Introduction
Immunoglobulin A nephropathy (IgAN) is considered to
be the most common type of glomerular disease worldwide (1). IgAN has been confirmed to be an
immune complex-mediated glomerulonephritis, defined morphologically
by the mesangial deposition of IgA (2,3).
Although defects in immune regulation are considered to be
important in the pathogenesis of IgAN, the pathogenetic mechanisms
remain to be fully elucidated. Previous studies have reported that
IgAN is regulated by B lymphocytes (4,5), and
increased numbers of T helper (CD4) lymphocytes and reduced numbers
of T suppressor (CD8) lymphocytes are associated with the
exacerbation of IgAN (6,7). However, overproduction of IgA is most
likely the consequence of the involvement of both T and B
lymphocytes.
The present study aimed to explore immune status
alterations during the progression of IgAN disease. The
distributions of different B-cell subsets and Tfh cells were
analyzed in patients with IgAN at various disease phases, and the
differential contributions of these lymphocytes to IgAN were
evaluated. To explain the imbalance of B-cell subsets, the main
effector of Tfh cells, IL-21 was also investigated.
IL-21 is not a classic T helper cell 1 or 2
cytokine, but is mainly produced by CD4+ follicular T helper cells,
which can be identified by their expression of the chemokine
receptor CXCR5. The high levels of IL-21 receptor (IL-21R)
expressed by B cells make B cells prime responders to IL-21. B
cells faced with IL-21 in the context of antigen-specific BCR
stimulation and T cell co-stimulation undergo class switch
recombination and differentiate into antibody producing plasma
cells (8,9). Activation-induced cytidine deaminase
(AID) induces somatic hypermutation, gene conversion, and
class-switch recombination of immunoglobulin genes in B cells
(10,11). Thus, it is important to clarify the
association between IL-21 and AID expression, which may aid
understanding of the functions of T helper cells and different
B-cell subsets in the process of IgAN disease.
Materials and methods
Patients
A total of 27 patients diagnosed with IgAN were
recruited for investigation from the Department of Gastroenterology
at the Affiliated Hospital of Changchun University of Chinese
Medicine (Changchun, China) between 2012 and 2013. The diagnoses of
IgAN in the patients were confirmed by biopsy and the presence of
proteinuria. The patients with IgAN were divided into two groups,
according to the extent of proteinuria: Group A (n=12), proteinuria
<4 g/24 h; group B (n=15), proteinuria ≥4 g/24 h. Patients who
had received immunosuppressive therapies in the 6 months preceding
the investigation were excluded. A total of 10 gender- and
age-matched healthy volunteers were selected as the HC group, each
of which were recruited from the Department of Physical Examination
Center of the Affiliated Hospital of Changchun University of
Chinese Medicine. None of the patients or controls had any systemic
disorders, viral infections or other autoimmune diseases. All study
participants provided written informed consent, and the ethical
committee of the Affiliated Hospital of Changchun University of
Chinese Medicine approved the experiment. The clinical
characteristics of the patients and HC individuals are presented in
Table I.
 | Table IClinical and pathological
manifestations of the patients with IgAN and the HC
individuals. |
Table I
Clinical and pathological
manifestations of the patients with IgAN and the HC
individuals.
| Parameter | IgAN group
A
(24-h proteinuria <4 g) | IgAN group
B
(24-h proteinuria ≥4 g) | HC |
|---|
| Number | 12 | 15 | 10 |
| Age (years) | 58 (40–63) | 47 (30–66) | 27 (18–40) |
| Female/male | 5/7 | 8/7 | 4/6 |
| Serum IgA
(g/l) | 4.97
(4.36–7.81)a | 5.85
(4.08–6.22)a | 1.82
(1.13–2.77) |
| Serum uric acid
(mol/l) | 343 (247–634) | 357 (271–603) | 320 (230–370) |
| eGFR (ml/min/1.73
m2) | 71 (32–89)a | 56 (40–126)a | 103 (90–129) |
PBMC culture and stimulation
Peripheral blood mononuclear cells (PBMCs) were
isolated from venous blood samples of all patients using standard
Ficoll-Paque Plus (GE Healthcare Life Sciences, Pittsburgh, PA,
USA) density-gradient centrifugation (1,500 × g for 10 min at room
temperature). Subsequent to washing in phosphate-buffered saline
(PBS), the PBMCs were diluted at 4×106/ml in Dulbecco's
modified Eagle's medium (GE Healthcare Life Sciences, Logan, UT,
USA), containing 10% fetal bovine serum (Gibco Life Technologies,
Carlsbad, CA, USA) and penicillin-streptomycin (100 U/ml) solution
(GE Healthcare Life Sciences) and were cultured in 24-well plates
(Corning Incorporated, Corning, NY, USA). The isolated PBMCs were
cultured with CpGB (3 µg/ml; R&D Systems, Inc.,
Minneapolis, MN, USA) and 10 ng/ml recombinant interleukin (IL)-2
(R&D Systems, Inc.) for 72 h to detect B cell sub-populations.
