Introduction
The Escherichia coli K5 capsular
polysaccharide (K5PS) is an acidic polysaccharide with a repeating
disaccharide unit consisting of β-D-glucuronic acid (GlcA) and
α-D-acetylglucosamine (GlcNAc) of (→4) GlcA (1→4) GlcNAc (1→). K5PS
has the same carbohydrate backbone as heparosan, which is a
precursor in the biosynthesis of heparin and heparan sulfate
(1). The K5 polysaccharide
exhibits low immunogenicity in humans by acting as a molecular
camouflage, which can facilitate bacterial infection of mammals by
evading the immune response (2).
It is well known that sulfation improves the biological activity of
polysaccharides. Several studies have found that sulfated
polysaccharides have numerous and marked biological activities
(3–5). Previous studies have demonstrated
that sulfated modifications of the K5 polysaccharide lead to the
synthesis of heparin-like compounds, which exhibit different
biological properties, including anticoagulant/antithrombotic,
anti-inflammatory, anticancer and antiviral activities (6). The epimerized N,O-sulfated heparosan
and O-sulfated heparosan exhibit potential anti-inflammatory
activity without the occurrence of bleeding as a side effect
(7). These two sulfated heparosan
derivatives also inhibit the replication of different HIV-1 strains
(8). Therefore, chemical
modifications, including partial or per-sulfonation can be applied
to obtain more active sulfated polysaccharides.
To the best of our knowledge, various
polysaccharides, particluarly following sulfation modification,
have immunoregulatory activities, which can be used in healthcare,
nutrition and medicine (9,10). Heparin is a known anti-coagulant
with a weak direct immunosuppressive action in vitro and
in vivo (11). The
inhibitory effects of heparin on human lymphoproliferative response
and natural killer (NK) cytotoxicity have been reported in previous
studies (12,13). Heparin has been reported to have
potential therapeutic immunomodulatory effects during severe sepsis
(14). However, heparan sulfate,
which is expressed on the cell surface, extracellular matrix and
basement membrane, contributes to the immune system by facilitating
leukocyte development, leukocyte migration, immune activation and
inflammatory processes (15).
Given the structural similarities between sulfated K5 derivatives,
heparin and heparan sulfate, the K5 polysaccharide with sulfated
modifications may also have immunomodulatory properties. However,
the effect of sulfated K5 polysaccharides on the immune system
remain to be fully elucidated.
The aim of the present study was to examine the
effects of sulfated K5 polysaccharide derivatives on RAW264.7
macrophage cells, and to examine macrophage phagocytosis, the
release of nitric oxide (NO), tumor necrosis factor-α (TNF-α), and
interleukin-1β (IL-1β). The gene expression levels of TNF-α, IL-1β,
and inducible nitric oxide synthase (iNOS) were also examined.
Morphological changes were analyzed to investigate the potential
roles of K5 polysaccharide in human immunity following sulfated
modifications. The immunomodulatory activities of sulfated K5
polysaccharide derivatives on murine RAW264.7 macrophage cells were
investigated to assist in the development of novel pharmaceutical
products. In addition, the presents study aimed to elucidate the
structure-activity associations among sulfated polysaccharides.
Materials and methods
Materials and chemicals
The pyridine-sulfotrioxyde complex,
tetrabutylammonium hydroxide,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
Dimethyl sulfoxide (DMSO), lipopolysaccharide (LPS; E. coli
0111:B4) and trypsin were from Sigma-Aldrich (St. Louis, MO, USA).
RPMI 1640 medium, Dulbecco's modified Eagle's medium (DMEM), bovine
serum albumin (BSA) and fetal bovine serum (FBS) were obtained from
Gibco Life Technologies (Carlsbad, CA, USA). All other chemicals
and solvents used were of analytical reagent grade and obtained
from Sinopharm Chemical Reagent Co, Ltd. (Shanghai China). The
enzyme-linked immunosorbent assay (ELISA) kits for IL-1β, TNF-α
were obtained from Shanghai Fu Sheng Industrial Co., Ltd.
(Shanghai, China).
Preparation of the K5PS and sulfated
modification
The capsular K5PS was produced from E. coli
strain 010:K5:H4 fermentation and purification from the culture
supernatant, as described previously (2,16).
The sulfation reagent was prepared in two
concentrations with ratios of K5PS:pyridine-sulfotrioxyde complex
at 1:0.5 and 1:2.5, respectively. Briefly, K5PS (1 g) was suspended
in anhydrous N,N-dimethylformamide (25 ml), followed by the
addition of the sulfation reagent. The mixture was maintained at
room temperature for 18 h with continuous stirring. Subsequently,
the mixture was cooled and recovered by precipitation with
NaCl-saturated acetone (20 ml). Finally, the sulfated
polysaccharide was dialyzed against distilled water, and was
freeze-dried as samples termed K5-OS1 and
K5-OS2. To obtain the N-deacetylated/N-sulfated K5PS
(K5-NS), K5PS (1 g) was dissolved in 2 M NaOH and incubated for 24
h at 60°C. The solution was then warmed to 40°C and added, in a
single step to the sodium carbonate and pyridine-sulfotrioxyde
complex for 4 h, and incubated for an additional hour at the same
temperature. The K5-NS was run through a cation exchange column
(IR-120 H+; Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 10°C
and was neutralized with 15% tetrabutylammonium hydroxide in water.
Following freeze-drying, the sample was dissolved in
N,N-dimethylformamide (40 ml), and the sulfation reagent was
added to synthesize N,O-sulfated K5PS (K5-N, OS), as described
above.
Lipopolysaccharode (LPS)-free samples of K5 and
sulfated K5PS were prepared using endotoxin removal resin (1.5 ml;
Genscript USA, Inc., Piscataway, NJ, USA). Endotoxin was removed,
resulting in levels <0.1 EU/ml, as indicated using a Limulus
Amebocyte Lysate assay (Xiamen Limulus Reagent Factory, Xiamen,
China), according to the manufacturer's protocol.
Characterization of sulfated K5
derivatives
Molecular weight determination was performed by gel
permeation chromatography using a multi-angle light scattering
detector (GPC-MALS; Wyatt Technology Corporation, Goleta, CA, USA).
Briefly, the concentration of polysaccharide solution was 3.0 mg/ml
in sodium chloride solution. The polysaccharide solution in sodium
chloride (200 µl) was detected after being filtered through
a 0.2 µm syringe filter. Astra software (Astra V; Wyatt
Technology Corporation) was utilized for data acquisition and
analysis. The content of uronic acid was estimated using a
carbazole method, with D-glucuronic acid as a standard (17). Sulfate content was determined using
a barium chloride-gelatin method (18). The degree of sulfation (DS), which
indicated the average number of sulfate ester groups on each
monosaccharide residue, was calculated based on the sulfur content
of the prepared samples (4).
