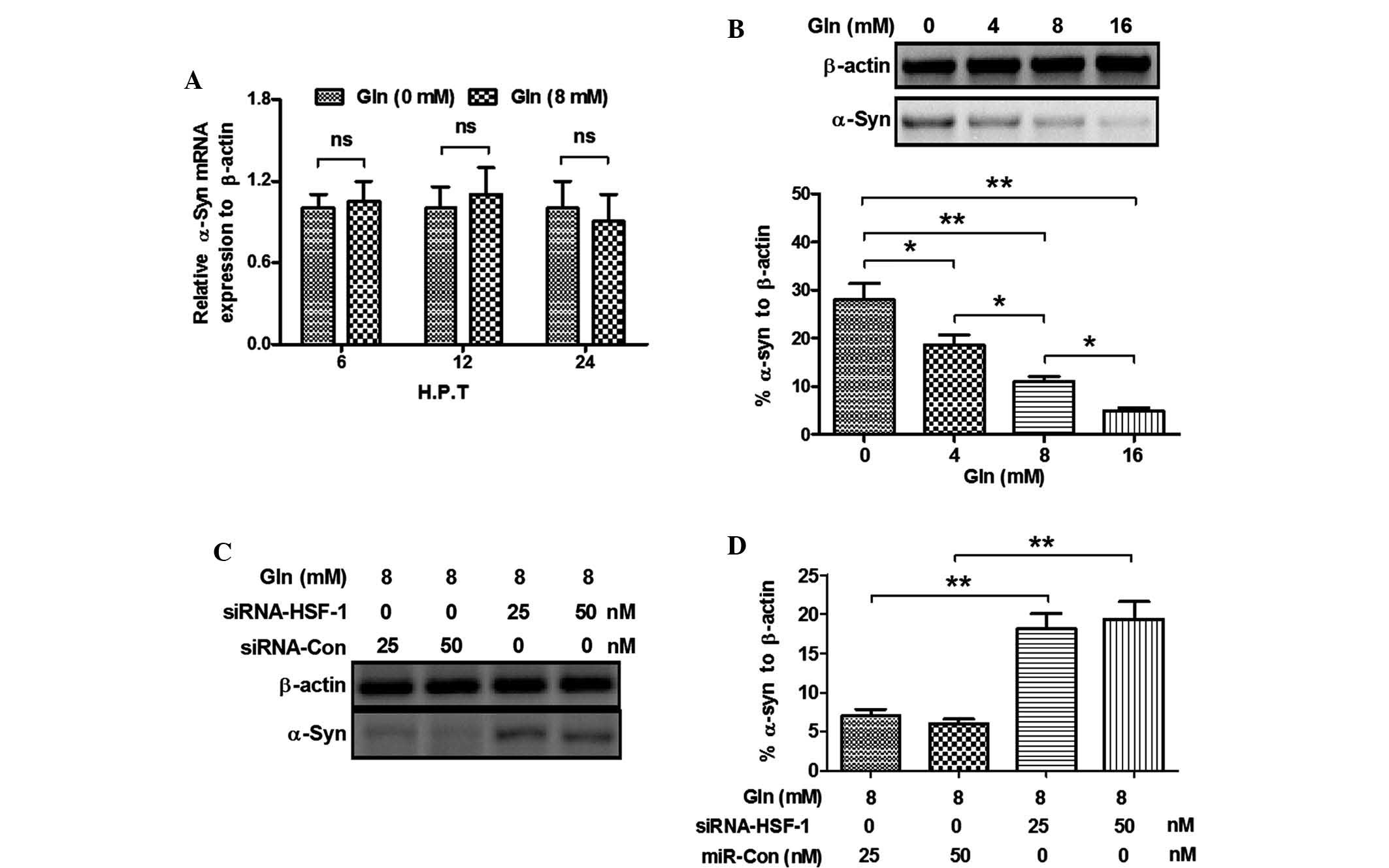 | Figure 4Gln-induced Hsp70 upregulation
increases α-Syn degradation. (A) mRNA levels of α-Syn in the
SH-SY5Y (Syn+) cells with or without 8 mM Gln treatment for 6, 12
or 24 h; (B) Western blot analysis indicated a significant
reduction of α-Syn levels the SH-SY5Y (Syn+) cells post-Gln
treatment (4, 8 or 16 mM for 24 h); (C) Western blot analysis of
α-Syn levels in the SH-SY5Y (Syn+) cells post-Gln treatment and/or
siRNA-HSF-1 transfection. (D) α-Syn reduction by Gln treatment in
the SH-SY5Y (Syn+) cells was abrogated by siRNA-HSF-1 transfection.