In order to stimulate T cells, 50 ng/ml phorbol myristate acetate,
1.0 mg/ml ionomycin and 5.0 mg/ml lipopolysaccharide (all from
Sigma-Aldrich, St. Louis, MO, USA) were cultured with the PBMCs for
2 h at 37°C. Subsequently, Brefeldin A (BD Biosciences, Franklin
Lakes, NJ, USA) was added to each well and they were incubated for
an additional 4 h at 37°C.
Flow cytometric analysis
To analyze the distribution of different B cells
subsets and Tfh cells, the PBMCs were stimulated in vitro,
as described above, and then harvested, washed with ice-cold PBS,
and stained with fluorescein-labeled monoclonal antibodies (1:500
dilution) against various B cell markers [anti-CD19 PerCP (cat. no.
340421), anti-CD27 APC (cat. no. 561786), anti-CD86 cy5.5 (cat. no.
561129), anti-CD138 fluorescein isothiocyanate (cat. no. 561703)]
and Tfh cells [anti-CXCR5 PerCP (cat. no. 562781) and anti-CD4 APC
(cat. no. 340443)]. All antibodies were from BD Pharmingen (San
Diego, CA, USA). The control PBMCs were cultured in medium alone.
Following a 1 h incubation with the primary antibodies at room
temperature, the cells were washed with PBS, and at least 20,000
events were recorded. The data was obtained using a FACSCalibur
analytical instrument (BD Biosciences) and four-color analysis was
performed using FlowJo software, version 7.6 (FlowJo, LLC, Ashland,
OR, USA).
ELISA
The serum level of IL-21 was quantified in the IgAN
patients and HC individuals using a Human IL-21 ELISA kit,
according to the manufacturers' instructions (Roche Diagnostics,
Ltd, Burgess Hill, UK). The plate was read at 450 nm using an
Infinite M200pro plate reader (Tecan, Männedorf, Switzerland) and
the sensitivity of the ELISA kits used in the experiment was 19
pg/ml. All samples were analyzed in duplicate using the average
optical density values to calculate the concentrations.
Determination of the effect of IL-21 on
levels of AID
To detect the expression of AID, the B cells were
collected from the PBMCs of the HC group, were stained with
anti-CD19 PerCP and anti-CD3 PE antibodies, and were sorted using a
FACSAria flow cytometer (BD Biosciences). The purified B cells were
identified as CD3−CD19+ cells. The isolated B cells were cultured
with recombinant human IL-21 (20 ng/ml; Peprotech, Inc., Rocky
Hill, NJ, USA), CpGB (3 µg/ml) and recombinant IL-2 (10
ng/ml) for 72 h, and were then lysed on ice for 30 min in
radioimmunoprecipitation assay buffer (Beytoime Institute of
Biotechnology, Haimen, China), containing 50 mM Tris-HCl (pH 7.4),
150 mM NaCl, 0.1% SDS, 1% deoxycholate, 1% Triton X-100, 1 mM EDTA,
5 mM NaF, 1 mM sodium vanadate and protease inhibitor cocktail.