Macrophage activation by sulfated K5PS
derivatives in vitro
Cell culture, morphological
observations and assessment of cell viability
The RAW264.7 cell line was obtained from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). The cells were maintained in DMEM supplemented with 10% FBS
containing 100 U/ml penicillin and 100 µg/ml streptomycin at
37°C in a 5% CO2 incubator. Polymyxin B (50
µg/ml) was added to each well to eliminate LPS
contamination. In brief, the cells were seeded at 1×105
cells/ml in a 96-well plate for the MTT assay. After 24 h at 37°C,
different concentrations of sulfated K5 derivatives (1, 10, 50 and
100 µg/ml) were added and the mixtures were cultured for 24
h at 37°C. Subsequently, the cells were visualized using light
microscopy with a Leica DMIL LED microscope (Leica Microsystems,
Inc., Buffalo Grove, IL, USA; magnification, ×400). Following
morphological observation, the medium was removed and replaced with
fresh medium containing 0.5 mg/ml MTT. The plates were further
incubated for 4 h at 37°C. Following the production of formazan
crystals, the crystals were dissolved in DMSO, and the absorbance
at 570 nm was measured using a microplate reader (Multiskan GO;
Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Phagocytosis of sulfated K5
polysaccharide derivative-stimulated RAW264.7 macrophages
The RAW264.7 cells were seeded (1×105
cells/ml) in a 96-well plate with DMEM medium (10% FBS) and
incubated at 37°C in 5% CO2 for 24 h. The cells were
cultured with the samples at final concentrations of 1, 10, 50 and
100 µg/ml for 24 h at 37°C. DMEM and 10 µg/ml LPS
were used as negative and positive controls, respectively. The
stimulated cells were washed twice with phosphate-buffered saline
(PBS), and 100 µl of 0.075% neutral red solution was added
to each well, followed by incubation for 4 h at 37°C. Following
removal of the unphagocytized neutral red by PBS, cell lysis buffer
(acetic acid/ethanol; 1:1) was added to each well at 4°C for 2 h.
The optical density (OD) value of each well was measured at 570 nm
using a micro-plate reader. Cell phagocytosis (%) was calculated as
follows: ODS / ODC × 100, where
ODS and ODC represent the OD values of the
stimulated and control wells, respectively. The percentage of
phagocytosis in the untreated cells was designated as 100%. All
experiments were performed in triplicate.
Measurement of NO in the RAW264.7
Cells A total of 5×104 cells/well were
seeded into a 96-well culture plate. After 24 h, the cells were
pre-incubated with different sample concentrations, as mentioned
above. LPS (10 µg/ml) was used as a positive control. The NO
concentrations were estimated by calculating the quantity of
released nitrite, using Griess reagent, in the cell supernatant.
The OD was determined at 540 nm using a microplate reader, with
data expressed as the mean ± standard deviation of three
experiments.
Measurement of cytokines in the
RAW264.7
The levels of TNF-α and IL-1β were determined using
ELISA kits, according to the manufacturer's instructions. Briefly,
the RAW264.7 cells were cultured for 24 h with different
concentrations of the sample, as described above. The supernatants
were harvested and standard curves were produced for calculation of
the cytokine concentrations using Origin 8.0 (OriginLab
Corporation, Northampton, MA, USA).
Gene expression levels of iNOS, TNF-α,
and IL-1β using reverse transcription-polymerase chain reaction
(RT-PCR)
In the present study, RT-PCR was performed to
determine changes in the gene expression levels of iNOS, TNF-α and
IL-1β. The macrophages were cultured in the presence or absence of
the samples for the indicated time-periods. The macrophages were
lysed using TRIzol reagent (Invitrogen Life Technologies) and the
total RNA was extracted, according to the manufacturer's
instructions. Reverse transcription of the RNA was performed using
RevertAid First Strand cDNA Synthesis kit (Fermentas, Thermo Fisher
Scientific, Inc.). PCR was carried out using a Bio-Rad MyCycler
thermal cycler (Bio-Rad Laboratories, Inc.) as follows: 12.5
µl Premix Taq (Takara Biotechnology, Inc., Dalian,
China), 1 µl template cDNA, 0.5 µl each primer
(Shanghai Sangon Biotechnology Co., Ltd., Shanghai, China), and
water up to 25 µl. PCR cycling conditions were as follows:
95°C for 3 min, followed by 95°C for 45 sec, 55°C (or 53°C, 57°C or
52°C) for 45 sec, and 72°C for 45 sec for 35 cycles, and a final
extension for 10 min at 72°C. The PCR product was maintained at 4°C
until further use. PCR products were electrophoresed on a 2%
agarose gel and an image was captured using a Bio-Rad Gel Doc XR+
imaging system (Bio-Rad Laboratories, Inc.). Optical density of the
bands was quantified using Quantity One 4.6 software (Bio-Rad
Laboratories, Inc.).
The sequences of the primers used are presented in
Table I.
 | Table IPrimer sequences and cycling
parameters used for reverse transcription-polymerase chain
reaction. |
Table I
Primer sequences and cycling
parameters used for reverse transcription-polymerase chain
reaction.
| Gene | Primer sequence (5′
to 3′) | Annealing
temperature (°C) |
|---|
| iNOS | Forward:
GAGCGAGTTGTGGATTGTC | 55 |
| Reverse:
CCAGGAAGTAGGTGAGGG | |
| TNF-α | Forward:
CACCCTTATTCTCGCTCAC | 53 |
| Reverse:
CCCGCTTACAGTTCCTCT | |
| IL-1β | Forward:
CTCGTGCTGTCGGACCCAT | 57 |
| Reverse:
CAGGCTTGTGCTCTGCTTGTGA | |
| GAPDH | Forward:
CTCTGCTCCTCCCTGTTC | 52 |
| Reverse:
CAATCTCCACTTTGCCACT | |
Measurement of cytotoxicity induced by
sulfated K5PS-treated macrophages
Macrophage-mediated tumor cell cytotoxicity was
measured, as described previously (19). The sulfated K5PSs-treated
macrophages, which were treated for 24 h followed by PBS washing,
were co-incubated with cancer cells (1.0×104 cells/well
murine B16 melanoma cells or human cervical cancer HeLa cells; Type
Culture Collection of the Chinese Academy of Sciences, Shanghai,
China) for 24 h at a cancer cell:macrophage ratio of 1:10. The
viability of the cancer cells was then determined using an MTT
assay, to measure macrophage-induced cytotoxicity.
Statistical analysis
The results are presented as the mean ± standard
deviation. One-way analysis of variance for was performed using
SPSS version 19.0 (IBM SPSS, Armonk, NY, USA). Tukey's method for
multiple comparisons among groups was used to determine significant
differences. P<0.05 was considered to indicate a statistically
significant differences.