All experiments were performed in triplicate.
*P<0.05; **P<0.01. ns, no significance;
siRNA, small interfering RNA; Con, control; SH-SY5Y (Syn+),
α-Syn-overexpressing SH-SY5Y cells; HSP, heat shock protein; HSF,
heat shock factor; Gln, glutamine; α-Syn, α-synuclein. |
Discussion
Accumulating evidence supports the key role of
impaired α-Syn degradation pathways (18,19),
followed by amyloid-like aggregation of α-Syn, in PD (32,33).
The importance of molecular chaperones has been underlined by the
fact that overexpression of these molecules, including HSPs, leads
to re-folding of the aberrant α-Syn aggregates to generate
non-toxic and non-aggregated α-Syn (20,21).
Therefore, functional defects of HSPs may have key roles in PD
(21,22), and promotion of HSP expression may
be a potential strategy to prevent or ameliorate the aberrant α-Syn
aggregation, and thus control the progression of PD. Hsp70 is the
most recognized molecular chaperone and has been linked with PD and
α-Syn aggregation. Substantial studies have confirmed the
preventive role of Hsp70 in α-Syn aggregation in PD (22,23).
Hsp70 is subject to transcriptional regulation upon various
stresses and is regulated by a variety of molecules (34–36).
Stress-inducible protein 1, an Hsp70/Hsp90-organizing protein, was
confirmed to independently regulate the expression of Hsp70
(34); Parathyroid hormone
activates adenylate cyclase and phospho-lipase C and subsequently
promotes Hsp70 expression (35).
Phorbol esters were also reported to deregulate the expression of
Hsp70 and Hsp90 (36). Therefore,
investigation of the deregulation of Hsp70 and its influence on
α-Syn degradation may shed light on the pathogenesis of PD.
HSF-1 has also been reported to be activated in
response to chemical or thermal stresses and to promote the
expression of HSPs, including Hsp70 (37–39).
Following a cascade of post-translational modifications, including
trimerization, nuclear translocation, DNA binding, and
phosphorylation of its transactivation domain, activated HSF-1
binds to conserved regulatory sequences known as heat shock
response elements and promotes HSP transcription (40,41).
Gln has been shown to safely enhance HSP expression in in
vitro and in vivo settings (25–28).
Gln mediates cellular protection against heat-stress injury to the
lung via promoting HSF-1 expression, increasing HSF-1 promoter
binding and phosphorylation, and then activating an HSP response
(25). The protective effect of
Gln was also confirmed in vivo, and was shown to proceed
through the enhancement of HSF-1 phosphorylation/activation and
promotion of HSP expression (26),
particularly the promotion of Hsp70 expression (27). However, to the best of our
knowledge, the protective effects of Gln against PD have not yet
reported.
The present study reported the upregulation of Hsp70
expression by Gln in SH-SY5Y neuroblastoma cells. Gln treatment
significantly upregulated Hsp70 expression at the mRNA and protein
level in a dose-dependent and time-dependent manner. Given the key
regulatory role of HSF-1 in Hsp70 expression, the effect of Gln on
Hsp70 expression was re-evaluated following HSF-1 knockdown. It was
shown that HSF-1-specific siRNA blocked HSF-1 expression at the
mRNA and protein level, and this HSF-1 blockage blunted the
upregulation of Hsp70 by Gln in the SH-SY5Y cells. The results
therefore confirmed that the Gln-induced Hsp70 upregulation was
HSF-1-dependent. Furthermore, the present study demonstrated that
the Gln-induced Hsp70 upregulation facilitated the degradation of
α-Syn, while it had no influence on α-Syn mRNA levels in SH-SY5Y
(Syn+) cells, implying a novel strategy for preventing the
progression of PD, which is thought to be caused by functional
defects of HSPs, impairing the degradation of α-Syn (21,22).
In conclusion, the present study confirmed that Gln
upreg-ulated Hsp70 expression in SH-SY5Y neuroblastoma cells in an
HSF-1-dependent manner. The upregulation of Hsp70 expression by
glutamine increased the degradation of α-Syn in
α-Syn-overexpressing SH-SY5Y cells. Therefore, Gln may be a
potential therapeutic agent to prevent α-Syn aggregation in PD. The
use of Gln for the treatment and prevention of PD requires further
investigation; in particular, Gln-mediated upregulation of HSP70
expression and α-Syn degradation require validation in
vivo.