Following quantification of the proteins with a Bicinchoninic Acid
Protein Assay kit (Beyotime Institute of Bioechnology), the total
proteins (70 µg) were then subjected to 10% SDS-PAGE
(Beyotime Institute of Biotechnology), and transferred onto a
nitrocellulose membrane (Pall Corporation, Port Washington, NY,
USA). The membrane was incubated with primary antibodies targeting
AID and GAPDH (1:2,000 dilution; cat. nos. sc-25620 and sc-25778;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The
animal-matched horseradish peroxidase-conjugated secondary antibody
was also purchased from Santa Cruz Biotechnology, Inc. (1:2,000
dilution; cat. no. sc-2370). The total RNA of these cultured cells
was extracted using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). Total RNA (1 µg) was used for cDNA
synthesis using the RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). PCR was
performed using Hot Start Taq DNA Polymerase (Takara Bio
Inc., Otsu, Japan), and PCR cycling conditions were as follows:
94°C for 3 min, followed by 35 cycles at 94°C for 30 sec, 58°C for
30 sec and 72°C for 45 sec, and a final extension step at 72°C for
10 min. Differences in the expression levels of cDNA were
normalized to GAPDH. The primer [Generay Biotech (Shanghai) Co.,
Ltd., Shanghai, China] sequences were as follows: AID, forward
5′-CAATAAGAACGGCTGCCAC -3′ and reverse 5′-TTGCGGTCCTCACAGAAGTAG-3′;
and GAPDH, forward 5′-CACCAACTGGGACGACAT-3′ and reve rse
5′-ACAGCCTGGATAGCAACG-3′.
Statistical analysis
All data are expressed as the mean ± standard
deviation in the text and figures. Statistical analyses were
conducted using GraphPad Prism 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA). Significant differences were calculated
using the two-tailed, unpaired Student's t-test, with a 95%
confidence interval. P<0.05 (two-tailed) was considered to
indicate a statistically significant difference.
Results and Discussion
Increased frequencies of different B cell
subsets in IgAN
IgAN is characterized by circulating immune
complexes, composed of galactose-deficient IgA1 and a
glycan-specific IgG antibody (12,13),
which is secreted by plasma cells. To determine the immune status
of B cells in IgAN, 27 patients with IgAN were recruited in the
present study and were divided into two groups: Group A,
proteinuria/24 h <4 g and group B, proteinuria/24 h ≥4 g. No
statistically significant differences were observed in the level of
glomerular filtration rate or the distribution of age and gender