Results
Characterization of sulfated K5PSs
The chemical compositions of the K5-OS1,
K5-OS2, K5-NS, and K5-N,OS samples are presented in
Table II. K5-OS1 and
K5PS contained similar total sugar (uronic acid) contents. Compared
with K5-OS1, the total sugars in the sulfation
derivative K5-OS2, K5-NS and K5-N,OS samples decreased
following modification. The sulfate content and DS of the sulfated
K5PSs are listed in decreasing order, as follows:
K5-N,OS>K5-NS>K5-OS2>K5-OS1. This
finding indicated that the sulfation of different degrees had been
successful. As shown in Table II,
the molecular weights following sulfation was lower than that of
the K5PS, which was possibly due to hydrolysis of the dominant
chains during the sulfation process.
 | Table IIChemical composition of the K5
samples. |
Table II
Chemical composition of the K5
samples.
| Sample | Uronic acid
(%) | Sulfate (%) | DS | Mw (Da) |
|---|
| K5 | 33.14 | 0 | 0 |
7.22×104 |
|
K5-OS1 | 32.95 | 0.21 | 0.01 |
6.58×104 |
|
K5-OS2 | 27.33 | 2.87 | 0.16 |
6.89×104 |
| K5-NS | 26.92 | 5.56 | 0.34 |
9.31×103 |
| K5-N,OS | 26.67 | 6.01 | 0.38 |
1.02×104 |
Effects of sulfated K5PSs on the growth
of RAW264.7 cells
To examine whether sulfated K5PSs were capable of
modulating the functional activation of macrophages, the RAW264.7
cells were treated with the samples and morphological changes were
observed after 24 h (Fig. 1). When
the RAW264.7 cells were cultured in the medium (Fig. 1A), the majority of the cells were
observed to exhibit a circular morphology, whereas the morphologies
of the cells cultured in the presence of LPS were altered (Fig. 1B). When the cells were co-cultured
with K5PS and the sulfated derivatives (Fig. 1C–G), morphological alterations
associated with macrophage activation were observed after 24 h.
 | Figure 1Morphology of RAW 264.7 cells 24 h
after treatment with (A) medium, (B) 10 µg/ml
lipopolysaccharide, (C) 100 µg/ml K5, (D) 100 µg/ml
K5-OS1 (E) 100 µg/ml K5-OS2, (F) 100
µg/ml K5-NS, and (G) 100 µg/ml K5-N,OS (magnification
×400). K5, K5 capsular polysaccharide; K5-OS1, 1:0.5
K5PS:pyridine-sulfotrioxyde complex; K5-OS2, 1:2.5
K5PS:pyridine-sulfotrioxyde complex; K5-NS,
N-deacetylated/N-sulfated K5PS; K5-N, OS, N,O-sulfated K5PS. |
To determine whether the sulfated K5PSs were
cytotoxic to RAW264.7 cells, the present study analyzed cell
viability at various concentrations of K5 and sulfated K5PSs (1,
10, 50 and 100 µg/ml) using an MTT assay. Following 24 h of
incubation with varying concentrations, the A570 values
of each group were assessed (Table
III). The A570 values of all samples at
concentrations of 100 µg/ml were significantly higher,
compared with those in the cell control group (P<0.05).
Furthermore, a significant difference (P<0.05) was observed
between the A570 values of the 50 and 100 µg/ml
concentrations of K5-OS2 and those of the other groups.
No cytotoxic effects were observed of K5PS or the sulfated
derivatives on the RAW264.7 cells.
 | Table IIIMacrophage viability changes in each
group in the presence of polysaccharide stimulation. |
Table III
Macrophage viability changes in each
group in the presence of polysaccharide stimulation.
| Concentration
(µg/ml) | A570
value
|
|---|
| K5 |
K5-OS1 |
K5-OS2 | K5-NS | K5-N,OS |
|---|
| 0 (control) | 0.653±0.004 | 0.656±0.003 | 0.657±0.007 | 0.651±0.002 | 0.652±0.003 |
| 1 | 0.643±0.004 | 0.641±0.010 | 0.674±0.003 | 0.629±0.013 | 0.629±0.009 |
| 10 | 0.652±0.018 | 0.653±0.002 | 0.685±0.003 | 0.629±0.003 | 0.669±0.009 |
| 50 | 0.662±0.005 | 0.665±0.012 | 0.804±0.006a | 0.664±0.003 | 0.674±0.030 |
| 100 | 0.695±0.014a | 0.712±0.007a | 0.842±0.017a | 0.693±0.008a | 0.711±0.007a |
Effects of sulfated K5PSs on phagocytic
activity
Based on the results described above, the present
study subsequently investigated the effect of K5,
K5-OS1, K5-OS2, K5-NS and K5-N,OS on the
phagocytic activity of RAW 264.7 cells. Neutral red solution is
commonly used in phagocytosis assays due to its ease of
manipulation and determination through examination of OD values
(20,21). The effects of the neutral
red-ingested macrophages in the different treatment groups on