Acknowledgments
The present study was supported by a grant from
Renmin Hospital of Wuhan University (Wuhan, China).
References
|
1
|
Bertram L and Tanzi RE: The genetic
epidemiology of neurode-generative disease. J Clin Invest.
115:1449–1457. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wirdefeldt K, Adami HO, Cole P,
Trichopoulos D and Mandel J: Epidemiology and etiology of
Parkinson's disease: A review of the evidence. Eur J Epidemiol.
26(Suppl 1): S1–S58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Irizarry MC, Growdon W, Gomez-Isla T,
Newell K, George JM, Clayton DF and Hyman BT: Nigral and cortical
Lewy bodies and dystrophic nigral neurites in Parkinson's disease
and cortical lewy body disease contain alpha-synuclein
immunoreactivity. J Neuropathol Exp Neurol. 57:334–337. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spillantini MG, Crowther RA, Jakes R,
Hasegawa M and Goedert M: Alpha-Synuclein in filamentous inclusions
of Lewy bodies from Parkinson's disease and dementia with lewy
bodies. Proc Natl Acad Sci USA. 95:6469–6473. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jellinger KA: Neuropathology of sporadic
Parkinson's disease: Evaluation and changes of concepts. Mov
Disord. 27:8–30. 2012. View Article : Google Scholar
|
|
6
|
Kruger R, Kuhn W, Muller T, Woitalla D,
Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L and Riess O:
Ala30Pro mutation in the gene encoding alpha-synuclein in
Parkinson's disease. Nat Genet. 18:106–108. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chartier-Harlin MC, Kachergus J, Roumier
C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J,
Hulihan M, et al: Alpha-synuclein locus duplication as a cause of
familial Parkinson's disease. Lancet. 364:1167–1169. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uéda K, Fukushima H, Masliah E, Xia Y,
Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y and Saitoh T:
Molecular cloning of cDNA encoding an unrecognized component of
amyloid in Alzheimer disease. Proc Natl Acad Sci USA.
90:11282–11286. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jakes R, Spillantini MG and Goedert M:
Identification of two distinct synucleins from human brain. Febs
Lett. 345:27–32. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mueller A, Ziegler K, Amsharov KY and
Jansen M: Perchloropyracylene and its fusion with C60 by
chlorine-assisted radio-frequency furnace synthesis. Chemistry.
17:11797–11804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartels T, Choi JG and Selkoe DJ:
α-Synuclein occurs physiologically as a helically folded tetramer
that resists aggregation. Nature. 477:107–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chandra S, Gallardo G, Fernández-Chacón R,
Schlüter OM and Südhof TC: Alpha-synuclein cooperates with CSPalpha
in preventing neurodegeneration. Cell. 123:383–396. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fauvet B, Mbefo MK, Fares MB, Desobry C,
Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, et al:
α-Synuclein in central nervous system and from erythrocytes,
mammalian cells and Escherichia coli exists predominantly as
disordered monomer. J Biol Chem. 287:15345–15364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eichner T and Radford SE: A diversity of
assembly mechanisms of a generic amyloid fold. Mol Cell. 43:8–18.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conway KA, Harper JD and Lansbury PT:
Accelerated in vitro fibril formation by a mutant alpha-synuclein
linked to early-onset Parkinson disease. Nat Med. 4:1318–1320.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alvarez-Erviti L, Seow Y, Schapira AH,
Rodriguez-Oroz MC, Obeso JA and Cooper JM: Influence of microRNA
deregulation on chaperone-mediated autophagy and α-synuclein
pathology in Parkinson's disease. Cell Death Dis. 4:e5452013.
View Article : Google Scholar
|
|
17
|
Singleton AB, Farrer M, Johnson J,
Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra
A, Nussbaum R, et al: alpha-Synuclein locus triplication causes
Parkinson's disease. Science. 302:8412003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cuervo AM, Stefanis L, Fredenburg R,
Lansbury PT and Sulzer D: Impaired degradation of mutant
alpha-synuclein by chaperone-mediated autophagy. Science.
305:1292–1295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alvarez-Erviti L, Rodriguez-Oroz MC,
Cooper JM, Caballero C, Ferrer I, Obeso JA and Schapira AH:
Chaperone-mediated autophagy markers in Parkinson disease brains.