between the IgAN groups and the HC group (Table I).
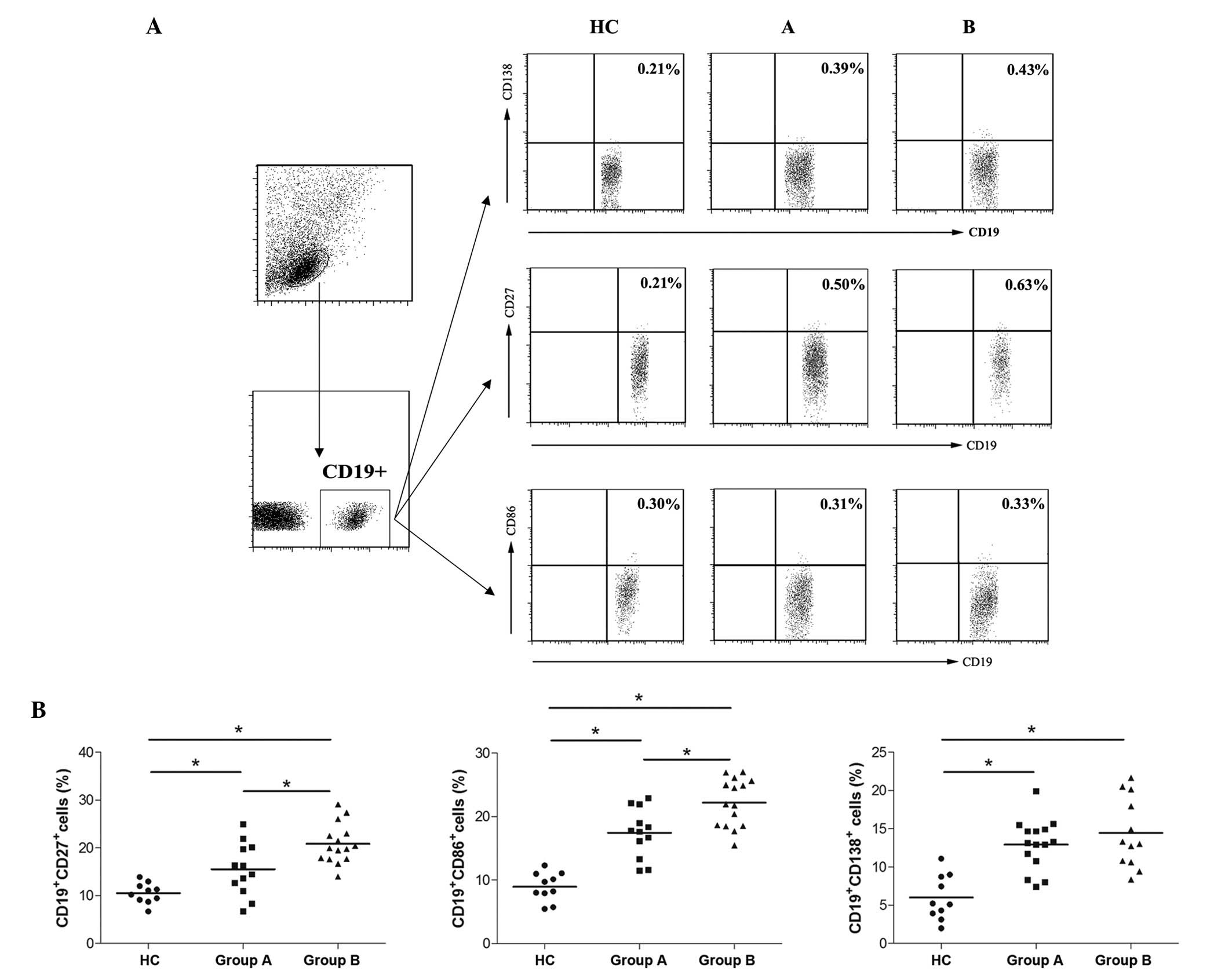
Marked increases in the percentages of CD19+CD138+
plasma cells, CD19+CD27+ memory B cells and CD19+CD86+ activated B
cells (14–16) were observed in the IgAN groups,
compared with the HC group. Although no significant difference in
the percentage of plasma B cells was observed between the two IgAN
groups, higher levels of memory and activated B cells were observed
in the IgAN group B, compared with the group A (Fig. 1). These data indicated that the
frequencies of memory and activated B cells were associated with
the progression of IgAN.
Frequencies of circulating Tfh cells are
higher in IgAN
Tfh cells are a subset of T cells that localize in
the GC and specialize in assisting B cell growth, differentiation
and class switching (17). Tfh
cells are characterized by increased expression levels of
molecules, including CXCR5, PD-1, ICOS, CD40L and IL-21, and
reduced expression of CCR7. The CD4+CXCR5+ T cells have been
previously identified as Tfh cells (18–20),
and the present study demonstrated that the frequency of CD4+CXCR5+
Tfh cells was significantly higher in the two IgAN groups, compared
with that in the HC group. In addition, the percentage of Tfh cells
was higher in the IgAN group B than group A (Fig. 2). These data indicated that high
levels of Tfh cells may accelerate IgAN exacerbation by promoting
the proliferation and function of B cells.
Serum IL-21 levels are increased in
patients with IgAN
As a key effector of Tfh cells, IL-21 is involved in
the process of promoting the growth, differentiation and class
switching of B cells (21,22). Due to the high frequency of Tfh
cells observed in the patients with IgAN, the concentration of
IL-21 in the serum was also determined in the in above-mentioned
groups. The results of the ELISA indicated that the concentration
of serum IL-21 in group B was significantly higher than that in
either group A or the HC group (Fig.
3A). Furthermore, the serum levels of IL-21 were positively
correlated with the extent of 24-h proteinuria (Fig. 3B), which indicated serum IL-21 may
act as a biomarker for IgAN.
Effect of IL-21 on AID in IgAN
AID belongs to the APOBEC family of cytidine
deaminases and is capable of deaminating dCs into dUs in
vitro on ssDNA substrates and ssDNA generated by the formation
of RNA-DNA hybrids (10,23). The key function of AID is to induce
somatic hypermutation (SHM), Ig class-switch recombination (CSR)
and gene conversion (11,24,25).