phagocytosis are shown in Fig. 2.
During the single period of polysaccharide stimulation, the
phagocytic activity of the K5-OS2 group was
significantly higher, compared with the activities in the untreated
control and remaining treatment groups (P<0.05), exhibiting a
dose-dependent pattern. K5-OS2 increased phagocytosis
between 22.8±3.8 and 46.0±4.6%. Compared with the control group,
K5-N,OS (100 µg/ml) and LPS (10 µg/ml) increased the
number of macrophages exhibiting neutral red ingestion, which had
higher OD values. The K5-OS1 and K5-NS groups were
unable to individually augment the phagocytic activity of the
RAW264.7 cells by 105.8±3.1% and 101.7±7.2%.
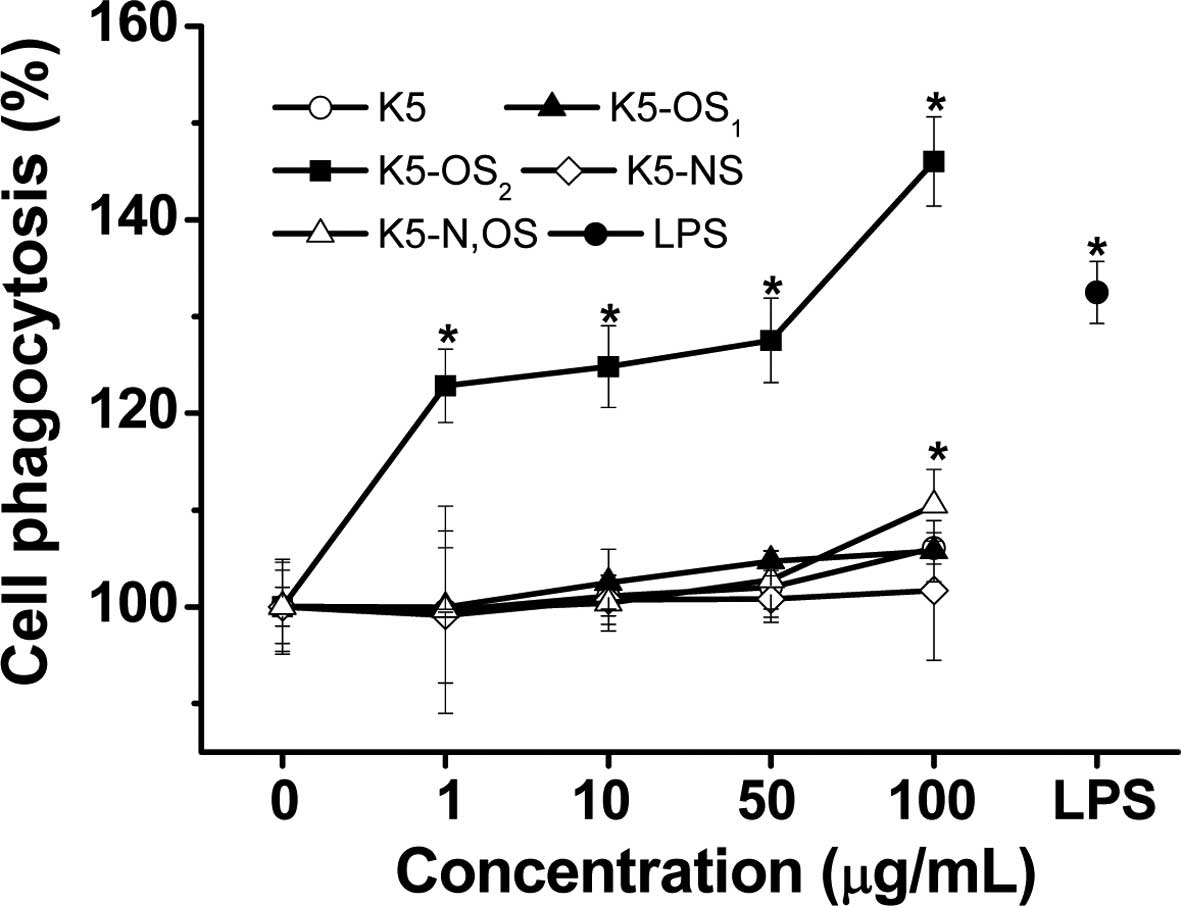 | Figure 2Effects of sulfated K5PSs on the
phagocytosis of RAW 264.7 cell, determined using a neutral red
uptake assay. Each value is expressed as the mean ± standard
deviation of three independent experiments. *P<0.05,
compared with the untreated control group. K5, K5 capsular
polysaccharide; K5-OS1, 1:0.5
K5PS:pyridine-sulfotrioxyde complex; K5-OS2, 1:2.5
K5PS:pyridine-sulfotrioxyde complex; K5-NS,
N-deacetylated/N-sulfated K5PS; K5-N, OS, N,O-sulfated K5PS; LPS,
lipopolysaccharide. |
Effects of sulfated K5PSs on the
production of NO in RAW264.7 macrophages
The effects of the sulfated K5PSs on the production
of NO in the RAW264.7 macrophages were investigated using the
Griess reaction in the culture medium (21). The production of NO was low in the
untreated RAW264.7 cells, however, the production increased
moderately with the sulfated K5PSs, and increased markedly with
LPS. As shown in Fig. 3, when the
RAW264.7 cells were treated with the four polysaccharides,
synthesized with different sulfation patterns, the production of NO
increased in a dose-dependent manner (P<0.05). However, K5PS had
no effect on inducting the production of NO. K5-N,OS minimally
increased the production of NO at concentrations of 1 or 10
µg/ml, and moderately stimulated NO at higher
concentrations. By contrast, K5-OS2 and
K5-OS1 were highly active and stimulated levels of
macrophage NO production, which were comparable to that in the
control. The production of NO by the RAW264.7 cells incubated with
K5-OS2 or K5-OS1 at concentrations of 100
µg/ml for 24 h were 249.2±11.2 and 221.2±17.1% of the
control value, respectively (P<0.05; Fig. 3).
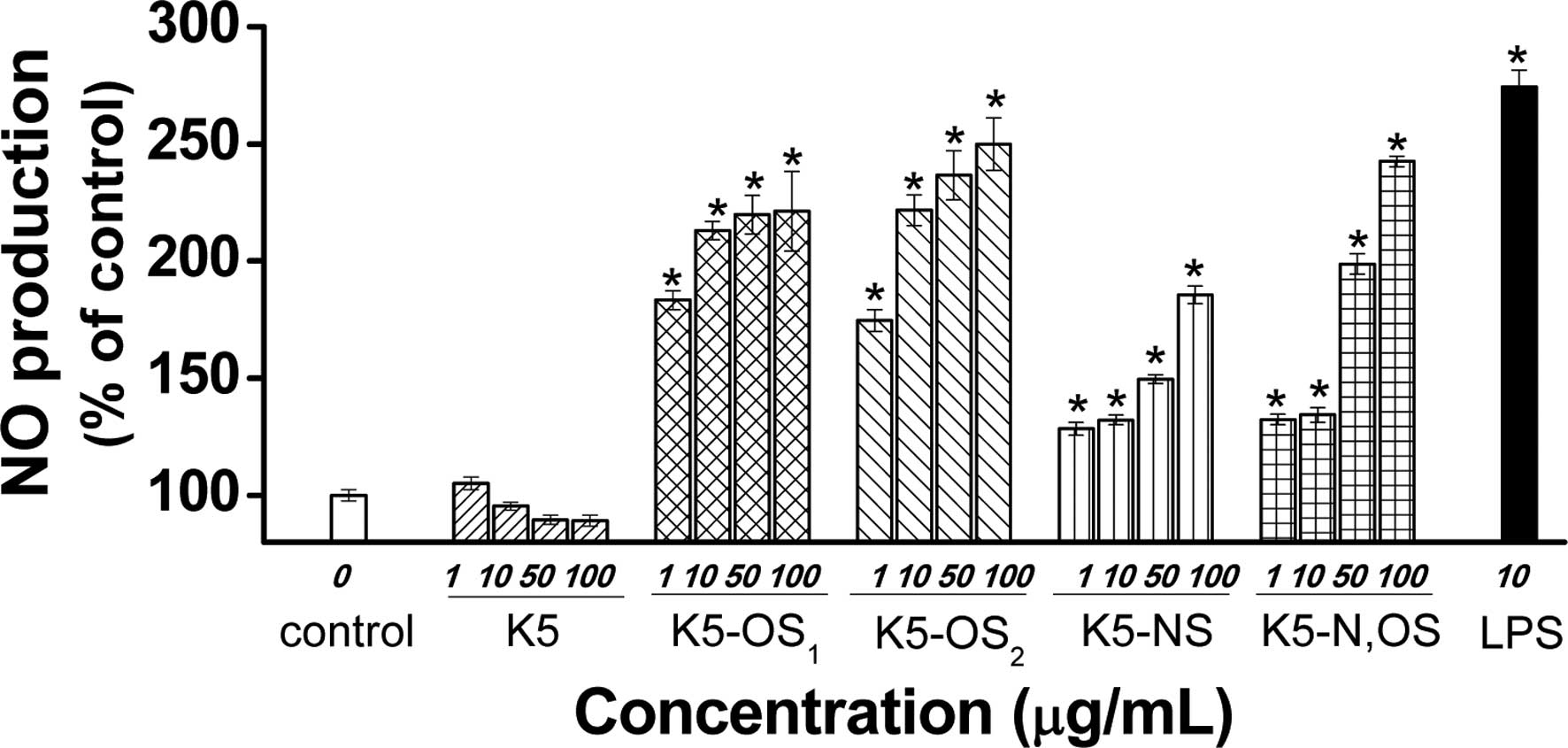 | Figure 3Effects of sulfated K5PSs on the
production of NO in RAW 264.7 cells. NO production was measured
using a Griess reaction assay and data are expressed as a
percentage of the untreated control cells, and as the mean ±
standard deviation of three independent experiments. The
differences between the control group and treatment groups were
determined using one-way analysis of variance
*P<0.05, compared with the control. NO, nitrous
oxide; K5, K5 capsular polysaccharide; K5-OS1, 1:0.5
K5PS:pyridine-sulfotrioxyde complex; K5-OS2, 1:2.5
K5PS:pyridine-sulfotrioxyde complex; K5-NS,
N-deacetylated/N-sulfated K5PS; K5-N, OS, N,O-sulfated K5PSl; LPS,
lipopolysaccharide. |
Effects of sulfated K5PSs on macrophage
production of TNF-α and IL-1β
To determine the effect of sulfated K5PS-activated
RAW264.7 cells on the expression of cytokines, the culture
supernatants were collected following 24 h of exposure, and the
levels of TNF-α and IL-1β were determined using ELISA kits. As
shown in Table IV, among the four
sulfated K5PS groups, K5-OS2 significantly increased
cytokine release from the macrophages at every concentration,
whereas K5-OS1 only increased the level of IL-1β between
113.0±0.5 and 114.1±0.4 pg/ml (P<0.05). In addition, K5-N,OS
also increased the release of IL-1β from the macrophages in a
dose-dependent manner and markedly increased the production of
IL-1β up to 120.5±3.5 pg/ml at high concentrations. The RAW264.7
cells treated with K5-N,OS produced considerably higher
concentrations of TNF-α, with levels reaching ~1161.8±113.4 pg/ml
at the 100 µg/ml concentration (P<0.05). By contrast, no
significant increases in TNF-α and IL-1β were observed in the K5
and K5-NS groups, at any concentration, in RAW264.7 cells.