Arch Neurol. 67:1464–1472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Periquet M, Fulga T, Myllykangas L,
Schlossmacher MG and Feany MB: Aggregated alpha-synuclein mediates
dopaminergic neurotoxicity in vivo. J Neurosci. 27:3338–3346. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Auluck PK, Chan HY, Trojanowski JQ, Lee VM
and Bonini NM: Chaperone suppression of alpha-synuclein toxicity in
a Drosophila model for Parkinson's disease. Science. 295:865–868.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klucken J, Shin Y, Masliah E, Hyman BT and
McLean PJ: Hsp70 reduces alpha-synuclein aggregation and toxicity.
J Biol Chem. 279:25497–25502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luk KC, Mills IP, Trojanowski JQ and Lee
VM: Interactions between Hsp70 and the hydrophobic core of
alpha-synuclein inhibit fibril assembly. Biochemistry.
47:12614–12625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Morrison AL, Dinges M, Singleton KD, Odoms
K, Wong HR and Wischmeyer PE: Glutamine's protection against
cellular injury is dependent on heat shock factor-1. Am J Physiol
Cell Physiol. 290:C1625–C1632. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singleton KD, Serkova N, Beckey VE and
Wischmeyer PE: Glutamine attenuates lung injury and improves
survival after sepsis: Role of enhanced heat shock protein
expression. Crit Care Med. 33:1206–1213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singleton KD and Wischmeyer PE:
Glutamine's protection against sepsis and lung injury is dependent
on heat shock protein 70 expression. Am J Physiol Regul Integr Comp
Physiol. 292:R1839–R1845. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wischmeyer PE, Kahana M, Wolfson R, Ren H,
Musch MM and Chang EB: Glutamine induces heat shock protein and
protects against endotoxin shock in the rat. J Appl Physiol (1985).
90:2403–2410. 2001.
|
|
29
|
Pelham HR: A regulatory upstream promoter
element in the Drosophila hsp 70 heat-shock gene. Cell. 30:517–528.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amin J, Ananthan J and Voellmy R: Key
features of heat shock regulatory elements. Mol Cell Biol.
8:3761–3769. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sorger PK: Heat shock factor and the heat
shock response. Cell. 65:363–366. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gupta A, Dawson VL and Dawson TM: What
causes cell death in Parkinson's disease? Ann Neurol. 64(Suppl 2):
S3–S15. 2008. View Article : Google Scholar
|
|
33
|
Jellinger KA: Basic mechanisms of
neurodegeneration: A critical update. J Cell Mol Med. 14:457–487.
2010.PubMed/NCBI
|
|
34
|
Song Y and Masison DC: Independent
regulation of Hsp70 and Hsp90 chaperones by Hsp70/Hsp90-organizing
protein Sti1 (Hop1). J Biol Chem. 280:34178–34185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fukayama S, Lanske B, Guo J, Kronenberg HM
and Bringhurst FR: Regulation of HSP70 by PTH: A model of gene
regulation not mediated by changes in cAMP levels. Am J Physiol.
271:C121–C129. 1996.PubMed/NCBI
|
|
36
|
Jacquier-Sarlin MR, Jornot L and Polla BS:
Differential expression and regulation of hsp70 and hsp90 by
phorbol esters and heat shock. J Biol Chem. 270:14094–14099. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sorger PK: Heat shock factor and the heat
shock response. Cell. 65:363–366. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sarge KD, Murphy SP and Morimoto RI:
Activation of heat shock gene transcription by heat shock factor 1
involves oligomerization, acquisition of DNA-binding activity and
nuclear localization and can occur in the absence of stress. Mol
Cell Biol. 13:1392–1407. 1993.PubMed/NCBI
|
|
39
|
Cotto JJ, Kline M and Morimoto RI:
Activation of heat shock factor 1 DNA binding precedes
stress-induced serine phosphory-lation. Evidence for a multistep
pathway of regulation. J Biol Chem. 271:3355–3358. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sasi BK, Sonawane PJ, Gupta V, Sahu BS and
Mahapatra NR: Coordinated transcriptional regulation of Hspa1a gene
by multiple transcription factors: Crucial roles for HSF-1, NF-Y,
NF-κB and CREB. J Mol Biol. 426:116–135. 2014. View Article : Google Scholar
|
|
41
|
Tetievsky A and Horowitz M:
Posttranslational modifications in histones underlie heat
acclimation-mediated cytoprotective memory. J Appl Physiol (1985).
109:1552–1561. 2010. View Article : Google Scholar
|