IgA class switching occurs in IgAN (26,27),
therefore, the present study investigated whether the high levels
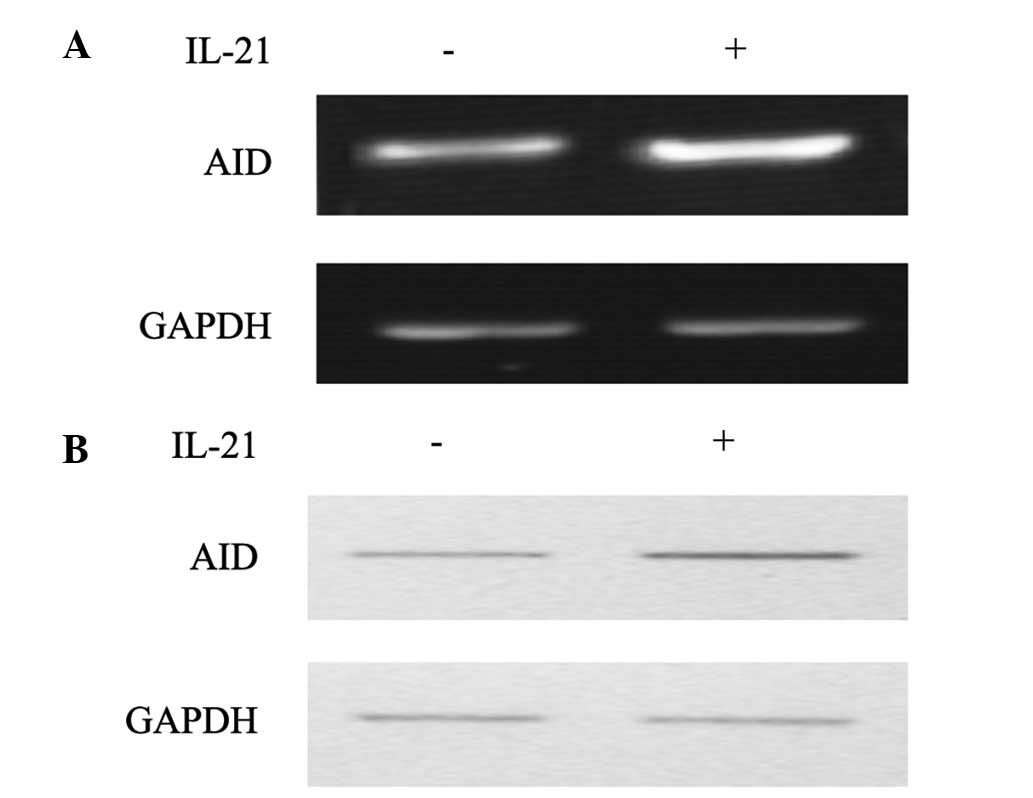
of IL-21 were associated with the expression of AID. Following
culture of the B cells in vitro, a significantly increasing
level of AID was observed in the IL-21-treated group (Fig. 4). This suggested that the excessive
production of IgA may be mediated by the high levels of IL-21
secreted by Tfh cells.
IgAN is characterized by circulating immune
complexes, which are composed of galactose-deficient IgA1 and a
glycan-specific IgG antibody. In addition, antigen-antibody immune
complexes against hepatitis B (HB) surface, HB core or HB e
antigens, together with complement components, have been
demonstrated to be deposited in renal tissue (28,29).
B cells are considered to be the key regulators of IgA production
in mucosal tissues, and CD138, CD86 and CD27 are reliable markers,
which are expressed by different subsets of B cells (30,31).
In the present study, CD19+CD138+ cells were defined as plasma
cells, CD19+CD27+ cells as memory B cells and CD19+CD86+ cells as
activated B cells, and the frequencies of these subsets of B cells
were analyzed in patients with IgAN. Significant increases in the
percentage of these three subsets of B lymphocytes were observed in
the two IgAN groups, compared with the HC group. Furthermore, the
frequencies of memory and activated B cells in the advanced IgAN
group (group B) were markedly higher than that of the less advanced
IgAN group (group A). Notably, although the IgA produced by plasma
cells mediated immune complex deposition in the glomerular
mesangial area, resulting in IgAN, the numbers of plasma cells were
not significantly different between the IgAN groups. These data
indicated that high levels of memory and activated B cells resulted
in the progression of IgAN, however, but that the IgA immune
complex deposition was not solely associated with excessive IgA
secretion by the plasma cells. Hernández et al (32), demonstrated that levels of plasma
von Willebrand Factor, a specific marker for endothelial cell
injury, are abnormal in patients with IgAN, who exhibit elevated
levels or defective molecules. Elevated levels of sFlt-1, a
receptor for vascular endothelial growth factor, may lead to
widespread endothelial dysfunction and also contributes to the
progression of IgAN (33). Thus,
the IgA immune complex deposition is associated with immune cells
and abnormal endothelial function.