 | Table IVEffects of sulfated K5PSs on cytokine
production by stimulated RAW264.7 cells. |
Table IV
Effects of sulfated K5PSs on cytokine
production by stimulated RAW264.7 cells.
| Sample | Concentration
(µg/ml) | Production (pg/ml)
|
|---|
| TNF-α | IL-1β |
|---|
| Control | 0 | 871.0±13.3 | 96.1±2.0 |
| LPS | 10 | 974.0±15.0a | 117.2±1.7a |
| K5 | 1 | 867.3±16.5 | 97.0±3.1 |
| 10 | 877.3±12.1 | 97.7±2.1 |
| 50 | 879.3±22.3 | 99.6±3.9 |
| 100 | 908.4±23.6 | 102.4±1.6 |
|
K5-OS1 | 1 | 877.3±27.0 | 113.0±0.5a |
| 10 | 918.5±57.9 | 114.1±1.0a |
| 50 | 922.6±57.2 | 114.2±0.5a |
| 100 | 935.3±26.7 | 114.1±0.4a |
|
K5-OS2 | 1 |
1,042.3±31.9a | 117.4±1.4a |
| 10 |
1,045.8±49.3a | 120.0±1.7a |
| 50 |
1,111.8±72.1a,b | 121.6±1.8a |
| 100 |
1,180.4±34.3a,b | 123.1±0.3a |
| K5-NS | 1 | 844.3±46.0 | 96.0±2.1 |
| 10 | 895.4±12.0 | 97.8±3.0 |
| 50 | 894.8±17.0 | 99.9±4.9 |
| 100 | 949.5±18.2 | 100.4±2.7 |
| K5-N,OS | 1 |
1,049.2±34.2a | 98.3±1.5 |
| 10 |
1,055.7±33.6a | 103.2±3.5 |
| 50 |
1,095.8±14.5a | 108.0±1.9a |
| 100 |
1,161.8±113.4a,b | 120.5±3.5a |
Effects of sulfated K5PSs on the mRNA
levels of iNOS, TNF-α and IL-1β
Activated macrophages are known to release immune
factors, including NO, TNF-α and IL-1β (21–23).
To assess the activation of macrophages by the sulfated K5PSs in
the present study, RT-PCR analysis was performed to evaluate the
mRNA expression levels of iNOS, TNF-α and IL-1β (Fig. 4A). Following treatment of the
samples at 100 µg/ml concentrations for 24 h, the RAW264.7
cells expressed mRNA encoding the genes for iNOS, TNF-α and IL-1β,
which are established markers of macrophage activation (Fig. 4A). As estimated using densitometric
measurement, K5-OS2 stimulated the expression of iNOS,
TNF-α and IL-1βin macrophages at three times the level observed in
the normal control (Fig. 4B).
Compared with the control, treatment with K5-N,OS by itself
significantly increased the mRNA levels of iNOS, TNF-α and IL-1β by
216, 202, and 134% (Fig. 4B),
which were comparable to the levels observed in the LPS treatment
group. By contrast, K5 had no significant effect on mRNA
expression, which was consistent with the ELISA assay data.
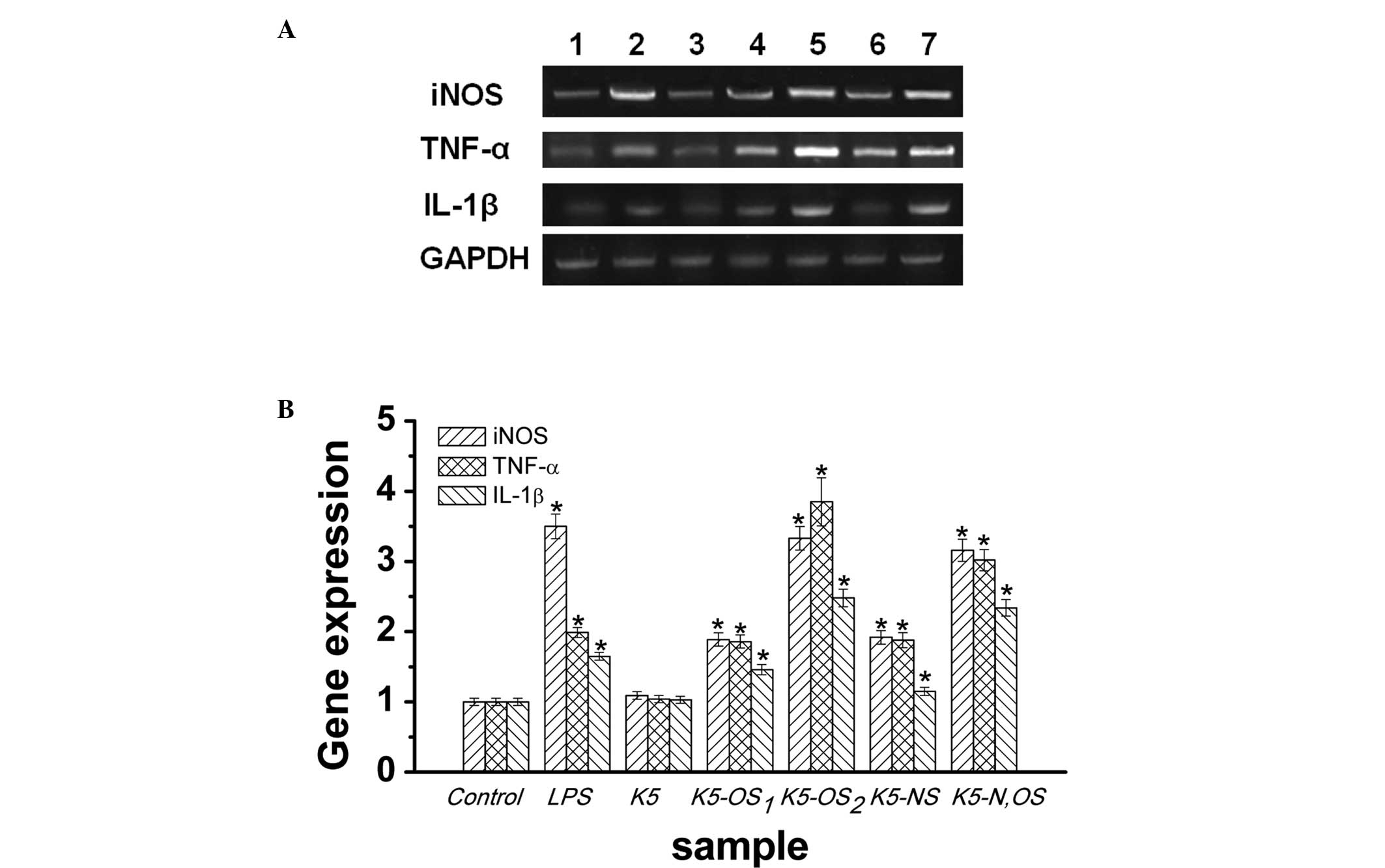 | Figure 4Effects of sulfated K5PSs on the mRNA
expression levels of iNOS, TNF-α, and IL-1β. RAW264.7 cells were
incubated for 24 h with samples (100 µg/ml) or LPS (10
µg/ml). (A) mRNA levels were determined using reverse
transcription-polymerase chain reaction.. Lanes 1–7 indicate the
control, LPS, K5, K5-OS1, K5-OS2, K5-NS, and
K5-N,OS groups, respectively. (B) Semi-quantification of the mRNA
expression levels of iNOS, TNF-α, and IL-1β, as the ratio of
densitometric measurement with that of the internal standard
(GAPDH). Significance was determined using one-way analysis of
variance (*P<0.05, compared with the control group).