Regarding the effect of Tfh cells on B cell
differentiation and activation, the frequency of Tfh cells was
analyzed in PBMCs in the present study. CD4+CXCR5+ T cells were
identified as Tfh cells, and the frequency of circulating Tfh cells
was higher in the advanced IgAN group than in the less advanced
IgAN group, which was similar to the results observed in the memory
and activated B cells. Although Tfh cells activate the
proliferation and differentiation of B cells, the selective
stimulation targeting different subsets of B cells requires further
investigation. Tfh cells are classified into three subsets: Tfh1,
Tfh2 and Tfh17, (CXCR3+CCR6−,
CXCR3−CCR6− and CXCR3−CCR6+ cells,
respectively) (20). In addition,
Tfh2 and Tfh17 cells have been reported to assist in the activation
of B cells via the production of IL-21, resulting in the secretion
of various isotypes, including IgM, IgA and IgG, and IgE for Tfh2
cells (20). Thus, the present
study hypothesized that different B cell subsets are stimulated by
different Tfh cell subsets, warranting investigation of the
mechanism underlying the regulation of Tfh cells.
As a key effector of activated Tfh cells, IL-21
induces B cell proliferation, mediates the differentiation of
activated B cells into plasma and promotes IgM, IgG and IgA
production (34,35). Thus, the concentration of serum
IL-21 was investigated, which revealed that increased levels of
IL-21 were positively correlated with the extent of 24 h
proteinuria. Thus, the activation of B cells was suggested to be
predominantly mediated by high levels of IL-21 secreted by Tfh
cells, and IL-21 may act as a biomarker of IgAN progression.
The association between high levels of IL-21 and the
expression of AID was subsequently investigated to clarify the
function of IL-21. By stimulating B cells using recombinant human
IL-21, a significant upregulation of AID was observed. This
indicated that IL-21 was positively associated with the expression
of AID expression in B cells. As a cytidine deaminase, the
predominant function of AID is inducing SHM and Ig CSR (11,24,25)
in GC or GC-like states at extrafollicular locations (36). In the GC, B cells differentiate
into either plasma cells, which secrete antibodies, or memory
cells, enabling long-term memory of antigens (37). IgA CSR is important during the
process of IgA production. B cell activation factor has been
reported to induce the expression of germline AID and IgA class
switching in a CD40-independent manner (38). Therefore, a high level of AID is a
key mediator for excessive IgA production, resulting in the
development of IgAN. The activity of AID is regulated at the
transcriptional level by HoxC4 and nuclear factor-κB, and at the
translational level by AID phosphorylation and ubiquitination
(39–42). The results of the present study
confirmed that IL-21 enhanced the expression of AID. Therefore, it
was hypothesized that high levels of IL-21 secreted by Tfh cells
may promote the differentiation of B cells and induce IgA CSR in
patients with IgAN.
The data of the present study demonstrated that the
progression of IgAN was closely associated with high levels of
memory B cells, activated B cells and Tfh cells, and that the
potential mechanism was predominantly associated with the selective
effect of Tfh cells on different B cell subsets. The significantly
increased levels of IL-21 upregulated the expression of AID in the
B cells, which mediated IgA class switching during the
differentiation of activated B cells into plasma B cells. However,
further investigation is required to fully elucidate the selective
mechanism underlying the stimulation of different types of Ig.
Acknowledgments
The authors would like to thank Dr Munan Sun (Jilin
Province People's Hospital) for the collection of clinical samples
and supporting information.
References
|
1
|
D'Amico G: The commonest
glomerulonephritis in the world: IgA nephropathy. Q J Med.
64:709–727. 1987.PubMed/NCBI
|
|
2
|
Endo Y and Kanbayashi H: Etiology of IgA
nephropathy syndrome. Pathol Int. 44:1–13. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galla JH: IgA nephropathy. Kidney Int.
47:377–387. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Das A, Ellis G, Pallant C, Lopes AR,
Khanna P, Peppa D, Chen A, Blair P, Dusheiko G, Gill U, et al:
IL-10 producing regulatory B cells in the pathogenesis of chronic
hepatitis B virus infection. J Immunol. 189:3925–3935. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki Y, Suzuki H, Nakata J, Sato D,
Kajiyama T, Watanabe T and Tomino Y: Pathological role of tonsillar
B cells in IgA Nephropathy. Clin Dev Immunol. 2011:6390742011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki H, Suzuki Y, Narita I, Aizawa M,
Kihara M, Yamanaka T, Kanou T, Tsukaguchi H, Novak J, Horikoshi S,
et al: Toll-like receptor 9 affects severity of IgA nephropathy. J
Am Soc Nephrol. 19:2384–2395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iwata Y, Wada T, Uchiyama A, Miwa A,
Nakaya I, Tohyama T, Yamada Y, Kurokawa T, Yoshida T, Ohta S, et
al: Remission of IgA nephropathy after allogeneic peripheral blood
stem cell transplantation followed by immunosuppression for acute
lymphocytic leukemia. Intern Med. 45:1291–1295. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ozaki K, Kikly K, Michalovich D, Young PR
and Leonard WJ: Cloning of a type I cytokine receptor most related
to the IL-2 receptor beta chain. Proc Natl Acad Sci USA.
97:11439–11444. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leonard WJ: Cytokines and immunodeficiency
diseases. Nat Rev Immunol. 1:200–208. 2001. View Article : Google Scholar
|
|
10
|
Chaudhuri J, Tian M, Khuong C, Chua K,
Pinaud E and Alt FW: Transcription-targeted DNA deamination by the
AID antibody diversification enzyme. Nature. 422:726–730. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muramatsu M, Kinoshita K, Fagarasan S,
Yamada S, Shinkai Y and Honjo T: Class switch recombination and
hypermutation require activation-induced cytidine deaminase (AID),
a potential RNA editing enzyme. Cell. 102:553–563. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barratt J, Feehally J and Smith AC:
Pathogenesis of IgA nephropathy. Semin Nephrol. 24:197–217. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang NS, Wu ZL, Zhang YE, Guo MY and Liao
LT: Role of hepatitis B virus infection in pathogenesis of IgA
nephropathy. World J Gastroenterol. 9:2004–2008. 2003.PubMed/NCBI
|
|
14
|
Arpin C, Déchanet J, Van Kooten C,
Merville P, Grouard G, Brière F, Banchereau J and Liu YJ:
Generation of memory B cells and plasma cells in vitro. Science.
268:720–722. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
aan de Kerk DJ, Jansen MH, ten Berge IJ,
van Leeuwen EM and Kuijpers TW: Identification of B cell defects
using age-defined reference ranges for in vivo and in vitro B cell
differentiation. J Immunol. 190:5012–5019. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klein U, Rajewsky K and Küppers R: Human
immunoglobulin (Ig)M+IgD+ peripheral blood B-cells expressing the
CD27 cell surface antigen carry somatically mutated variable region
genes: CD27 as a general marker for somatically mutated (memory)
B-cells. J Exp Med. 188:1679–1689. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fazilleau N, Mark L, McHeyzer-Williams LJ
and McHeyzer-Williams MG: Follicular helper T cells: Iineage and
location. Immunity. 30:324–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Linterman MA, Liston A and Vinuesa CG:
T-follicular helper cell differentiation and the co-option of this
pathway by non-helper cells. Immunol Rev. 247:143–159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Linterman MA and Vinuesa CG: T follicular
helper cells during immunity and tolerance. Prog Mol Biol Transl
Sci. 92:207–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morita R, Schmitt N, Bentebibel SE,
Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S,
Sabzghabaei N, et al: Human blood CXCR5 (+) CD4 (+) T cells are
counterparts of T follicular cells and contain specific subsets
that differentially support antibody secretion. Immunity.
34:108–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ettinger R, Kuchen S and Lipsky PE: The
role of IL-21 in regulating B-cell function in health and disease.
Immunol Rev. 223:60–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spolski R and Leonard WJ: Interleukin-21:
Basic biology and implications for cancer and autoimmunity. Annu
Rev Immunol. 26:57–79. 2008. View Article : Google Scholar
|
|
23
|
Ramiro AR, Stavropoulos P, Jankovic M and
Nussenzweig MC: Transcription enhances AID-mediated cytidine
deamination by exposing single-stranded DNA on the nontemplate
strand. Nat Immunol. 4:452–456. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jacob J, Kelsoe G, Rajewsky K and Weiss U:
Intraclonal generation of antibody mutants in germinal centres.