Data are expressed as the mean ± standard deviation. iNOS,
inducible nitric oxide synthase; TNF, tumor necrosis factor; IL,
interleukin; K5, K5 capsular polysaccharide; K5-OS1,
1:0.5 K5PS:pyridine-sulfotrioxyde complex; K5-OS2, 1:2.5
K5PS:pyridine-sulfotrioxyde complex; K5-NS,
N-deacetylated/N-sulfated K5PS; K5-N, OS, N,O-sulfated K5PS; LPS,
lipopolysaccharide. |
Effects of sulfated K5PSs on
macrophage-mediated cytotoxicity against cancer cells in vitro
The immunomodulatory effect of polysaccharides is
suggested to occur in cancer via macrophage-mediated cytotoxicity
against cancer cells (24).
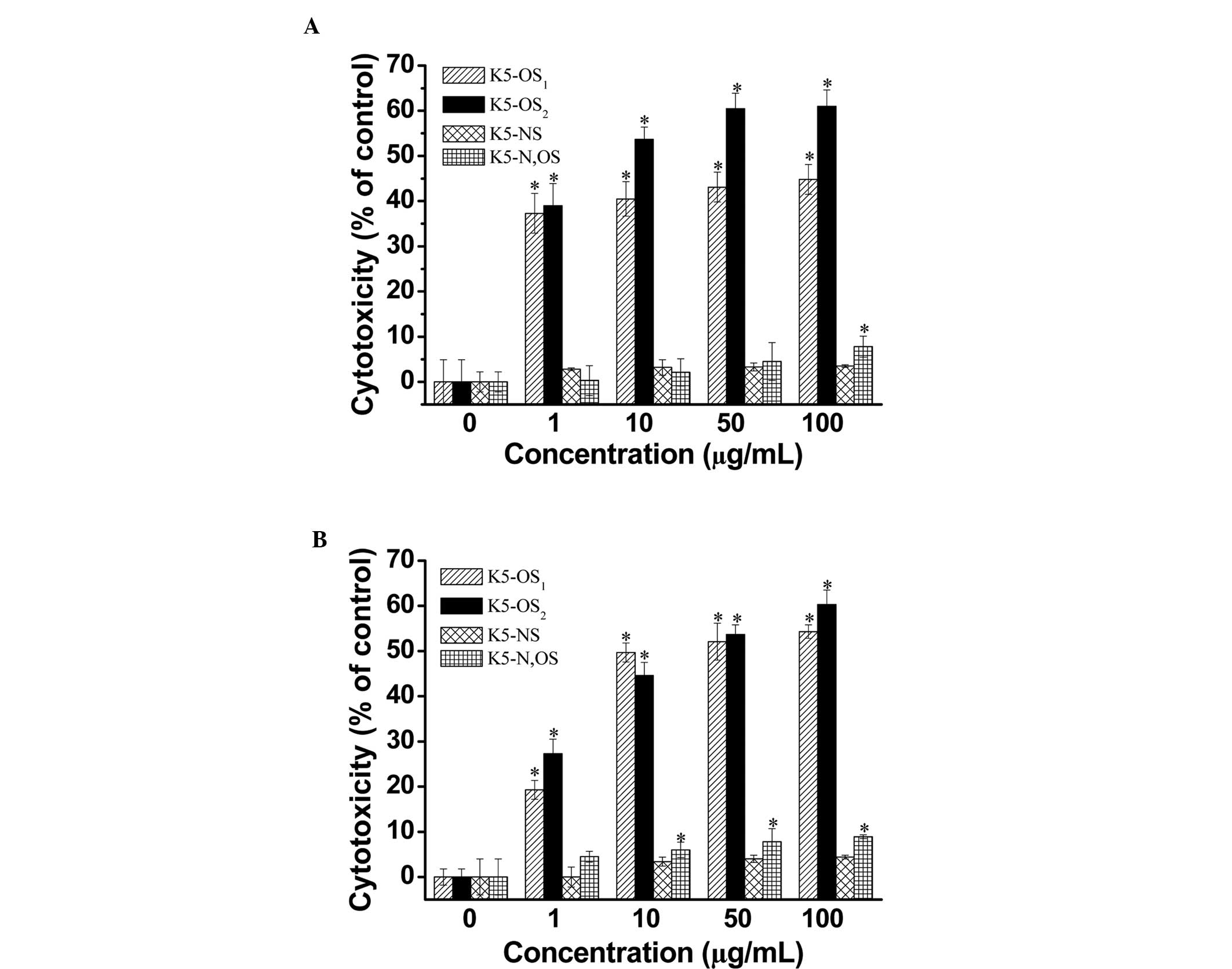
Therefore, the present study examined the cytotoxicity of activated
RAW264.7 cells against tumor cells. As shown in Fig. 5, K5-OS2 and
K5-OS1 enhanced macrophage-induced cytotoxicity against
the B16 (Fig. 5A) and Hela
(Fig. 5B) tumor cell lines in the
concentration range of 1–100 µg/ml. The macrophage-induced
cytotoxicity of K5-OS2 was increased in a dose-dependent
manner, with levels reaching ~62%, compared with the control, at
the 100 µg/ml concentration (P<0.05). K5-N,OS only
enhanced cytotoxicity (7.8%) against B16 cell lines at the highest
dose (P<0.05; Fig. 5A).
However, K5-N,OS improved the cytotoxicity against Hela cells in
the concentration range of 10–100 µg/ml (P<0.05; Fig. 5B). The results also revealed a
significant difference between the K5-OS2 and the K5-NS,
K5-N,OS, and control groups at all concentrations assessed
(P<0.05). However, no significant difference was observed
between the K5-NS group and the control at any concentration
(P>0.05). Similarly, unmodified K5PS had no effect on
macrophage-mediated cytotoxicity against cancer cells (data not
shown).
Discussion
To the best of our knowledge, macrophages are the
first cells to recognize infectious agents. Macrophages are
involved in specific and non-specific immune reactions (25). Therefore, the identification of
agents, which can modulate macrophages is of significant interest.
A number of polysaccharides have been reported to exhibit
beneficial pharmacological effects due to their ability to modulate
macrophage function (26). In the
present study, K5PS was prepared from E. coli, and sulfated
derivatives of K5PS with different sulfation patterns were prepared
via chemical modifications, the effects of which were investigated
in mouse macrophage RAW264.7 cells, a model, which is closer to
humans (21). Based on the results
of the MTT assay and morphological observations, the present study
demonstrated that sulfated K5PSs induced macrophage activation,
without cytotoxicity, in the RAW264.7 cells.
In addition, the sulfated K5PSs significantly
enhanced the production of NO in the RAW264.7 cells in a
dose-dependent manner in vitro. The release of NO from
macrophages is an intracellular messenger molecule in the
nonspecific immune response (25,27).
The release of NO has been suggested to be important in the immune
response (28). Therefore,
sulfated K5PSs may be an immune mediator/modulator with multiple
biological functions, including macrophage-mediated activity.
Activated macrophages are considered to be associated with cytokine
release. Cytokines are signaling molecules, which control
homeostasis in organisms by the regulation of cell differentiation,
proliferation and apoptosis, and also regulate defense functions,
including immune responses (29).
TNF-α, produced by activated macrophages, T lymphocytes and NK
cells, functions as a key cytokine in immune and inflammatory
reactions (30). IL-1β is one of
the most important cytokines that facilitates activated macrophage
release and functions as a mediator of the immune system (29). In the present study,
K5-OS2 and K5-N,OS were observed to markedly stimulate
RAW264.7 cells to release TNF-α and IL-1β. Therefore, K5-OS2 and
K5-N,OS may be investigated as novel immune-stimulants for drugs in
the future (28).
K5-OS2 and K5-N,OS also upregulated the expression
levels of iNOS, TNF-α and IL-1β mRNA, which may explain the
immunostimulatory properties of K5-OS2 and K5-N,OS. Our
previous study also confirmed that the stimulation of macrophages
by sulfated polysaccharides was superior to the stimulation
provided by unmodified polysaccharides (5).
The production of NO by activated macrophages can
lead to cytostatic and cytotoxic activities on malignant cells
(31). The results of the present
study revealed that K5-OS1 and K5-OS2
markedly induced macrophage-mediated cytotoxicity against cancer
cells. Therefore, macrophages activated by K5-OS1 and
K5-OS2 may exert tumoricidal activity through
NO-dependent pathways (32).
K5-OS2 promoted phagocytosis, which is the first step in
the macrophage response to invading microorganisms, followed by the
activation of phagocytosis, which elevates the innate immune
response (33,34). The results of the present study
indicated that K5-OS2 may have indirect antitumor
activity by improving immunologic function.
Previous investigations have demonstrated that
polysaccharides have several bioactivities and that appropriate
molecular modification or structure reform may lead to the
generation or enhancement of activity in polysaccharides (5). Sulfated modification of
polysaccharides is one of the commonly used modification methods.
Sulfated polysaccharides exert potent biological properties, which
are relative to the degree of substitution and the position of
sulfated groups (4). Therefore,
the DS of polysaccharides may be an important parameter that
affects bioactivities. In the present study, K5-OS2
exhibited the most marked activity, which can be attributed to a
higher DS. K5-OS1 had a lower DS, which indicated that
the sulfate esters were important in its biological activity
(4,35), however, previous evidence suggests
that the position of the sulfate substituent is important for
bioactivity (27), consistent with
the present study. K5-NS, with a higher DS at the N-position,
exhibited minimal stimulation of the RAW264.7 cells in
vitro, whereas K5-N,OS induced macrophage formation and
exhibited immunoregulation activity. Comparison between the
structures and activities of K5-NS and K5-N,OS indicated that the
sulfate substitution position was one of the key structural factors
that determined the efficacy of RAW264.7 macrophage stimulation
in vitro (36). Sulfation
in the O-position of the K5 polysaccharide appeared to be more
effective, compared with sulfation in the N-position.
In conclusion, sulfation modification was observed
to significantly enhance the macrophage immunomodulatory activity
of E. coli K5PSs. High levels of sulfation in the O-position
of K5PSs may be required to produce immunomodulatory activities.
The results of the present study also suggested that
K5-OS2 may be a promising novel immunopotentiator.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 21306066
and 51303068), and the Natural Science Foundation of Jiangsu
Province (grant no. BK2012557).
References
|
1
|
Vann WF, Schmidt MA, Jann B and Jann K:
The structure of the capsular polysaccharide (K5 antigen) of
urinary-tract-infective Escherichia coli 010:K5:H4.A polymer
similar to desulfo-heparin. Eur J Biochem. 116:359–364. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Z, Ly M, Zhang F, Zhong W, Suen A,
Hickey AM, Dordick JS and Linhardt RJ: E. coli K5 fermentation and
the preparation of heparosan, a bioengineered heparin precursor.
Biotechnol Bioeng. 107:964–973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun Y, Liang H, Cai G, Guan S, Tong H,
Yang X and Liu J: Sulfatedmodificationof the water-soluble
polysaccharides from Polyporus albicans mycelia and its potential
biological activities. Int J Biol Macromol. 44:14–17. 2009.
View Article : Google Scholar
|
|
4
|
Wang X, Zhang Z, Yao Z, Zhao M and Qi H:
Sulfation, anticoagulant and antioxidant activities of
polysaccharide from green algae Enteromorpha linza. Int J Biol
Macromol. 58:225–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao X, Hu Y, Wang D, Liu J and Guo L: The
comparison of immune-enhancing activity of sulfated
polysaccharidses from Tremella and Condonpsis pilosula. Carbohydr
Polym. 98:438–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li P, Sheng J, Liu Y, Li J, Liu J and Wang
F: Heparosan-derived heparan sulfate/heparin-like compounds: One
kind of potential therapeutic agents. Med Res Rev. 33:665–692.
2013. View Article : Google Scholar
|
|
7
|
Gori AM, Attanasio M, Gazzini A, Rossi L,
Lucarini L, Miletti S, Chini J, Manoni M, Abbate R and Gensini GF:
Cytokine gene expression and production by human LPS-stimulated
mono-nuclear cells are inhibited by sulfated heparin-like
semi-synthetic derivatives. J Thromb Haemost. 2:1657–1662. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Urbinati C, Bugatti A, Oreste P, Zoppetti
G, Waltenberger J, Mitola S, Ribatti D, Presta M and Rusnati M:
Chemically sulfated Escherichia coli K5 polysaccharide derivatives
as extracellular HIV-1 Tat protein antagonists. FEBS Lett.