Nature. 354:389–392. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muramatsu M, Sankaranand VS, Anant S,
Sugai M, Kinoshita K, Davidson NO and Honjo T: Specific expression
of activation-induced cytidine deaminase (AID), a novel member of
the RNA-editing deaminase family in germinal center B cells. J Biol
Chem. 274:18470–18476. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Vlassaelaer P, Punnonen J and de Vries
JE: Transforming growth factor-beta directs IgA switching in human
B cells. J Immnol. 148:2062–2067. 1992.
|
|
27
|
Baskin B, Pettersson E, Rekola S, Smith CI
and Islam KB: Studies of the molecular basis of IgA production,
subclass regulation and class-switch recombination in IgA
nephropathy patients. Clin Exp Immunol. 106:509–517. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He XY, Fang LJ, Zhang YE, Sheng FY, Zhang
XR and Guo MY: In situ hybridization of hepatitis B DNA in
hepatitis B-associated glomerulonephritis. Pediatr Nephrol.
12:117–120. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu ZL, Wang NS, Xu XH, Qiu LQ, Zhou Q and
Zhang YE: Positive and negative hepatitis B virus in renal biopsies
of IgA nephropathy: An 85-case clinicopathological analysis.
Nephrology. 6:185–189. 2001. View Article : Google Scholar
|
|
30
|
LeBien TW and Tedder TF: B lymphocytes:
how they develop and function. Blood. 112:1570–1580. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Odendahl M, Jacobi A, Hansen A, Feist E,
Hiepe F, Burmester GR, Lipsky PE, Radbruch A and Dörner T:
Disturbed peripheral B lymphocyte homeostasis in systemic lupus
erythematosus. J Immunol. 165:5970–5979. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hernández E, Toledo T, Alamo C, Mon C,
Rodicio JL and Praga M: Elevation of von Willebrand factor levels
in patients with IgA nephropathy: Effect of ACE inhibition. Am J
Kidney Dis. 30:397–403. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhai YL, Zhu L, Shi SF, Liu LJ, Lv JC and
Zhang H: Elevated soluble VEGF receptor sFlt-1 correlates with
endothelial injury in IgA nephropathy. PLoS One. 9:e1017792014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Konforte D, Simard N and Paige CJ: IL-21:
An executor of B cell fate. J Immunol. 182:1781–1787. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bryant VL, Ma CS, Avery DT, Li Y, Good KL,
Corcoran LM, de Waal Malefyt R and Tangye SG: Cytokine-mediated
regulation of human B cell differentiation into Ig-secreting cells:
Predominant role of IL-21 produced by CXCR5+ T follicular helper
cells. J Immunol. 179:8180–8190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cattoretti G, Büttner M, Shaknovich R,
Kremmer E, Alobeid B and Niedobitek G: Nuclear and cytoplasmic AID
in extrafollicular and germinal center B cells. Blood.
107:3967–3975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Good-Jacobson KL, Szumilas CG, Chen L,
Sharpe AH, Tomayko MM and Shlomchik MJ: PD-1 regulates germinal
center B cell survival and the formation and affinity of long-lived
plasma cells. Nat Immunol. 11:535–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu H, Peng Y, Liu F, Xiao W, Zhang Y and
Li W: Expression of IgA class switching gene in tonsillar
mononuclear cells in patients with IgA nephropathy. Inflamm Res.
60:869–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu Z, Zan H, Pone EJ, Mai T and Casali P:
Immunoglobulin class-switch DNA recombination: Induction, targeting
and beyond. Nat Rev Immunol. 12:517–531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McBride KM, Gazumyan A, Woo EM, Schwickert
TA, Chait BT and Nussenzweig MC: Regulation of class switch
recombination and somatic mutation by AID phosphorylation. J Exp
Med. 205:2585–2594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Basu U, Wang Y and Alt FW: Evolution of
phosphorylation-dependent regulation of activation-induced cytidine
deaminase. Mol Cell. 32:285–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aoufouchi S, Faili A, Zober C, D'Orlando
O, Weller S, Weill JC and Reynaud CA: Proteasomal degradation
restricts the nuclear lifespan of AID. J Exp Med. 205:1357–1368.
2008. View Article : Google Scholar : PubMed/NCBI
|