568:171–177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmed AB, Adel M, Karimi P and Peidayesh
M: Pharmaceutical, cosmeceutical, and traditional applications of
marine carbohydrates. Adv Food Nutr Res. 73:197–220. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raveendran S, Yoshida Y, Maekawa T and
Kumar DS: Pharmaceutically versatile sulfated polysaccharide based
bionano platforms. Nanomedicine. 9:605–626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Górski A, Wasik M, Nowaczyk M and
Korczak-Kowalska G: Immunomodulating activity of heparin. FASEB J.
5:2287–2291. 1991.PubMed/NCBI
|
|
12
|
Górski A, Wasik M, Stepień-Sopniewska B
and Glapiński T: Heparin and platelet interactions influence
alloantigen-induced proliferative responses of human T lymphocytes
and immunoglobulin synthesis in vivo. Immunol Lett. 28:161–165.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto H, Fuyama S, Arai S and Sendo F:
Inhibition of mouse natural killer cytotoxicity by heparin. Cell
Immunol. 96:409–417. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robertson MS: Heparin: The cheap
alternative for immunomodulation in sepsis. Crit Care Resusc.
8:235–238. 2006.PubMed/NCBI
|
|
15
|
Simon Davis DA and Parish CR: Heparan
sulfate:A ubiquitous glycosaminoglycan with multiple roles in
immunity. Front Immunol. 4:4702013. View Article : Google Scholar
|
|
16
|
Zhang C, Liu L, Teng L and Chen J, Liu J,
Li J, Du G and Chen J: Metabolic engineering of Escherichia coli
BL21 for biosynthesis of heparosan, a bioengineered heparin
precursor. Metab Eng. 14:521–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bitter T and Muir HM: A modified uronic
acid carbazole reaction. Anal Biochem. 4:330–334. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawai Y, Seno N and Anno K: A modified
method for chondrosulfatase assay. Anal Biochem. 32:314–321. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nguyen HY, Vo BH, Nguyen LT, Bernad J,
Alaeddine M, Coste A, Reybier K, Pipy B and Nepveu F: Extracts of
Crinum latifolium inhibit the cell viability of mouse lymphoma cell
line EL4 and induce activation of anti-tumour activity of
macrophages in vitro. J Ethnopharmacol. 149:75–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yi Y, Zhang M, Liao S, Zhang R, Deng Y,
Wei Z, Tang X and Zhang Y: Structural features and immunomodulatory
activities of polysaccharides of logan pulp. Carbohydr Polym.
87:636–643. 2012. View Article : Google Scholar
|
|
21
|
Wu J, Li M, Liu L, An Q, Zhang J, Zhang J,
Li M, Duan W, Liu D, Li Z, et al: Nitric oxide and interleukins are
involved in cell proliferation of RAW264.7 macrophages activated by
viili exopolysaccharides. Inflammation. 36:954–961. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang CX and Dai ZR: Immunomodulatory
activities on macrophage of a polysaccharide from Sipunculus nudus
L. Food Chem Toxicol. 49:2961–2967. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Wang J, Xu Z, Li Z, Feng S and Lu
H: The impact of rhubarb polysaccharides on Toll-like receptor
4-mediated activation of macrophages. Int Immunopharmacol.
17:1116–1119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takeda K, Tomimori K, Kimura R, Ishikawa
C, Nowling TK and Mori N: Anti-tumor activity of fucoidan is
mediated by nitric oxide released from macrophages. Int J Oncol.
40:251–260. 2012.
|
|
25
|
Kim HK, Lee JY, Han HS, Kim YJ, Kim HJ,
Kim YS, Kim HM, Ko SG, An HJ, Lee YJ, et al: Immunomodulatory
effects of Liriope platyphylla water extract on
lipopolysaccharide-activated mouse macrophage. Nutrients.
4:1887–1897. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schepetkin IA, Xie G, Kirpotina LN, Klein
RA, Jutila MA and Quinn MT: Macrophage immunomodulatory activity of
polysaccharides isolated from Opuntia polyacantha. Int
Immunopharmacol. 8:1455–1466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Byeon SE, Lee J, Kim JH, Yang WS, Kwak YS,
Kim SY, Choung ES, Rhee MH and Cho JY: Molecular mechanism of
macrophage activation by red ginseng acidic polysaccharide from
Korean red ginseng. Mediators Inflamm. 2012:7328602012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meng LZ, Lin BQ, Wang B, Feng K, Hu DJ,
Wang LY, Cheong KL, Zhao J and Li SP: Mycelia extracts of fungal
strains isolated from Cordyceps sinensis differently enhance the
function of RAW 264.7 macrophages. J Ethnopharmacol. 148:818–825.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park HY, Yu AR, Choi IW, Hong HD, Lee KW
and Choi HD: Immunostimulatory effects and characterization of a
glycoprotein fraction from rice bran. Int Immunopharmacol.
17:191–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jacques A, Bleau C, Turbide C, Beauchemin
N and Lamontagne L: Macrophage interleukin-6 and tumour necrosis
factor-alpha are induced by coronavirus fixation to Toll-like
receptor 2/heparan sulphate receptors but not carcinoembryonic cell
adhesion antigen 1a. Immunology. 128(1 Suppl): e181–e192. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin YS, Lin CH, Huang LD, Chao T, Kuo CD,
Hung LC, Wong FH, Lin CC and Fu SL: The suppression of thoc1 in
cancer cell apoptosis mediated by activated macrophages is nitric
oxide-dependent. Biochem Pharmacol. 86:242–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao T, Mao G, Mao R, Zou Y, Zheng D, Feng
W, Ren Y, Wang W, Zheng W, Song J, et al: Antitumor and
immunomodulatory activity of a water-soluble low molecular weight
polysaccharide from Schisandra chinensis (Turcz.) Baill. Food Chem
Toxicol. 55:609–616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang PY, Zhu XL and Lin ZB: Antitumor and
immunomodulatory effects of polysaccharides from broken-spore of
Ganoderma lucidum. Front Pharmacol. 3:1352012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nguyen HY, Vo BH, Nguyen LT, Bernad J,
Alaeddine M, Coste A, Reybier K, Pipy B and Nepveu F: Extracts of
Crinum latifolium inhibit the cell viability of mouse lymphoma cell
line EL4 and induce activation of anti-tumour activity of
macrophages in vitro. J Ethnopharmacol. 149:75–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang J, Zhu B, Zheng J, Sun L, Zhou D,
Dong X and Yu C: Stimulation of lymphocyte proliferation by oyster
glycogen sulfated at C-6 position. Carbohydr Polym. 94:301–308.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cardozo FT, Camelini CM, Cordeiro MN,
Mascarello A, Malagoli BG, Larsen IV, Rossi MJ, Nunes RJ, Braga FC,
Brandt CR, et al: Characterization and cytotoxic activity of
sulfated derivatives of polysaccharides from agaricus brasiliensis.
Int J Biol Macromol. 57:265–272. 2013. View Article : Google Scholar : PubMed/NCBI
|